Application of Ultrasound Combined with DSA-guided Single-incision Technique via Axillary Vein Access in Implantation of Totally Implantable Venous Access Port
-
摘要:目的
探讨超声联合数字减影血管造影技术(DSA)引导下行单切口经腋静脉(AV)入路植入输液港(TIVAP)在临床应用中的可行性及安全性。
方法回顾性分析在福建省肿瘤医院就诊并接受超声联合DSA引导下行单切口经AV入路TIVAP植入术的240例患者临床资料。记录手术相关信息,包括AV宽度、AV穿刺成功率、植入成功率、超声引导穿刺时间、手术时间及术中、术后并发症等。
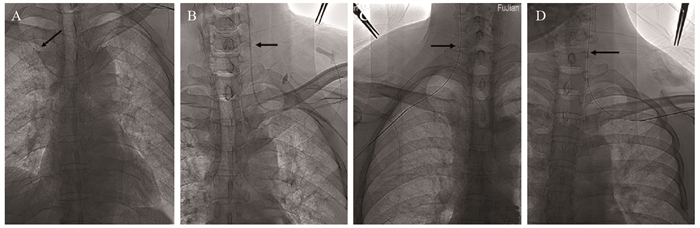
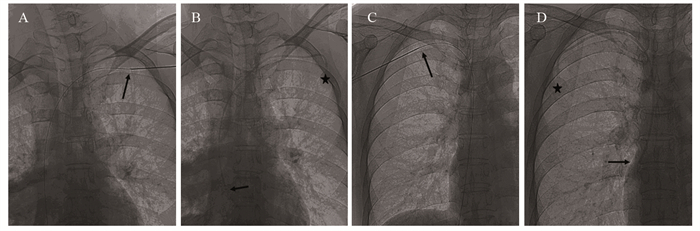
结果240例患者均成功植入TIVAP,植入成功率100%。229例患者于超声联合DSA引导下行单切口经AV穿刺植入TIVAP,AV穿刺成功率95.42%(229/240),11例因AV穿刺失败,改超声联合DSA引导下经同侧颈内静脉(IJV)穿刺植入TIVAP。240例患者术前超声探查定位下测得拟穿刺段AV平均宽度为(7.56±1.26)mm,其中AV 1次穿刺成功195例,2次穿刺成功26例,3次穿刺成功8例,成功率分别为81.25%、10.83%、3.34%;超声引导下平均穿刺时间(0.85±0.52)min,手术平均时间(25.9±4.8)min。术中并发症发生率为1.67%(4/240),未发生血胸、血气胸及严重致死性并发症;TIVAP留置期间相关并发症发生率为2.92%(7/240),未见导管相关血流感染、导管相关静脉血栓、导管断裂/移位、夹闭综合征、药物外渗等并发症发生。
结论超声联合DSA引导下行单切口经AV植入TIVAP术是一种可行且安全的植入方式,具有较高的成功率、较短的手术时长和较低的并发症风险,可作为TIVAP植入方式的另一种选择。
Abstract:ObjectiveTo evaluate the technical feasibility and safety of a single-incision technique via axillary vein (AV) for placement of totally implantable venous access port (TIVAP) guided by ultrasound combined with DSA in clinical application.
MethodsWe retrospectively analyzed clinical data of 240 patients who received TIVAP by single incision technique via AV access guided by ultrasound combined with DSA. We observed and recorded operation-related information such as AV width, AV puncture success rate, implantation success rate, ultrasound-guided puncture time, operation time and intraoperative and postoperative complications, etc.
ResultsAll 240 patients were successfully implanted with TIVAP, and the success rate was 100%. In 229 cases, TIVAP was implanted through single-incision AV puncture under the guidance of ultrasound combined with DSA, and the success rate of AV puncture was 95.42% (229/240). In 11 cases, TIVAP was implanted through the ipsilateral internal jugular vein (IJV) under the guidance of ultrasound combined with DSA due to the failure of AV puncture. In the 240 patients, the average width of AV of the intended puncture segment was (7.56±1.26) mm measured by preoperative ultrasound exploration and positioning, in which 195 cases were successfully punctured once, 26 cases were successfully punctured twice, and 8 cases were successfully punctured three times, with the success rate of 81.25%, 10.83% and 3.34%, respectively. The average puncture time under ultrasound guidance was (0.85±0.52) min, and the average operation time was (25.9±4.8) min. The incidence of intraoperative complications was 1.67% (4/240). No hemothorax, hemopneumothorax or serious fatal complications occurred. The incidence of complications during TIVAP retention was 2.92% (7/240). No complication such as catheter-related bloodstream infection, catheter-related venous thrombosis, catheter rupture/displacement, clipping syndrome or drug extravasation was observed.
ConclusionUltrasound combined with DSA guided single-incision technique via AV access in the implantation of TIVAP is a feasible and safe implantation method with high technical success rate, short operation time and low risk of complications. It can be used as another choice of TIVAP implantation method.
-
Key words:
- Ultrasound /
- DSA /
- Single-incision /
- Axillary vein /
- Totally implantable venous access port
-
Competing interests: The authors declare that they have no competing interests.作者贡献:刘伟夫:选题设计、试验执行、病例资料及数据收集、文章撰写及修改张孔志:指导选题设计及试验执行余文昌:指导试验执行陈示光:病例资料及数据收集王小珑:病例资料收集
-
表 1 240例患者的恶性肿瘤类型
Table 1 Types of malignancies in 240 patients

表 2 AV不同穿刺次数成功的一般资料比较(x±s)
Table 2 General information of patients with different times of successful axillary vein puncture (x±s)

表 3 患者一般资料与腋静脉宽度之间相关性分析
Table 3 Correlation between general date of patients and width of axillary vein

-
[1] Ignatov A, Hoffman O, Smith B, et al. An 11-year retrospective study of totally implanted central venous access ports: Complications and patient satisfaction[J]. Eur J Surg Oncol, 2009, 35(3): 241-246. doi: 10.1016/j.ejso.2008.01.020
[2] Tumay LV, Guner OS. Availability of totally implantable venous access devices in cancer patients is high in the long term: a seven-year follow-up study[J]. Support Care Cancer, 2021, 39(7): 3531-3538. doi: 10.1007/s00520-020-05871-6
[3] 中心静脉通路上海协作组, 上海市抗癌协会实体肿瘤聚焦诊疗专委会血管通路专家委员会. 完全植入式输液港上海专家共识[J]. 介入放射学杂志, 2019, 28(12): 1123-1128. Shanghai cooperation group on central venous access: expert committee on vascular access, Committee of experts on focused diagnosis and treatment of solid tumors, Shanghai anti-cancer association. Consensus of shanghai experts on totally implantable access port(2019)[J]. Jie Ru Fang She Xue Za Zhi, 2019, 28(12): 1123-1128.
[4] Wu S, Huang J, Jiang Z, et al. Internal jugular vein versus subclavian vein as the percutaneous insertion site for totally implantable venous access devices: a meta-analysis of comparative studies[J]. BMC Cancer, 2016, 16(1): 747. doi: 10.1186/s12885-016-2791-2
[5] Velioğlu Y. Yüksel A, Sınmaz E. Complications and management strategies of totally implantable venous access port insertion through percutaneous subclavian vein[J]. Turk Gogus Kalp Damar Cerrahisi Derg, 2019, 27(4): 499-507. doi: 10.5606/tgkdc.dergisi.2019.17972
[6] Ribeiro RC, Abib SC, Aguiar AS, et al. Long-term complications in totally implantable venous access devices: randomized study comparing subclavian and internal jugular vein puncture[J]. Pediatric Blood Cancer, 2012, 58(2): 274-277. doi: 10.1002/pbc.23220
[7] Chen YB, Bao HS, Deng HR, et al. Comparison of comfort and complications in breast cancer patients of implantable venous access port (IVAP) with ultrasound guided internal jugular vein (IJV) and axillary vein/subclavian vein (AxV/SCV) puncture: a randomized controlled study protocol[J]. Ann Palliat Med, 2020, 9(6): 4323-4331. doi: 10.21037/apm-20-1752
[8] Hong S, Seo TS, Song MG, et al. Clinical outcomes of totally implantable venous access port placement via the axillary vein in patients with head and neck malignancy[J]. J Vasc Access, 2019, 20(2): 134-139. doi: 10.1177/1129729818781270
[9] Jiang M, Gong XR, Zhou SH, et al. A Comparison of Steep and Shallow Needle Trajectories in Blind Axillary Vein Puncture[J]. Pacing Clin Electrophysiol, 2013, 36(9): 1150-1155. doi: 10.1111/pace.12156
[10] Seo TS, Song MG, Kim JS, et al. Long-term clinical outcomes of the single-incision technique for implantation of implantable venous access ports via the axillary vein[J]. J Vasc Access, 2017, 18(4): 345-351. doi: 10.5301/jva.5000751
[11] Pardo I, Rager EL, Bowling MW, et al. Central Venous Port Placement: A Comparison of Axillary Versus Anterior Chest Wall Placement[J]. Ann Surg Oncol, 2011, 18(2): 468-471. doi: 10.1245/s10434-010-1353-0
[12] Jiang M, Mao JL, He B. Clinical definition of the axillary vein and experience with blind axillary puncture[J]. Int J Cardiol, 2012, 159(3): 243-245. doi: 10.1016/j.ijcard.2012.05.089
[13] Galloway S, Bodenham A. Ultrasound imaging of the axillary vein-Anatomical basis for central venous access[J]. Br J Anaesth, 2003, 90(5): 589-595. doi: 10.1093/bja/aeg094
[14] Sharma A, Bodenham AR, Mallick A. Ultrasound-guided infraclavicular axillary vein cannulation for central venous access[J]. Br J Anaesth, 2004, 93(2): 188-192. doi: 10.1093/bja/aeh187
[15] O'Leary R, Ahmed SM, McLure H, et al. Ultrasound-guided infraclavicular axillary vein cannulation: a useful alternative to the internal jugular vein[J]. Br J Anaesth, 2012, 109(5): 762-768. doi: 10.1093/bja/aes262
[16] Osawa H, Hasegawa J, Yamakawa K, et al. Ultrasound-guided infraclavicular axillary vein puncture is effective to avoid pinch-off syndrome: A long-term follow-up study[J]. Surg Today, 2013, 43(7): 745-750. doi: 10.1007/s00595-012-0309-3
[17] 刘孜卓, 柴艳芬, 寿松涛, 等. 超声定位法在腋静脉穿刺中的安全性评价[J]. 中华急诊医学杂志, 2019, 28(12): 1520-1523. doi: 10.3760/cma.j.issn.1671-0282.2019.12.012 Liu ZZ, Chai YF, Shou ST, et al. Safety evaluation of ultrasound location in axillary venipuncture[J]. Zhonghua Ji Zhen Yi Xue Za Zhi, 2019, 28(12): 1520-1523. doi: 10.3760/cma.j.issn.1671-0282.2019.12.012
[18] 王敏欢, 何敏, 谢红. 超声引导下腋-锁骨下静脉穿刺的临床效果[J]. 临床麻醉学杂志, 2018, 34(4): 356-358. https://www.cnki.com.cn/Article/CJFDTOTAL-LCMZ201804014.htm Wang MH, He M, Xie H. Clinical effect of ultrasound-guided axillary-subclavian vein catheterization[J]. Lin Chuang Ma Zui Xue Za Zhi, 2018, 34(4): 356-358. https://www.cnki.com.cn/Article/CJFDTOTAL-LCMZ201804014.htm
[19] 盛阮妹, 王海燕, 高彦定, 等. 老年患者超声引导下锁骨下静脉与腋静脉穿刺的比较[J]. 临床麻醉学杂志, 2020, 36(6): 540-543. https://www.cnki.com.cn/Article/CJFDTOTAL-LCMZ202006004.htm Sheng RM, Wang HY, Gao YD, et al. Comparison of ultrasound guided subclavian vein and axillary vein puncture in elderly patients[J]. Lin Chuang Ma Zui Xue Za Zhi, 2020, 36(6): 540-543. https://www.cnki.com.cn/Article/CJFDTOTAL-LCMZ202006004.htm
[20] Kim IS, Kang SS, Park JH, et al. Impact of sex, age and BMI on depth and diameter of the infraclavicular axillary vein when measured by ultrasonography[J]. Eur J Anaesthesiol, 2011, 28(5): 346-350. doi: 10.1097/EJA.0b013e3283416674
[21] Pittiruti M, Biasucci DG, Greca AL, et al. How to make the axillary vein larger? Effect of 90° abduction of the arm to facilitate ultrasound-guided axillary vein puncture[J]. J Critical Care, 2016, 33: 38-41. doi: 10.1016/j.jcrc.2015.12.018
[22] Ahn JH, Kim IS, Shin KM, et al. Influence of arm position on catheter placement during real-time ultrasound-guided right infraclavicular proximal axillary venous catheterization[J]. Br J Anaesth, 2016, 116(3): 363-369. doi: 10.1093/bja/aev345
[23] Ma LI, Liu Y, Wang J, et al. Totally implantable venous access port systems and associated complications: A single-institution retrospective analysis of 2, 996 breast cancer patients[J]. Mol Clin Oncol, 2016, 4(3): 456-460. doi: 10.3892/mco.2016.726
[24] Li Y, Ca I Y, Gan X, et al. Application and comparison of different implanted ports in malignant tumor patients[J]. World J Surg Oncol, 2016, 14(1): 251. doi: 10.1186/s12957-016-1002-6
[25] Wang YC, Lin PL, Chou WH, et al. Long-term outcomes of totally implantable venous access devices[J]. Support Care Cancer, 2017, 25(7): 2049-2054. doi: 10.1007/s00520-017-3592-0
[26] Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th Edition[J]. J Infus Nurs, 2021, 44(1S Suppl 1): S1-S224. http://journals.lww.com/journalofinfusionnursing/Fulltext/2021/01001/Infusion_Therapy_Standards_of_Practice,_8th.1.aspx?context=LatestArticles
[27] Li J, Chen W, Zhao W, et al. Surface measurement, intracardiac electrocardiogram and tracheal bifurcation techniques for locating the catheter tips of totally implantable venous access port[J]. Comput Methods Programs Biomed, 2020, 187: 105238. doi: 10.1016/j.cmpb.2019.105238
[28] Orsi F, Grasso RF, Arnaldi P, et al. Ultrasound guided versus direct vein puncture in central venous port placement[J]. J Vasc Access, 2000, 1(2): 73-77. doi: 10.1177/112972980000100209
[29] Shin HJ, Na HS, Koh WU, et al. Complications in internal jugular vs subclavian ultrasound-guided central venous catheterization: a comparative randomized trial[J]. Intensive Care Med, 2019, 45(7): 968-976. doi: 10.1007/s00134-019-05651-9
[30] Dubberke ER, Carling P, Carrico R, et al. Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 Update[J]. Infect Control Hosp Epidemiol, 2014, 35(6): 628-645. doi: 10.1086/676023
[31] Pinelli F, Cecero E, Degl'Innocenti D, et al. Infection of totally implantable venous access devices: A review of the literature[J]. J Vasc Access, 2018, 19(6): 230-242. http://www.onacademic.com/detail/journal_1000040228839310_c865.html



 下载:
下载: