Effect of LncRNA LINC00857 Knockdown on Migration, Proliferation and Apoptosis of Pancreatic Cancer PANC-1 Cells
-
摘要:目的
探讨LncRNA LINC00857在胰腺细胞系中的表达及其下调对胰腺癌PANC-1细胞增殖、迁移和凋亡的影响,并探讨其机制。
方法构建慢病毒载体GV112,转染胰腺癌细胞株PANC-1为实验组,转染空白质粒为阴性对照组,未干预的细胞作为正常对照组;CCK-8法、Transwell实验、划痕实验、流式细胞术、Western blot检测下调LINC00857对细胞增殖、迁移、凋亡、细胞周期以及上皮间质转化(EMT)相关蛋白的影响。
结果胰腺癌细胞系中LINC00857的表达显著高于胰腺正常上皮细胞(P < 0.01)。敲低LINC00857可显著抑制PANC-1细胞的增殖和迁移能力(P < 0.0001);与阴性对照组相比,实验组细胞的凋亡率明显增加(P < 0.05),G0/G1期细胞数量增多(P < 0.01),S期细胞数目减少(P < 0.05),细胞被阻滞在G1期;E-cadherin表达显著上调(P < 0.05),N-cadherin和Vimentin表达显著下调(N-cadherin P < 0.01, Vimentin P < 0.05)。
结论LINC00857可通过调控G1/S期转化EMT信号通路促进PANC-1细胞的增殖和迁移,并抑制其凋亡。
Abstract:ObjectiveTo investigate the expression of LncRNA LINC00857 in pancreatic cells and the effect of lncRNA LINC00857 down-regulation on proliferation, migration and apoptosis of pancreatic cancer PANC-1 cells and possible mechanism.
MethodsThe lentiviral vector GV112 was constructed and infected PANC-1 cells to obtain experimental group, while the blank plasmid was transfected as a negative control group and the cells without intervention were taken as normal control group. CCK-8 assay, Transwell assay, scratch test, flow cytometry, Western blot were used to detect the effect of LINC00857 down-regulation on cell proliferation, migration, apoptosis, cell cycle and EMT-related proteins.
ResultsLINC00857 expression in pancreatic cancer cells were significantly higher than that in normal pancreatic epithelial cells (P < 0.001). Knockdown of LINC00857 could significantly inhibit the proliferation and migration of PANC-1 cells (P < 0.0001); compared with the negative control group, the apoptosis rates of cells in the experimental groups were significantly increased (P < 0.05), the number of cells in G0/G1 phase increased (P < 0.01), the number of cells in S phase decreased (P < 0.05), and the cells were blocked in the G1 phase, E-cadherin expression was significantly up-regulated (P < 0.05) and N-cadherin and Vimentin expression were significantly down-regulated (N-cadherin: P < 0.01, Vimentin: P < 0.05).
ConclusionLINC00857 can promote proliferation and migration of PANC-1 cells and inhibit its apoptosis by regulating G1/S phase transition and EMT signaling pathway.
-
Key words:
- Pancreatic cancer /
- LncRNA LINC00857 /
- EMT /
- Apoptosis /
- Cell cycle /
- Proliferation /
- Migration
-
0 引言
胰腺癌(pancreatic cancer, PC)是一种常见的消化系统恶性肿瘤,发病率逐年上升,5年生存率仅9%[1-2],其高死亡率与早期症状不典型且易转移有关,大多数患者确诊时即在晚期,缺乏有效的治疗手段。因此,寻找PC早诊的生物标志物及治疗靶点是近年研究的热点。长链非编码RNA(long non-coding RNA, lncRNA)长度超过200个核苷酸,最初被认为是“垃圾序列”,没有特定的生物学功能,但越来越多的研究证实lncRNA与肿瘤的发生发展密切相关[3]。LINC00857是新近研究中发现的与多种肿瘤的增殖、转移、凋亡和预后相关的lncRNA[4-10],但在PC中鲜有研究。我们通过GEPIA数据库发现,LINC00857在PC组织中高表达,其表达水平越高,PC患者预后越差[11]。然而LINC00857影响PC进展的机制尚不清楚。本研究拟检测LINC00857在胰腺细胞系中的表达水平,并通过慢病毒转染下调LINC00857的表达,探讨LINC00857对胰腺癌PANC-1细胞增殖、迁移和凋亡的影响及其可能的机制。
1 材料与方法
1.1 实验材料
胰腺癌细胞株AsPC-1、PANC-1和胰腺正常上皮细胞HPNE购自上海中科院,胰腺癌细胞株CFPAC-1由甘肃省生物治疗与再生医学重点实验室提供。胰蛋白酶和DMEM培养基(Gibco,美国);胎牛血清和RPMI 1640培养基(BioInd,以色列);TRIzol(Invitrogen,美国);引物GAPDH和LINC00857(宝日生物,中国);反转录和PCR扩增试剂盒(TaKaRa,日本);PVDF膜、ECL发光液(Millipore,美国);LV-LINC00857-RNAi慢病毒(上海吉凯生物,中国);E-cadherin和N-cadherin抗体(Abcam,美国);Vimentin(赛维尔,中国);鼠抗人GAPDH(Proteintech,美国);羊抗兔、鼠二抗(SAB,美国);细胞周期试剂盒(索莱宝,中国);细胞凋亡试剂盒(江苏碧云天,中国);CCK-8试剂(日本同仁,日本);Transwell小室(Corning,美国)。
1.2 方法
1.2.1 细胞培养
AsPC-1细胞使用含10%胎牛血清的RPMI1640培养基,PANC-1、CFPAC-1和HPNE细胞使用含10%胎牛血清的DMEM培养基,置于恒温培养箱(37℃、5%CO2)中培养。
1.2.2 慢病毒的设计和细胞转染
本实验中使用的LINC00857 shRNA慢病毒由上海吉凯基因公司构建,载体为GV112,元件顺序为hU6-MCS-CMV-puromycin,其中LINC00857-sh1序列为5'-CCGGCTCCTAGAATACCACGTTTATCTCGAGATAAACGTGGTATTCTAGGAGTTTTTG-3',LINC00857-sh2序列为5'-CCGGAAGGTGGAGAAGAGTACCCAACTCGAGTTGGGTACTCTTCTCCACCTTTTTTTG-3',空载体序列为5'-TTCTCCGAACGTGTCACGT-3'。根据慢病毒转染指南,六孔板中细胞汇合度在30%~40%时进行感染,MOI值为10。共计分为4组:Normal control组为未处理的正常对照组,Negative control组为转染空白载体的阴性对照组,LINC00857-sh1和LINC00857-sh2为实验组。转染16 h后换液,3天后用嘌呤霉素(2 µg/ml)筛选,隔天换液,筛选9天获得稳定转染细胞株。
1.2.3 RNA提取和qPCR法实验
待细胞融合度达80%~90%时,TRIzol提取细胞的RNA,检测浓度和纯度,随即将RNA反转录为cDNA,将cDNA原液5倍稀释后按10 μl反应体系进行PCR扩增。引物序列如下,LINC00857:F: 5'-CCCCTGCTTCATTGTTTCCC-3',R: 5'-AGCTTGTCCTTCTTGGGTACT-3';GAPDH:F: 5'-GTCTCCTCTGACTTCAACAGCG-3',R: 5'-ACCACCCTGTTGCTGTAGCCAA-3'。扩增参数:95℃ 30 s,1个循环;95℃ 5 s,60℃ 34 s,40个循环;95℃ 15 s,60℃ 1 min,95℃ 15 s,1个循环。运用2-ΔΔCt计算检测结果。
1.2.4 CCK-8法检测转染后PANC-1细胞增殖
消化处于对数生长期的细胞,取每孔100 µl细胞悬液(含2 000个细胞)接种于96孔板,每组重复5次,细胞贴壁后弃掉原液,CCK-8溶液和无血清的DMEM按1:10配置,每孔加100 µl配置液,恒温培养箱中培养2 h,酶标仪测450 nm波长处的吸光度(OD)值,每天检测1次,隔天换液,连续测5天。采用GraphPad软件绘制增殖曲线。
1.2.5 Transwell和划痕实验检测转染后PANC-1细胞的迁移能力
将对数生长期的细胞饥饿处理24 h,随即胰酶消化,取200 µl细胞无血清悬液(含6×l04个细胞)接种于Transwell上室,600 µl(含30%胎牛血清)完全培养基加入下室,孵箱中培养48 h,随即用4%多聚甲醛固定、结晶紫避光染色,放置在浸没PBS的24孔板中,倒置显微镜(200倍)下多视野拍照。
六孔板中细胞融合度在90%左右时,在超净台中用10 μl枪头垂直划痕。轻柔洗去漂浮细胞,加入2 ml DMEM(不含血清),0 h和24 h于倒置显微镜(100倍)下拍照。利用Image J软件测细胞迁移前后的面积。划痕愈合率=(0 h划痕面积-24 h划痕面积)/0 h划痕面积×100%。
1.2.6 流式细胞仪检测转染后PANC-1细胞的细胞周期和凋亡
待细胞汇合度达70%~80%时,收集消化的各组细胞,取1 ml含1×106个细胞的细胞悬液,离心后预冷PBS洗两遍,逐滴加入70%预冷的无水乙醇重悬细胞,随后置于4℃冰箱过夜,清洗后重悬于100 µl RNase A溶液,加400 µl碘化丙啶(PI)固定,上流式细胞仪检测细胞DNA含量。取胰酶(不含EDTA)消化的5~10万个细胞重悬于195 μl Annexin V-FITC结合液中,加入5 μl Annexin V-FITC、10 μl PI,4℃避光静置20 min,加入300 μl结合液,上流式细胞仪检测细胞凋亡情况。使用ModFit软件分析细胞周期,FlowJo软件分析细胞凋亡情况。
1.2.7 Western blot实验检测转染后PANC-1细胞EMT相关蛋白的表达
提取细胞总蛋白,BCA法测蛋白浓度,加入适量5×SDS蛋白上样缓冲液和强RIPA将待测蛋白样品定量至合适浓度,的100℃沸水中煮10 min使蛋白质变性。每孔加50 µg蛋白进行SDS-PAGE凝胶电泳(80 V 30 min,120 V 1.5 h,恒压)、湿转法转膜(200 mA,2~3 h, 恒流),5%BSA封闭1 h,TBST洗PVDF膜3次,每次10 min,按分子量拆剪条带,放置于一抗(稀释比Vimentin 1:500,GAPDH、E-cadherin和N-cadherin均为1:1 000)中,4℃冰箱保存过夜;TBST清洗后在二抗(稀释比1:2000)中孵育1 h,ECL化学发光A、B液按1:1配置,曝光仪下显影并拍照。采用Image J软件核算蛋白条带灰度值。
1.3 统计学方法
采用SPSS22.0和GraphPad Prism 7.00统计软件对实验数据进行分析,定量资料用(x±s)表示,数据均进行正态分布检验,每个实验至少重复3次,两组间比较采用t检验,多组间比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果
2.1 LINC00857在胰腺细胞系中的表达及其在PANC-1细胞中的敲低情况
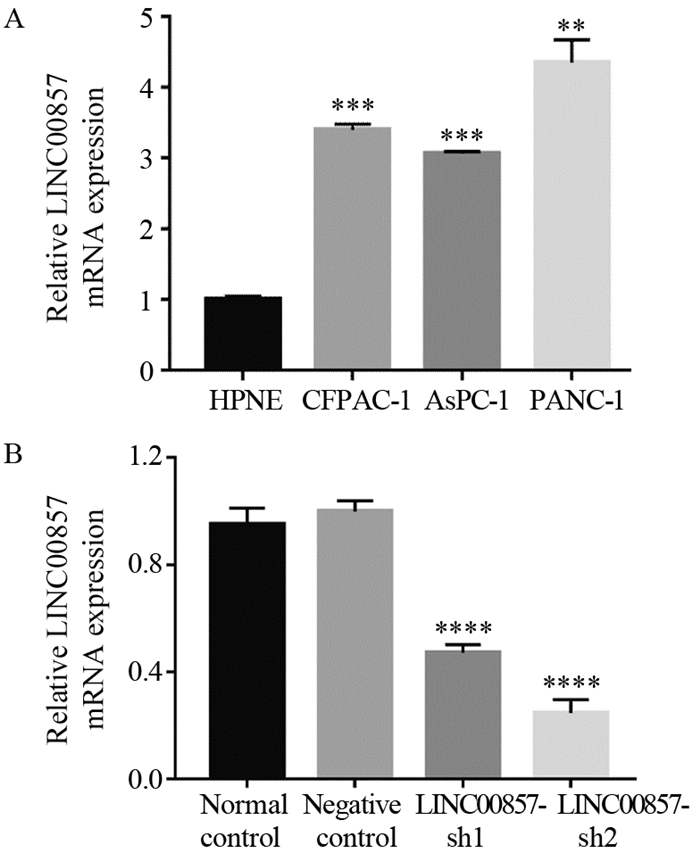
LINC00857在三种胰腺癌细胞系中的表达显著高于胰腺正常上皮细胞HPNE,差异有统计学意义(P < 0.01),其中以PANC-1细胞本底表达最高,故选择PANC-1细胞进行后续实验,见图 1A。对照组间LINC00857表达水平未见明显差异(P =0.5946),与对照组相比,实验组中LINC00857的表达水平均显著下调,差异有统计学意义(P < 0.0001),见图 1B。
2.2 CCK-8法检测细胞增殖结果
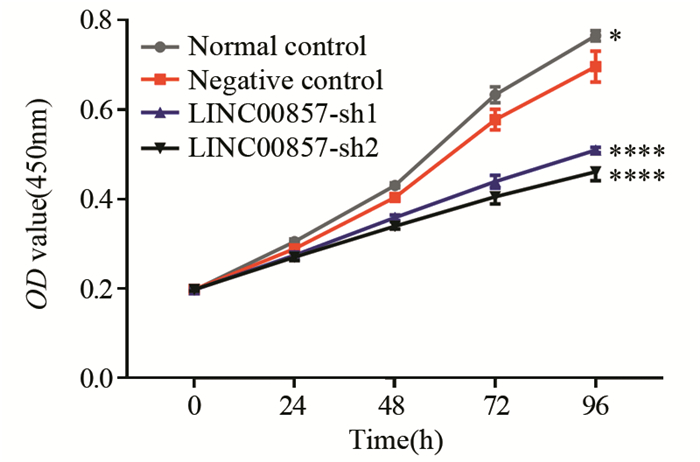
与Normal control组相比,Negative control组细胞OD值减小(24 h P=0.115,余P < 0.05);与对照组相比,实验组细胞OD值均明显减小(均P < 0.0001);实验组间OD值仅在48 h差异有统计学意义(P < 0.05);表明下调LINC00857可抑制PANC-1细胞的增殖能力,见图 2。
2.3 Transwell和划痕实验检测细胞迁移结果
与对照组相比,实验组穿过的细胞数目均明显减少(P < 0.0001),实验组和对照组间各自组间差异无统计学意义(P=0.7835和P=0.7279),见图 3A、3C。划痕实验也同样证实了敲低LINC00857可以显著抑制PANC-1细胞的迁移能力,见图 3B、3D。与Normal control组(78.54±40.25)%和Negative control组(78.06±1.263)%的划痕愈合率相比,LINC00857-sh1组(44.92±2.14)%和LINC00857-sh2组(42.75±1.425)%的划痕愈合率均显著下降(P < 0.0001),实验组和对照组间各自组间差异无统计学意义(P=0.9990和P=0.9226)。
2.4 流式细胞仪检测细胞周期和凋亡的结果
细胞周期结果显示,与Negative control组G0/G1期细胞的百分比(55.4±1.045)%相比,LINC00857-sh1组(64.91±1.185)%和LINC00857-sh2组(69.77±0.775)%显著上升(P=0.0058和P=0.0012),差异有统计学意义;实验组和对照组各自组间差异无统计学意义(P=0.9743和P=0.0599)。与Negative control组S期细胞的百分比(22.15±1.51)%相比,LINC00857-sh1组(14.24±0.02)%和LINC00857-sh2组(9.9±0.38)%明显减少(P=0.0493和P=0.0109),差异有统计学意义;实验组和对照组各自组间差异无统计学意义(P=0.6489和P=0.254),见图 4A、4C。说明敲低LINC00857后PANC-1细胞被阻滞在G1期。
细胞凋亡结果所示,与Negative control组凋亡(早期凋亡+晚期凋亡)比例(26.84±0.97)%相比,LINC00857-sh1组(41.45±0.55)%和LINC00857-sh2组(50.3±2.6)%明显增加(P=0.0178和P=0.0031),实验组和对照组各自组间差异无统计学意义(P=0.8005和P=0.0914),见图 4B、4D。说明敲低LINC00857后PANC-1细胞凋亡增加。
2.5 Western blot检测上皮间质转化相关蛋白表达的结果
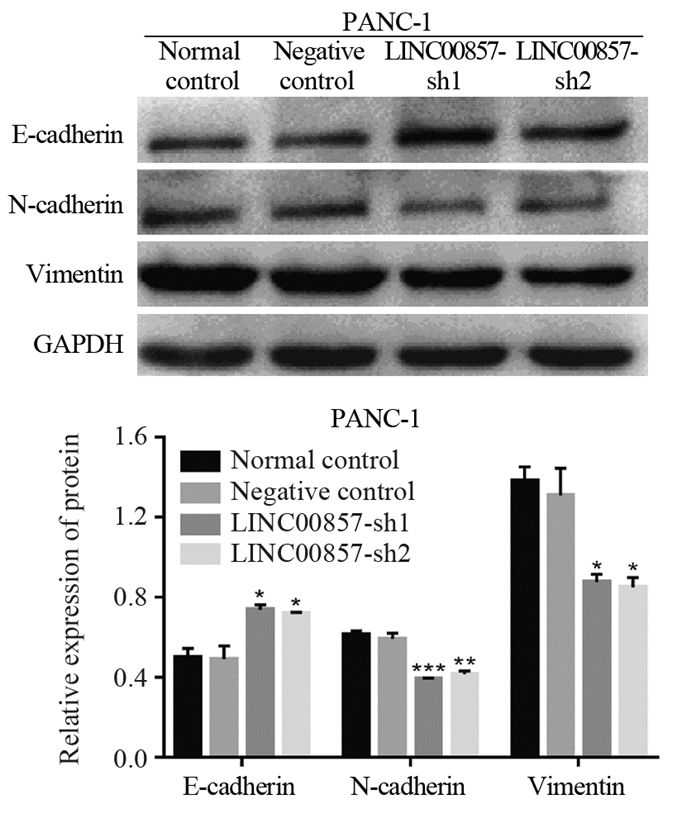
对照组间EMT相关蛋白表达未见明显异常(均P > 0.05);与Negative control组相比,实验组EMT相关的蛋白E-cadherin表达显著升高(P=0.0152和P=0.0196),N-cadherin表达显著下调(P=0.0001和P=0.0016),Vimentin表达显著降低(P=0.0208和P=0.0170),差异有统计学意义,见图 5。
3 讨论
近些年研究发现LncRNA与PC的恶性行为及治疗密切相关。如LncRNA DGCR5可诱导胰腺癌细胞凋亡,抑制细胞增殖[12];Wang等[13]研究发现lncRNA H19不仅参与维持胰腺癌细胞干性,还与胰腺癌细胞的侵袭、迁移、EMT和化疗耐药性密切相关。纳米颗粒介导的致癌LncRNA DANCR已被证明有助于三阴性乳腺癌的治疗[14]。LINC00857是一种新发现的LncRNA,位于人类染色体10q22.3的正链上,不能编码蛋白质[4]。LINC00857在肺癌[4-5]、胃癌[6-7]、膀胱癌[8]、肝癌[9]、食管癌[10]等癌组织或细胞中高表达,通过不同机制促进肿瘤的恶性进展。已有研究[15]通过生物信息学分析发现LINC00857在PC组织中高表达,与PC预后呈负相关,提示LINC00857可能作为促癌基因参与PC的恶性进展。然而,LINC00857在胰腺癌细胞中的表达和作用机制尚无报道。
本研究发现,LINC00857在胰腺癌细胞系中高表达。下调LINC00857后,细胞增殖能力明显减弱,说明LINC00857在PC中是一种促癌因素。细胞周期和凋亡实验发现实验组较对照组细胞凋亡率明显增加,G0/G1期比例增加,S期比例下降,细胞被阻滞在G1期。Wang等[4]和Pang等[7]研究发现,LINC00857下调后,细胞周期信号通路失活,细胞周期蛋白依赖性激酶2(CDK2)、细胞周期蛋白D1(cyclin D1)、细胞周期蛋白E1(cyclin E1)表达减少,细胞被阻滞在G1期,细胞增殖受到抑制;LINC00857表达缺失后肺癌和食管癌细胞凋亡比例增加,细胞增殖被抑制[5, 10]。由此可见,G1期阻滞或诱导凋亡可能是LINC00857下调抑制肿瘤生长的机制。本研究Transwell和划痕实验同时证实,下调LINC00857可抑制PANC-1细胞的迁移能力;为了探究其原由,我们检测了EMT相关蛋白的表达,与对照组相比,实验组的E-cadherin表达明显上升,N-cadherin和Vimentin表达显著下降,EMT过程被抑制。这与Xia等[9]在肝癌中的研究一致。肿瘤早期转移与细胞的EMT过程紧密相关,EMT可加速肿瘤的侵袭和迁移[16],这种癌细胞的表型转换是胰腺癌细胞恶性生长所必需的[17]。LncRNA SNHG12可通过EMT增加胰腺癌细胞的恶性行为[18]。因此,EMT可能是实验组和对照组迁移能力差异的原因。
综上所述,LINC00857在胰腺癌细胞系中高表达,下调LINC00857可抑制胰腺癌细胞的增殖和迁移能力,促进细胞的凋亡,其机制可能与G1期阻滞和EMT信号通路有关。LINC00857对PC的发生发展起促进作用,可能成为PC治疗的新靶点,但LINC00857在PC中的具体作用机制仍需进一步探究。
Competing interests: The authors declare that they have no competing interests.作者贡献:张波:设计与实施实验、收集与分析数据、撰写论文任龙飞:指导数据分析和论文写作胡进静、白仲添:指导实验周文策:指导实验设计、修改和审核论文 -
-
[1] Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019[J]. CA Cancer J Clin, 2019, 69(1): 7-34. doi: 10.3322/caac.21551
[2] Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. doi: 10.3322/caac.21492
[3] Zhou W, Chen L, Li C, et al. The multifaceted roles of long noncoding RNAs in pancreatic cancer: an update on what we know[J]. Cancer Cell Int, 2020, 20: 41. doi: 10.1186/s12935-020-1126-1
[4] Wang L, He Y, Liu W, et al. Non-coding RNA LINC00857 is predictive of poor patientsurvival and promotes tumor progression via cell cycle regulation in lung cancer[J]. Oncotarget, 2016, 7(10): 11487-11499. doi: 10.18632/oncotarget.7203
[5] Wang L, Cao L, Wen C, et al. LncRNA LINC00857 regulates lung adenocarcinoma progression, apoptosis and glycolysis by targeting miR-1179/SPAG5 axis[J]. Hum Cell, 2020, 33(1): 195-204. doi: 10.1007/s13577-019-00296-8
[6] Zhang K, Shi H, Xi H, et al. Genome-Wide lncRNA Microarray Profiling Identifies Novel Circulating lncRNAs for Detection of Gastric Cancer[J]. Theranostics, 2017, 7(1): 213-227. doi: 10.7150/thno.16044
[7] Pang K, Ran MJ, Zou FW, et al. Long non-coding RNA LINC00857 promotes gastric cancer cell proliferation and predicts poor patient survival[J]. Oncol Lett, 2018, 16(2): 2119-2124.
[8] Dudek AM, van Kampen JGM, Witjes JA, et al. LINC00857 expression predicts and mediates the response to platinum-based chemotherapy in muscle-invasive bladder cancer[J]. Cancer Med, 2018, 7(7): 3342-3350. doi: 10.1002/cam4.1570
[9] Xia C, Zhang XY, Liu W, et al. LINC00857 contributes to hepatocellular carcinoma malignancy via enhancing epithelial-mesenchymal transition[J]. J Cell Biochem, 2018. Online ahead of print.
[10] Su W, Wang L, Niu F, et al. LINC00857 knockdown inhibits cell proliferation and induces apoptosis via involving STAT3 and MET oncogenic proteins in esophageal adenocarcinoma[J]. Aging (Albany NY), 2019, 11(9): 2812-2821.
[11] Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses[J]. Nucleic Acids Res, 2017, 45(W1): W98-W102. doi: 10.1093/nar/gkx247
[12] Li X, Zhou S, Fan T, et al. lncRNA DGCR 5/miR-27a-3p/BNIP3 promotes cell apoptosis in pancreatic cancer by regulating the p38 MAPK pathway[J]. Int J Mol Med, 2020, 46(2): 729-739. doi: 10.3892/ijmm.2020.4632
[13] Wang F, Rong L, Zhang Z, et al. LncRNA H19-Derived miR-675-3p Promotes Epithelial-Mesenchymal Transition and Stemness in Human Pancreatic Cancer Cells by targeting the STAT3 Pathway[J]. J Cancer, 2020, 11(16): 4771-4782. doi: 10.7150/jca.44833
[14] Vaidya AM, Sun Z, Ayat N, et al. Systemic Delivery of Tumor-Targeting siRNA Nanoparticles against an Oncogenic LncRNA Facilitates Effective Triple-Negative Breast Cancer Therapy[J]. Bioconjug Chem, 2019, 30(3): 907-919. doi: 10.1021/acs.bioconjchem.9b00028
[15] 张志鹏, 孙维佳, 陈泓西, 等. 基于生物信息学的胰腺导管腺癌预后风险长链非编码RNA筛选[J]. 中国普通外科杂志, 2018, 27(9): 1126-1134. https://www.cnki.com.cn/Article/CJFDTOTAL-ZPWZ201809010.htm Zhang ZP, Sun WJ, Chen HX, et al. Screening of long non-coding RNA for prognostic risk of pancreatic ductal adenocarcinoma based on bioinformatics[J]. Zhongguo Pu Tong Wai Ke Za Zhi, 2018, 27(9): 1126-1134. https://www.cnki.com.cn/Article/CJFDTOTAL-ZPWZ201809010.htm
[16] Expósito-Villén A, Aránega AE, Franco D. Functional Role of Non-Coding RNAs during Epithelial-To-Mesenchymal Transition[J]. Noncoding RNA, 2018, 4(2): 1-14.
[17] 成鉴晓, 江月萍, 任琳琳, 等. lncRNA MEG3通过调控miR-543/PTEN分子轴抑制胰腺癌细胞增殖及转移的机制[J]. 肿瘤防治研究, 2019, 46(7): 588-593. doi: 10.3971/j.issn.1000-8578.2019.18.1876 Cheng JX, Jiang YP, Ren LL, et al. LncRNA MEG3 suppresses proliferation and metastasis of pancreatic cancer cells via regulating miR-543/PTEN axis[J]. Zhong Liu Fang Zhi Yan Jiu, 2019, 46(7): 588-593. doi: 10.3971/j.issn.1000-8578.2019.18.1876
[18] Cao W, Zhou G. LncRNA SNHG12 contributes proliferation, invasion and epithelial-mesenchymal transition of pancreatic cancer cells by absorbing miRNA-320b[J]. Biosci Rep, 2020, 40(6): BSR20200805. doi: 10.1042/BSR20200805
-
期刊类型引用(1)
1. 李永宁,付雪芹,李英,潘耀振. 下调IQGAP1对胰腺癌细胞增殖、凋亡的影响及作用机制. 中国老年学杂志. 2024(11): 2716-2719 .  百度学术
百度学术
其他类型引用(3)



 下载:
下载: