Expression and Prognostic Value of Inward Rectifier Potassium Channel Subfamily J Member 11 mRNA in Gliomas
-
摘要:目的
分析KCNJ11 mRNA在脑胶质瘤中的表达及其预测预后价值。
方法收集CGGA数据库中273例脑胶质瘤患者临床、组织病理及分子病理特征,分析不同类型患者KCNJ11 mRNA表达差异,以及不同亚型患者中KCNJ11 mRNA高表达与低表达患者生存时间差异。
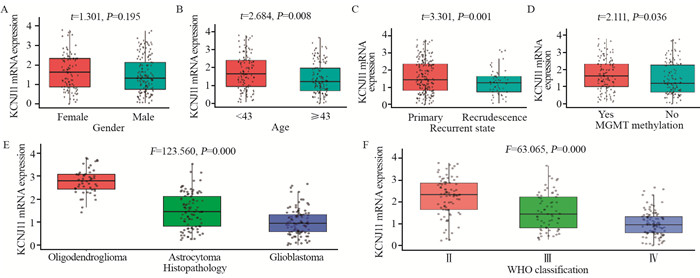
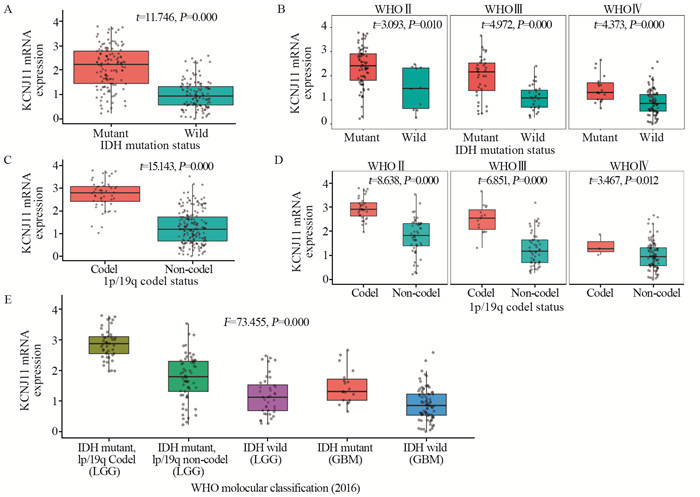
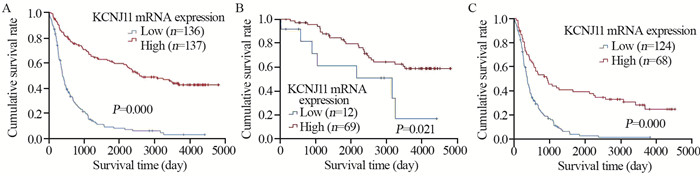
结果脑胶质瘤患者年龄越大KCNJ11 mRNA表达越低(P=0.008),原发胶质瘤KCNJ11 mRNA表达高于复发胶质瘤(P=0.001)。少枝胶质细胞瘤KCNJ11 mRNA表达 > 星形细胞瘤 > 胶质母细胞瘤(P=0.000)。WHOⅡ级KCNJ11 mRNA表达 > WHOⅢ级 > WHOⅣ级(P=0.000)。IDH突变型KCNJ11 mRNA表达高于IDH野生型(P=0.000)。1p/19q缺失型KCNJ11 mRNA表达高于1p/19q不缺失型(P=0.000)。MGMT甲基化KCNJ11 mRNA高于无甲基化(P=0.036)。KCNJ11 mRNA高表达患者(≥2.77)生存期长于低表达患者(< 2.77)(P=0.000)。多因素Cox分析提示KCNJ11 mRNA高表达是脑胶质瘤患者良好预后的独立影响因素。
结论脑胶质瘤恶性程度越高,KCNJ11 mRNA表达越低。KCNJ11 mRNA高表达是影响脑胶质瘤患者良好预后的独立影响因素。
Abstract:ObjectiveTo analyze the expression of KCNJ11 mRNA in gliomas and its prognostic value.
MethodsThe clinical, histopathological and molecular pathological features of 273 patients with gliomas were collected from CGGA. We analyzed the differences of KCNJ11 mRNA expression in different types of gliomas and the survival time of patients with high and low expression of KCNJ11 mRNA in different subtypes of gliomas.
ResultsThe expression levels of KCNJ11 mRNA in young glioma and primary glioma patients were higher than those in old glioma and recurrent glioma patients, respectively (P=0.008, 0.001). The expression of KCNJ11 mRNA in oligodendroglioma was the highest, astrocytoma was the second, and glioblastoma was the lowest (P=0.000). The expression of KCNJ11 mRNA in WHOⅡ grade glioma was the highest, WHOⅢ was the second, and WHOⅣ was the lowest (P=0.000). The expression levels of KCNJ11 mRNA in IDH-mutant type glioma patients were higher than those in IDH-wild type glioma patients (P=0.000). The expression of KCNJ11 mRNA in deletion of 1p/19q glioma patients was higher than that in non-deletion of 1p/19q ones (P=0.000). The expression of KCNJ11 mRNA in MGMT methylated glioma patients was higher than that in non-methylated ones (P=0.036). The survival time of patients with high expression of KCNJ11 mRNA (≥2.77) was longer than that with low expression (P=0.000). Multivariate Cox analysis showed that the high expression of KCNJ11 mRNA was an independent factor affecting the good prognosis of patients with glioma.
ConclusionThe expression of KCNJ11 mRNA is negatively related to the malignant degree of the tumor. The high expression of KCNJ11 mRNA is an independent factor affecting the good prognosis of patients with glioma.
-
Key words:
- Glioma /
- KCNJ11 /
- Molecular typing /
- Mechanism
-
0 引言
胶质瘤是颅内最常见的原发恶性肿瘤[1]。近年来,研究阐明了胶质瘤的一些遗传变化,如IDH1/2[2]和TP53[3]及ATRX[4]突变、tert启动子突变[5]、MET扩增[6]、MGMT启动子甲基化[7]和1p/19q共缺失[8],有助于指导胶质瘤的分类和治疗。世界卫生组织(WHO)于2016年结合分子标志物IDH及1p19q对其进行了分子分类[9]。但是高级别胶质瘤预后不良,尤其胶质母细胞瘤患者5年生存率仅为5%[1, 10],目前利用高通量技术寻找、筛查对临床诊断及预后判断有价值的分子指标,并据此对胶质瘤进行分子分类,进一步进行相应靶向药物研发成为研究热点[11-12]。
研究发现,跨细胞膜的离子通量的变化对于维持细胞的动态平衡至关重要。离子通道的失调和(或)功能异常是包括癌症在内的多种疾病的发病机制中的关键事件。包括电压门控、瞬时受体电位通道以及ATP结合盒转运蛋白在内的离子通道在癌症生物学中发挥相关作用,可能是进行临床干预的新标志物和靶点[13-15]。由内向整流型钾离子通道亚家族J成员11(inward rectifier potassium channel subfamily J member 11, KCNJ11)编码的Kir6.2和ATP结合盒亚家族C成员8(ATP binding cassette subfamily C member 8, ABCC8)编码的SUR1组成的ATP敏感度钾通道(KATP)对血糖浓度与胰岛素分泌的耦合起关键作用。ABCC8或KCNJ11突变引起通道功能下降会导致先天性高胰岛素血症[16],高胰岛素血症可促进结直肠癌发生[17]。而KCNJ11突变引起的通道功能增加可导致新生儿糖尿病[18],KCNJ11突变糖尿病患儿常合并有发育延迟、癫痫、认知功能下降神经系统症状[19]。先前研究发现,ABCC8 mRNA表达可以预测脑胶质瘤患者的预后及对替莫唑胺的敏感度[20],但KCNJ11在脑胶质瘤中的表达及作用价值未见报道。在此,我们研究了273例不同脑胶质瘤患者的KCNJ11 mRNA表达,探讨其作预后预测指标的应用价值。
1 资料与方法
1.1 一般资料
从中国脑胶质瘤基因组图谱计划(CGGA)中收集273例不同脑胶质瘤患者的临床信息(性别、年龄、复发与否、术后放化疗)、病理信息(组织病理类型、WHO分级、IDH突变、1p/19q缺失、MGMT甲基化)及随访信息(生存期)。根据2016年世界卫生组织标准对所有患者进行分子分级。从最初病理诊断到死亡或最后一次随访日期,计算临床终点事件的总生存率。本研究获得北京天坛医院机构伦理委员会批准(KYSB 2015-017),所有患者均签署了书面知情同意书。
1.2 研究方法
从CGGA数据库(http://www.cgga.org.cn/download.jsp)中下载入选的273例不同脑胶质瘤患者的KCNJ11 mRNA表达数据。所有入选患者病变组织均经病理证实为脑胶质瘤。数据库中通过转录组测序,获取KCNJ11 mRNA表达数据。所有实验方法均按照相关指南和规定进行。RNAseq文库的制备、测序和数据分析与本研究组之前的研究[21]一样。使用安捷伦2100生物分析仪检查总RNA的完整性。仅使用RNA完整性值(RIN)≥6.8的优质样本构建测序文库。使用2100生物分析仪测量DNA片段的长度,然后使用Illumina HiSeq 2000/2500/4000测序系统对RNA序列库进行测序。阅读长度分别为101、125或150 bp。
1.3 统计学方法
使用SPSS 25.0软件、R 3.3.2软件进行统计学分析和盒式散点图及生存曲线绘制。采用卡方检验比较不同临床及病理特征的脑胶质瘤患者KCNJ11 mRNA表达。对符合正态分布的KCNJ11 mRNA表达采用独立样本t检验、单因素方差分析及LSD-t两两比较法进行统计学比较。对不同组别脑胶质瘤患者生存期采用Kaplan-Meier曲线及Log rank检验进行分析。对脑胶质瘤患者的生存影响因素采用单因素及多因素Cox回归分析。所有统计检验均为双侧性,P < 0.05为差异有统计学意义。
2 结果
2.1 患者一般临床资料、病理特征及KCNJ11 mRNA表达
根据入选的273例脑胶质瘤患者KCNJ11 mRNA中位表达值(2.77)分类,≥2.77为高表达、 < 2.77为低表达。KCNJ11 mRNA表达与脑胶质瘤患者年龄、是否复发、组织病理类型、WHO分级、IDH突变状态、1p/19q共缺失状态、MGMT甲基化状态、术后有无接受化疗及WHO 2016年分子分级显著相关(P < 0.05),见表 1。
表 1 273例脑胶质瘤患者临床病理特征与KCNJ11 mRNA表达Table 1 Clinicopathological features and KCNJ11 mRNA expression in 273 patients with glioma
2.2 不同临床及组织病理特征脑胶质瘤患者的KCNJ11 mRNA表达比较
在预后较好的脑胶质瘤患者,如:年轻患者(P=0.008)、原发胶质瘤(P=0.001)、MGMT甲基化胶质瘤(P=0.036)患者中KCNJ11 mRNA表达较高。病理类型中KCNJ11 mRNA在少枝胶质细胞瘤患者中表达最高,星形细胞瘤患者次之,胶质母细胞瘤患者中最低(P=0.000)。在WHO分级中KCNJ11 mRNA在Ⅱ级胶质瘤中表达最高,Ⅲ级次之,Ⅳ级胶质瘤患者中最低(P=0.000),见图 1。
2.3 不同分子病理特征脑胶质瘤患者KCNJ11 mRNA表达比较
IDH突变型脑胶质瘤患者KCNJ11 mRNA表达高于IDH野生型患者(P=0.000);根据WHO分级分层后,各级别脑胶质瘤患者中仍出现同样的结果,见图 2A、B。1p/19q联合缺失脑胶质瘤患者KCNJ11 mRNA表达高于无联合缺失患者(P=0.000);根据WHO分级分层后,各级别脑胶质瘤患者中仍出现同样的结果,见图 2C、D。IDH突变、1p/19q缺失型较低级别胶质瘤(WHOⅡ~Ⅲ)KCNJ11 mRNA表达高于IDH突变、1p/19q无缺失型较低级别胶质瘤(WHOⅡ~Ⅲ)和IDH野生型较低级别胶质瘤(WHOⅡ~Ⅲ)(P=0.000),见图 2E。
2.4 脑胶质瘤患者KCNJ11 mRNA表达与生存期的关系
结果表明,KCNJ11 mRNA高表达脑胶质瘤患者比低表达患者生存期更长(χ2=92.891, P=0.000)。进一步亚组分析显示在低级别(χ2=5.313, P=0.021)和高级别脑胶质瘤患者(χ2=29.456, P=0.000)中,KCNJ11 mRNA高表达患者较低表达患者均有更长的生存期,见图 3。
2.5 影响脑胶质瘤患者生存期因素的单因素及多因素Cox回归分析
单因素Cox分析显示年龄、是否复发、组织病理、WHO分级、IDH突变状态、1p/19q共缺失状态、术后放疗、术后化疗和KCNJ11 mRNA表达是影响患者生存期的因素(均P < 0.05)。多因素Cox分析显示KCNJ11 mRNA高表达、1p/19q共缺失和术后化疗是良好生存期的独立影响因素(均P < 0.05, 均HR < 1),而年龄、复发和WHO分级是不良生存期的独立影响因素(均P < 0.05, 均HR > 1),见表 2。
表 2 影响脑胶质瘤患者生存期的单因素、多因素Cox回归分析Table 2 Univariate and multivariate Cox regression analyses of survival of glioma patients
3 讨论
KCNJ11基因编码三磷酸腺苷敏感度钾通道的Kir6.2亚基。KATP通道是独特进化的蛋白质复合物,先后在心肌、胰腺β细胞、脑、骨骼肌和血管平滑肌中被发现。通过将细胞腺嘌呤核苷酸浓度与膜电位耦合,起“代谢传感器”的作用,可将细胞能量水平与细胞兴奋性耦合,将代谢状态转化为电活动,控制着广泛的生理过程,包括激素分泌、神经元传递、血管扩张以及针对缺血性损伤的心脏和神经元预处理[22],以适应不断变化的代谢环境。分子克隆和功能表征揭示由内向整流钾通道(Kir)和磺酰脲受体(SUR)两种蛋白共同组成该通道,属于ATP结合盒(ABC)转运蛋白超家族[23]。Kir可分为Kir6.1和Kir6.2,SUR可分为SUR1和SUR2,SUR2又可分为SUR2A及SUR2B。不同组织中的KATP通道由于Kir6.1或Kir6.2与SUR1或SUR2的不同组合,具有不同的功能和药理学特性。Kir6.2/SUR1主要表达于胰腺内分泌胰岛和大脑,Kir6.2/SUR2A主要表达心肌细胞和骨骼肌,Kir6.1/SUR2B主要表达于血管平滑肌。SUR1由ABCC8基因编码,ABCC8和KCNJ11基因突变与一系列胰岛素分泌和神经系统疾病相关。本研究发现:KCNJ11 mRNA表达与脑胶质瘤恶性程度有关,恶性程度越高,表达越低;低表达于星形胶质细胞瘤、WHOⅢ~Ⅳ级、高分子分级胶质瘤、IDH野生型及1p19q无缺失型胶质瘤患者,提示其可以用于预测脑胶质瘤患者肿瘤的恶性程度。同时KCNJ11 mRNA表达与脑胶质瘤患者的生存期有关,表达越低,生存期越短,提示其可以用于预测患者的生存预后。同时本研究还发现:KCNJ11 mRNA表达是影响脑胶质瘤患者生存期的独立影响因素,可以作为预后指标预测脑胶质瘤患者生存期。类似的研究目前未见报道。
KCNJ11 mRNA在恶性脑胶质瘤中表达降低及低表达引起短生存期的具体机制目前尚不清楚。研究发现:几种离子通道的表达受激素、化学致癌物或人乳头瘤病毒癌基因等肿瘤相关因素的调节[24-26]。Sato等[27]研究发现:中度缺氧诱导胰腺β细胞凋亡,使胰岛中KCNJ11表达下调,揭示低氧是胰腺β细胞的新型应激源,并且低氧应激导致β细胞功能恶化。由此推测,随着恶性程度的增加,脑胶质瘤细胞增殖加速,细胞密度增加,内部坏死,肿瘤细胞处于饥饿缺氧状态,导致KCNJ11表达下调,有待进一步实验验证。Zhang等[28]研究发现,真核RNA G-四链体富含mRNA 3'-UTR,保证了KCNJ11 mRNA的正常表达。而microRNA通过结合mRNA 3'-UTR,破坏了心肌细胞中的RNA G-四链体稳定性,抑制了KCNJ11表达。
KCNJ11编码胰腺ATP敏感钾通道(KATP)的Kir6.2亚单位。该通道有两个重要的亚基:成孔的Kir6.2亚基和磺酰脲受体1调节亚基。KCNJ11 mRNA在β细胞和神经元中的表达升高。通道开放引起的KATP电流增加导致β细胞膜超极化,从而抑制胰岛素分泌。相反,在葡萄糖增加时关闭这一通道会触发胰岛素从β细胞释放到血液中,从而有助于控制血糖水平。在神经元中,KATP电流增加会导致电活动减少。KCNJ11突变与两种主要表型有关:中度发育迟缓和(或)肌肉无力但不伴有癫痫的新生儿糖尿病和发育迟缓、癫痫的新生儿糖尿病。本研究发现,在少突胶质细胞瘤患者中,KCNJ11 mRNA表达上调,而少突胶质细胞瘤患者常合并癫痫发作,两者之间是否存在一定的联系,有待进一步研究。
Griscelli等[29]提取KCNJ11突变的糖尿病患者诱导性多能干细胞,通过非整合型病毒转导重新编程的KCNJ11突变细胞具有正常的核型,而KCNJ11突变细胞具有表达多能性标志,并具有向三个胚层的分化能力。由此提示,在KCNJ11低表达的胶质瘤中,是否具有更多的幼稚干细胞,导致其增殖能力及恶性度更高,预后更差,有待进一步研究。
综上,本研究发现脑胶质瘤恶性程度越高,KCNJ11 mRNA表达越低。KCNJ11 mRNA高表达是脑胶质瘤患者良好预后的独立影响因素。但脑胶质瘤患者中KCNJ11 mRNA转录调控、蛋白翻译水平调控作用机制及针对该靶点的靶向药物研发等相关研究较少,值得进一步研究跟挖掘。
Competing interests: The authors declare that they have no competing interests.作者贡献:王芳:实验设计、数据收集及下载、分析及撰写论文周开甲:指导实验设计、数据统计分析、论文写作及校对论文赵征:指导数据下载、分析及修改论文梁晖:提供论文写作指导刘彦伟:对课题思路提出建议及指导论文写作 -
表 1 273例脑胶质瘤患者临床病理特征与KCNJ11 mRNA表达
Table 1 Clinicopathological features and KCNJ11 mRNA expression in 273 patients with glioma

表 2 影响脑胶质瘤患者生存期的单因素、多因素Cox回归分析
Table 2 Univariate and multivariate Cox regression analyses of survival of glioma patients

-
[1] Jiang T, Mao Y, Ma W, et al. CGCG clinical practice guidelines for the management of adult diffuse gliomas[J]. Cancer Lett, 2016, 375(2): 263-273. doi: 10.1016/j.canlet.2016.01.024
[2] Konteatis Z, Artin E, Nicolay B, et al. Vorasidenib (AG-881): A First-in-Class, Brain-Penetrant Dual Inhibitor of Mutant IDH1 and 2 for Treatment of Glioma[J]. ACS Med Chem Lett, 2020, 11(2): 101-107. doi: 10.1021/acsmedchemlett.9b00509
[3] Yu BX, Zou L, Li S, et al. LncRNA SAMD12-AS1 down-regulates P53 to promote malignant progression of glioma[J]. Eur Rev Med Pharmacol Sci, 2019, 23(19): 8456-8467. https://pubmed.ncbi.nlm.nih.gov/31646576/
[4] Xie YB, Tan YL, Yang C, et al. Omics-based integrated analysis identified ATRX as a biomarker associated with glioma diagnosis and prognosis[J]. Cancer Biol Med, 2019, 16(4): 784-796. https://pubmed.ncbi.nlm.nih.gov/31908895/
[5] Razis E, Kotoula V, Koliou GA, et al. Is There an Independent Role of TERT and NF1 in High Grade Gliomas?[J]. Transl Oncol, 2020, 13(2): 346-354. doi: 10.1016/j.tranon.2019.10.016
[6] Hu HM, Mu QH, Bao ZS, et al. Mutational Landscape of Secondary Glioblastoma Guides MET-Targeted Trial in Brain Tumor[J]. Cell, 2018, 175(6): 1665-1678. e18. doi: 10.1016/j.cell.2018.09.038
[7] Malmström A, Łysiak M, Kristensen BW, et al. Do we really know who has an MGMT methylated glioma? Results of an international survey regarding use of MGMT analyses for glioma[J]. Neurooncol Pract, 2020, 7(1): 68-76. https://pubmed.ncbi.nlm.nih.gov/32025325/
[8] Zacher A, Kaulich K, Stepanow S, et al. Molecular Diagnostics of Gliomas Using Next Generation Sequencing of a Glioma-Tailored Gene Panel[J]. Brain Pathol, 2017, 27(2): 146-159. doi: 10.1111/bpa.12367
[9] Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary[J]. Acta Neuropathol, 2016, 131(6): 803-820. doi: 10.1007/s00401-016-1545-1
[10] 李壮, 杨军, 苏文. 胶质母细胞瘤的治疗现状[J]. 临床医学进展, 2020, 10(12): 2875-2882. https://www.cnki.com.cn/Article/CJFDTOTAL-SXZL201924039.htm Li Z, Yang J, Su W. Current Status of Glioblastoma Treatment[J]. Lin Chuang Yi Xue Jin Zhan, 2020, 10(12): 2875-2882. https://www.cnki.com.cn/Article/CJFDTOTAL-SXZL201924039.htm
[11] Zacher A, Kaulich K, Stepanow S, et al. Molecular Diagnostics of Gliomas Using Next Generation Sequencing of a Glioma-Tailored Gene Panel[J]. Brain Pathol, 2017, 27(2): 146-159. doi: 10.1111/bpa.12367
[12] Konteatis Z, Artin E, Nicolay B, et al. Vorasidenib (AG-881): A First-in-Class, Brain-Penetrant Dual Inhibitor of Mutant IDH1 and 2 for Treatment of Glioma[J]. ACS Med Chem Lett, 2020, 11(2): 101-107. doi: 10.1021/acsmedchemlett.9b00509
[13] Anderson KJ, Cormier RT, Scott PM. Role of ion channels in gastrointestinal cancer[J]. World J Gastroenterol, 2019, 25(38): 5732-5772. doi: 10.3748/wjg.v25.i38.5732
[14] Chávez-López MG, Zúñiga-García V, Pérez-Carreón JI, et al. Eag1 channels as potential early-stage biomarkers of hepatocellular carcinoma[J]. Biologics, 2016, 10: 139-148. https://www.dovepress.com/eag1-channels-as-potential-early-stage-biomarkers-of-hepatocellular-ca-peer-reviewed-fulltext-article-BTT
[15] Hassan AY, Maulood IM, Salihi A. The vasodilatory mechanism of nitric oxide and hydrogen sulfide in the human mesenteric artery in patients with colorectal cancer[J]. Exp Ther Med, 2021, 21(3): 214. doi: 10.3892/etm.2021.9646
[16] Kandasamy B, Shyng SL. Methods for Characterizing Disease-Associated ATP-Sensitive Potassium Channel Mutations[J]. Methods Mol Biol, 2018, 1684: 85-104. https://pubmed.ncbi.nlm.nih.gov/29058186/
[17] Tanaka H, Imai H, Higashi T, et al. Splenic artery transposition for hepatic arterial reconstruction in conversion surgery of an initially unresectable, locally advanced pancreatic cancer after gemcitabine/nab-paclitaxel: A case report[J]. Int J Surg Case Rep, 2021, 78: 192-196. doi: 10.1016/j.ijscr.2020.12.041
[18] Bowman P, Day J, Torrens L, et al. Cognitive, Neurological, and Behavioral Features in Adults With KCNJ11 Neonatal Diabetes[J]. Diabetes Care, 2019, 42(2): 215-224. doi: 10.2337/dc18-1060
[19] Ludwig A, Enke S, Heindorf J, et al. Formal Neurocognitive Testing in 60 Patients with Congenital Hyperinsulinism[J]. Horm Res Paediatr, 2018, 89(1): 1-6. doi: 10.1159/000481774
[20] 周开甲, 刘彦伟, 赵征, 等. ABCC8在胶质瘤中的表达及其临床意义[J]. 中华神经医学杂志, 2020, 19(12): 1260-1266. doi: 10.3760/cma.j.cn115354-20200714-00563 Zhou KJ, Liu YW, Zhao Z, et al. Expression of ATP binding cassette subfamily C member 8 and its clinical significance in gliomas[J]. Zhonghua Shen Jing Yi Xue Za Zhi, 2020, 19(12): 1260-1266. doi: 10.3760/cma.j.cn115354-20200714-00563
[21] Zhao Z, Meng FL, Wang W, et al. Comprehensive RNA-seq transcriptomic profiling in the malignant progression of gliomas[J]. Sci Data, 2017, 4: 170024. doi: 10.1038/sdata.2017.24
[22] Martin GM, Sung MW, Shyng SL. Pharmacological chaperones of ATP-sensitive potassium channels: Mechanistic insight from cryoEM structures[J]. Mol Cell Endocrinol, 2020, 502: 110667. doi: 10.1016/j.mce.2019.110667
[23] Inagaki N, Gonoi T, Clement JP, et al. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor[J]. Science, 1995, 270(5239): 1166-1170. doi: 10.1126/science.270.5239.1166
[24] Díaz L, Ceja-Ochoa I, Restrepo-Angulo I, et al. Estrogens and human papilloma virus oncogenes regulate human ether-a-go-go-1potassium channel expression[J]. Cancer Res, 2009, 69(8): 3300-3307. doi: 10.1158/0008-5472.CAN-08-2036
[25] Lee JH, Park JW, Byun JK, et al. Silencing of voltage-gated potassium channel KV9.3 inhibits proliferation in human colon and lung carcinoma cells[J]. Oncotarget, 2015, 6(10): 8132-8143. doi: 10.18632/oncotarget.3517
[26] de Guadalupe Chávez-López M, Hernández-Gallegos E, Vázquez-Sánchez AY, et al. Antiproliferative and proapoptotic effects of astemizole on cervical cancer cells[J]. Int J Gynecol Cancer, 2014, 24(5): 824-828. doi: 10.1097/IGC.0000000000000151
[27] Sato Y, Inoue M, Yoshizawa T, et al. Moderate hypoxia induces β-cell dysfunction with HIF-1-independent gene expression changes[J]. PLoS One, 2014, 9(12): e114868. doi: 10.1371/journal.pone.0114868
[28] Zhang J, Wang JX, Li FY, et al. Normal expression of KCNJ11 is maintained by the G-quadruplex[J]. Int J Biol Macromol, 2019, 138: 504-510. doi: 10.1016/j.ijbiomac.2019.07.094
[29] Griscelli F, Feraud O, Ernault T, et al. Generation of an induced pluripotent stem cell (iPSC) line from a patient with maturity-onset diabetes of the young type 13 (MODY13) with a the potassium inwardly-rectifying channel, subfamily J, member 11 (KCNJ11) mutation[J]. Stem Cell Res, 2017, 23: 178-181. doi: 10.1016/j.scr.2017.07.023
-
期刊类型引用(1)
1. 江晓珍,邓葵,花美玲,郭芬棻,杨玄勇,万红萍. PRR11和MMP9在胶质瘤中的表达及与临床预后的关系. 实用临床医学. 2023(04): 1-6 .  百度学术
百度学术
其他类型引用(2)



 下载:
下载: