New Insight on Tumor Microenvironment Remodelling and Augmented Therapeutic Efficacy of Immunotherapy by Radiotherapy
-
摘要:
以免疫检查点抑制剂(ICIs)为主的免疫治疗改变了传统癌症治疗手段,但对于大多数类型的癌症,ICIs治疗受益十分有限(10%~30%),并且在某些癌症类型中基本无效(如胰腺癌、脑胶质瘤)。将ICIs治疗与现有及潜在的疗法相结合从而克服肿瘤原发性和获得性抵抗,对于提高治疗率、增加疗效的持久性和延长患者的生存期有重要意义。放射治疗能杀伤肿瘤细胞,同时引起促炎性分子的释放和免疫细胞的肿瘤浸润。此外,放射治疗能在肿瘤细胞中诱导产生微核,从而激活胞质核酸感应器,其中最重要的是环GMP-AMP合成酶-干扰素诱导基因通路,并且所产生的炎性反应信号效应重塑了肿瘤免疫微环境。肿瘤细胞在放射处理后还可通过新抗原的表达来影响免疫监测。本文将深入探讨放射治疗对于肿瘤微环境的免疫调节作用以及放疗与ICIs联合治疗作为一种潜在的癌症治疗策略,并介绍放射治疗引起的肿瘤微环境的重塑,包括对树突状细胞、T细胞浸润以及抑制性髓样细胞群的影响。
Abstract:Immune checkpoint inhibitors (ICIs)-based tumor immunotherapy has changed the traditional cancer treatment. However, ICI treatment benefits small percentage of patients in most types of cancer (10%-30%), and is basically ineffective in some cancers (such as pancreatic cancer and glioma). Combining ICIs with existing and potential therapies to overcome tumor innate and acquired resistance is of great significance for improving the treatment efficacy, increasing the durability of the therapeutic effect and prolonging patients' survival. Radiotherapy can not only kill tumor cells, but also cause the release of pro-inflammatory molecules and immune cell infiltration in tumors. In addition, radiotherapy can induce micronuclei in tumor cells, thereby activating cytosolic DNA/RNA sensors, the most important of which is the cyclic GMP-AMP synthase (cGAS)-STING pathway. Radiotherapy can also regulate immune surveillance through the expression of tumor neoantigens. In this review, we will discuss in depth the immunomodulatory effect of radiotherapy on the tumor microenvironment and its combination with ICI as a potential cancer treatment, and focus on the effects of radiotherapy on non-tumor cells in the tumor microenvironment, including dendritic cells, T cell infiltration, as well as myeloid-derived suppressor cells.
-
Key words:
- Radiotherapy /
- Tumor microenvironment /
- Immunotherapy
-
0 引言
抑制程序性细胞死亡受体蛋白1(PD-1)、程序性细胞死亡配体1(PD-L1)或细胞毒性T淋巴细胞相关蛋白4(CTLA4)免疫检查点是肿瘤免疫治疗的主要手段。尽管部分患者表现出明显的反应性和潜在的持久响应,但大多数肿瘤患者并没有从中受益。因此,目前很多临床前研究和临床试验的关注点是将现有的治疗手段与免疫治疗相结合从而改善疗效。临床上约50%的癌症患者接受放疗作为治疗的一部分。在过去的一个多世纪以来,放疗成为众多实体瘤的一线治疗手段。早在1979年,Stone等报道在免疫缺陷小鼠中减少50%移植瘤体积(TCD50)所需的放疗剂量是在免疫正常小鼠中的两倍[1]。目前,已知放疗可以发挥显著的免疫刺激作用,因此,放疗与免疫检查点抑制剂(immune checkpoint inhibitors, ICIs)免疫治疗相结合越来越被视为有前途的潜在肿瘤联合治疗方法[2-3]。
放疗不仅具有介导DNA损伤从而导致癌细胞死亡的作用,还可以通过触发促炎介质的释放调节产生免疫原性和佐剂性,增加免疫刺激抑制肿瘤细胞浸润并增强新抗原的表达[4-5]。总的来说,放疗所显示出的积极的免疫刺激作用,一定程度上能被免疫学上称作的“冷”肿瘤变为“热”肿瘤。通过驱动免疫细胞浸润和增强免疫原性,放疗有可能增加肿瘤的免疫反应。有研究表明,放疗后肿瘤细胞DNA损伤这一内在事件是驱动免疫调节的关键[4]。因此肿瘤细胞的自主效应及其如何指导未来联合治疗是本综述的重点。
本文总结了放射疗法诱发的肿瘤基因组片段化激活细胞质核酸传感器(DNA sensors)的反应从而引发细胞1型干扰素(Type 1interferon, T1IFN)等细胞因子的表达,还将讨论放疗引起的DNA损伤和基因转录的改变对肿瘤新抗原表达的调节作用。这些事件可以引起固有性和(或)适应性抗肿瘤免疫程序的启动,为放疗、DNA损伤修复(DNA damage response, DDR)抑制剂以及ICIs的联合使用提供理论依据。
1 细胞质核酸传感器
1.1 放疗通过cGAS-STING信号通路诱导细胞质DNA感应
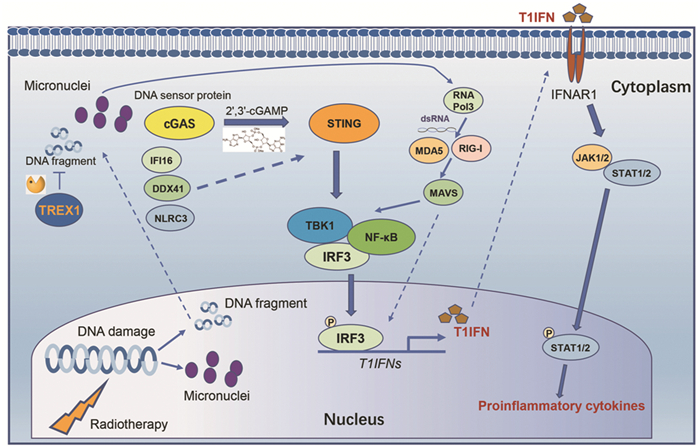
细胞质核酸传感器最初是细胞内模式识别受体(pattern recognition receptors, PRRs)启动对DNA病毒的天然免疫反应[6]。有研究证明,在放疗抗癌的机制中一个关键因素是放疗能够引起增殖的肿瘤细胞DNA损伤并释放至细胞质从而激活细胞内传感器。其中细胞质DNA传感器环GMP-AMP合成酶(cyclic GMP-AMP synthase, cGAS)-干扰素诱导基因(stimulator of interferon genes,STING)途径似乎在表型上占据了主导地位[7-8]。初步研究发现细胞质B型DNA与cGAS的结合触发了第二信使(或称作免疫递质)cGAMP(cyclic GMP-AMP)的合成,cGAMP结合下游STING蛋白并激活TBK1(TANK binding kinase 1)蛋白激酶和IRF3(interferon regulatory factor 3)转录因子,最终诱导T1IFN的表达,而IFN-α/β能够与干扰素α/β受体1(interferon alpha and beta receptor subunit 1, IFNAR1)结合在放疗引起的抗肿瘤免疫中发挥关键作用[9-10],见图 1。在小鼠模型中,外源性cGAMP和合成STING激动剂亦可增强放射的效应[7, 11]。2017年Demaria团队发现肿瘤细胞胞质内脱氧核糖核酸酶TREX1(three prime repair exonuclease 1)能够降解细胞质中双链DNA(double-stranded DNA,dsDNA),因此可以降低由cGAS产生cGAMP的水平,从而抑制放疗的抗肿瘤免疫效应。当高剂量的放疗(12~18 Gy)处理小鼠移植瘤时,TREX1蛋白能够被诱导表达,而采用多次低剂量放疗即连续3天8 Gy可以避免TREX1被诱导表达,最终使放疗抑制肿瘤的效果显著增加[12]。
![]() 图 1 放疗诱发肿瘤基因片段化与微核形成,从而激活细胞质DNA/RNA核酸感受器,上调1型干扰素及促炎性细胞因子的表达Figure 1 Radiation can induce tumor gene fragmentation and micronuclei formation, which then activates cytoplasmic DNA/RNA nucleic acid receptor and subsequently up-regulates the expression of type 1 interferon and proinflammatory cytokinescGAS: cyclic GMP-AMP synthase; cGAMP: cyclic GMP-AMP; STING: stimulator of interferon genes; TBK1: TANK binding kinase 1; IRF3: interferon regulatory factor 3; T1IFN: Type 1 interferon; IFNAR1: interferon alpha and beta receptor subunit 1; RIG-I: retinoic acid inducible gene I; MDA5: melanoma differentiation-associated gene 5; MAVS: adaptor protein; TREX1: three prime repair exonuclease 1; IFI16: interferon gamma inducible protein 16; DDX41: DEAD-box helicase 41; NLRC3: NLR family CARD domain containing 3.
图 1 放疗诱发肿瘤基因片段化与微核形成,从而激活细胞质DNA/RNA核酸感受器,上调1型干扰素及促炎性细胞因子的表达Figure 1 Radiation can induce tumor gene fragmentation and micronuclei formation, which then activates cytoplasmic DNA/RNA nucleic acid receptor and subsequently up-regulates the expression of type 1 interferon and proinflammatory cytokinescGAS: cyclic GMP-AMP synthase; cGAMP: cyclic GMP-AMP; STING: stimulator of interferon genes; TBK1: TANK binding kinase 1; IRF3: interferon regulatory factor 3; T1IFN: Type 1 interferon; IFNAR1: interferon alpha and beta receptor subunit 1; RIG-I: retinoic acid inducible gene I; MDA5: melanoma differentiation-associated gene 5; MAVS: adaptor protein; TREX1: three prime repair exonuclease 1; IFI16: interferon gamma inducible protein 16; DDX41: DEAD-box helicase 41; NLRC3: NLR family CARD domain containing 3.目前,有报道显示非肿瘤细胞中STING的激活是免疫激活的关键因素[7, 11, 13]。但在某些体内模型中,肿瘤细胞固有的STING激活是必要的。在这种情况下,临床前数据表明肿瘤细胞衍生的DNA和(或)源自cGAS催化产生的cGAMP可通过细胞间和外泌体传递到非肿瘤细胞中(如树突状细胞),从而扩大T1IFN信号,有助于增加放射治疗的抗肿瘤免疫效应[12, 14-16]。哺乳动物细胞中cGAMP可以被细胞膜外以及基质中的外核苷酸焦磷酸酶/磷酸二酯酶1(ectonucleotide pyrophosphatase/phosphodiesterase 1, ENPP1)降解,降低ENPP1的表达能够显著增强放疗产生的抗肿瘤免疫效应[17]。此外,最近的研究表明跨膜蛋白SLC19A1(solute carrier family 19 member 1)是第一个被人们所认知的cGAMP通道蛋白[18-19]。
1.2 其他细胞质DNA传感器及其与RNA传感器的串扰
cGAS-STING途径同时受到其他核酸传感器的正串扰。除cGAS以外,还有类似含CARD结构域的NOD囊样受体家族3(NLR family CARD domain containing 3, NLRC3)、IFNγ诱导蛋白的蛋白结构域16(interferon gamma inducible protein 16, IFI16)和DEAD-box解旋酶41(DEAD-box helicase 41, DDX41)等,它们都能够结合dsDNA对STING介导的T1IFN信号转导具有积极影响,见图 1。NLRC3通过与STING相互作用从而阻止其功能,而且NLRC3与dsDNA结合后能够有助于STING的释放[20]。IFI16可以通过两个HIN结构域结合dsDNA,随后通过吡啶结构域与STING相互作用[21]。虽然有研究发现IFI16在IFNβ表达中没有起到直接作用[22],但总体而言,IFI16-STING相互作用增强STING依赖性IFNβ的产生[23-24]。DDX41同样也可以结合dsDNA,并且与STING结合增强下游T1IFN的表达[25-26]。
小鼠肿瘤细胞在放射处理后还有许多RNA核酸传感器上调,包括模式识别受体视黄酸诱导型基因Ⅰ(RIG-Ⅰ,也称为DDX58)和黑色素瘤分化相关蛋白5(MDA5,也称为IFIH1)[27]。在DNA感应途径和细胞质RNA感应途径之间已经确定了一定程度的串扰。双链RNA(dsRNA)与细胞质中RIG-1或MDA5的结合通过衔接蛋白MAVS来诱导T1IFN的表达。已有研究证明,放疗诱导产生最大1型干扰素这一过程中,MAVS和RIG-Ⅰ的参与是必不可少的[28-29]。放射产生的具有5'-三磷酸并富含AT的dsDNA能够被RNA聚合酶Ⅲ转录形成dsRNA,也可以激活RNA感应途径中的MDA5和RIG-I途径,见图 1。
1.3 cGAS对微核的监测
最初人们发现细胞质DNA是通过cGAS-STING途径诱导T1IFN产生,但如何将这一过程与放疗相结合并不十分清楚。最近有研究揭示了cGAS通过监测放射处理所产生的微核和(或)小片段DNA,从而建立肿瘤细胞中放射处理与免疫系统之间的关系。
放疗在肿瘤细胞中诱导微核是一个公认的现象。Harding等发现放射处理后肿瘤细胞中能够产生微核,表明细胞分裂导致微核的产生。当敲除非同源末端连接(non-homologous end joining, NHEJ)通路中的关键分子或利用DNA-PK抑制剂时,微核的产生受到抑制。重要的是,cGAS蛋白能够与核膜破裂的微核中DNA相结合,激活下游STING以及诱导T1IFN的表达[4], 见图 1。在具有微核的细胞中,干扰素刺激基因(interferon-stimulated gene, ISG)依赖于cGAS和STING方式的转录显著增强[4-5, 30]。此外,cGAS能够被募集到DNA损伤位点并直接抑制DNA修复,从而可能进一步促进微核的形成,开启一个正反馈模式[31-32]。上述这些重要发现弥补了关于放疗如何通过细胞质微核衍生的DNA传播炎性T1IFN反应的知识空白。
2 放射诱导的抗原呈递
2.1 放射诱导肿瘤相关新抗原的呈递
细胞毒性CD8+T细胞清除肿瘤细胞需要识别肿瘤细胞表面主要组织相容性复合物Ⅰ(MHC-Ⅰ)上呈递的抗原。在这方面,放疗显示出较大的优势,既增加肿瘤细胞表面MHC-Ⅰ的表达,同时又调节肿瘤细胞上的抗原呈递。已有多项研究发现放射能够上调现有和新型抗原在肿瘤细胞表面的呈递。放疗引起的DNA损伤能够触发一系列转录的改变,可以使得某些平时表达水平很低以及发生突变的多肽大量表达,触发抗肿瘤免疫。在化疗抵抗的转移性非小细胞肺癌患者中利用CTLA4阻滞与放疗联合使用时,在具有完全反应的患者中可以检测到新抗原KPNA2(karyopherin subunit alpha 2)突变蛋白,且放疗上调了KPNA2基因的表达。突变型KPNA2产生的抗原可促进患者CD8+ T细胞产生大量的IFNγ[2]。
2.2 放射产生的新抗原和T细胞受体库
目前认为突变负荷和肿瘤新抗原负荷通常可预测肿瘤细胞对ICIs的临床反应。肿瘤细胞可能由于暴露于诱变剂(例如紫外线和吸烟)、DNA修饰和复制错误(APOBEC3B表达或POLE突变),以及遗传的或获得性的DNA修复缺陷而产生高突变负荷。在许多癌症类型中,DDR途径中基因的体细胞变异和表观遗传沉默普遍存在,这些患者的肿瘤可能同时具有高突变和新抗原负荷,以及细胞质DNA驱动的炎性反应信号[33]。
放射治疗也增加了T细胞中特异性TCR的表达和T细胞克隆的数量,再加上抗PD-1治疗,能够将这种增加的多样性扩展到放射之外的肿瘤部位,这一作用被称作远隔效应(abscopal effect)。在对小鼠的放疗和抗CTLA4阻断反应的TCR记忆库研究中,放疗增加了肿瘤内T细胞TCR记忆库的多样性,并与抗CTLA4结合使用增强了对肿瘤的抑制作用[34-35]。目前尚不清楚在临床上针对新的放射治疗产生的肿瘤抗原的免疫应答是否比增强既有肿瘤抗原的活性更重要,这也可以成为未来的一个研究方向。
3 放射引起肿瘤微环境的重塑
3.1 放疗对树突状细胞的影响
放疗除了可以增强肿瘤细胞表面MHC-Ⅰ的呈递作用外,还可以显著提高肿瘤抗原的交叉呈递。在OT-1小鼠模型中,放射能够增加淋巴结中被表达有MHC-I-SIINFEKL的树突状细胞和所激活的特异性效应记忆的CD8+T细胞[36]。免疫原性所导致的细胞死亡与损伤相关分子模式(damage-associated molecular patterns, DAMP)的释放,被人们了解较多的是Calreticulin、HMGB1(high mobility group box 1)和ATP,而放射能够明显增加Calreticulin、HMGB1和ATP的释放[37]。此外,树突状细胞对于放射的免疫应答至关重要。CD11c+CD8α+ BATF3系的树突状细胞是放疗和抗CTLA4反应的抗肿瘤效应产生的关键[12],而在小鼠树突状细胞中缺少IFNAR1受体的表达则会逆转这一抗肿瘤效应[7]。
3.2 T细胞浸润
放疗可促进T细胞浸润所必需的细胞因子分泌。放射诱导肿瘤细胞内源性CXCL16(C-X-C motif chemokine ligand 16)的分泌,CXCL16能与Th1(T helper 1)细胞上的C-X-C趋化因子受体6(C-X-C motif chemokine receptor 6, CXCR6)结合并激活CD8+T细胞[38]。放射可以上调肿瘤细胞中T细胞趋化因子CXCL9和CXCL10的表达,而CXCL9和CXCL10则能够与T细胞上的CXCR3结合,促进T细胞的浸润。放射治疗后,体内肿瘤细胞还可以被诱导表达ICAM1(intercellular adhesion molecule 1)和NKG2D配体RAE-1γ(由Raet1g编码)。在T细胞浸润后,MHC-Ⅰ、ICAM1、RAE-1γ和NKG2D导致小鼠的T细胞在肿瘤微环境中停滞,肿瘤细胞表达在放疗和抗CTLA4的抗肿瘤效应起到重要的作用[39]。但另一方面,放射在促进抗原呈递、树突状细胞功能和CD8+T细胞浸润等方面的积极作用也会被上调的抑制性信号部分抵消。放射治疗后,小鼠肿瘤中的调节性CD4+ FOXP3+ T细胞(Treg细胞)也同时增加[27]。Treg细胞通过产生CTLA4信号、TGFβ而表现出免疫抑制作用[40-41]。因此,靶向Treg细胞联合放疗可能是有益的[34-35, 42]。
3.3 抑制性髓样细胞群(myeloid-derived suppressor cells, MDSCs)
经典的炎性单核细胞在炎性反应过程中被募集到组织中,它们可以分化为巨噬细胞或DCs,这些巨噬细胞或DCs可以表现出促炎或抗炎特性。同时,在肿瘤微环境某些细胞因子的作用下,炎性单核细胞还可以分化为MDSCs[40]。放射在肿瘤细胞中产生的CCL2-CCR2信号上调能够诱导小鼠MDSCs的分化,从而增加放射治疗后的免疫抑制作用[43-45]。小鼠中敲除Ccr2(C-C motif chemokine receptor 2)或Ccl2(C-C motif chemokine ligand 2)可以降低肿瘤微环境中MDSCs细胞的水平并增加放疗的抗肿瘤效应[41]。此外,在小鼠体内实验中,放射治疗还能够增加肿瘤微环境中MDSCs细胞PD-L1的表达[46-47]。因此这一现象也支持靶向PD-1或PD-L1联合放疗是有益的[48-49]。
4 结论
综上所述,放射治疗后肿瘤细胞所产生的内在信号在重塑炎性肿瘤微环境中具有举足轻重的作用。放射引起的肿瘤细胞DNA损伤显著增加了细胞质微核和小片段DNA的水平,从而激活细胞质核酸传感器,尤其是cGAS及其下游STING蛋白信号通路,诱导肿瘤细胞及肿瘤微环境中特定细胞表达1型干扰素。同时,放射治疗能够引起肿瘤细胞原有及特异新抗原的表达和递呈,增加肿瘤细胞的免疫原性。上述生物学效应将促进肿瘤微环境中CD8+ T细胞等具有抗肿瘤作用的免疫细胞浸润,降低MDSCs的免疫抑制作用。因此,以抑制免疫检查点为手段的免疫治疗与放疗联用具有潜在协同抗肿瘤作用。
放疗引起的DNA损伤促使肿瘤细胞启动DNA修复及应激,从而修复损伤的DNA并产生放疗抵抗(radioresistance)。因此,DNA修复及应激中关键激酶的小分子抑制剂不仅能够抑制DNA修复,增加放疗的敏感度,同时也能够增加放疗引起的肿瘤细胞中微核和(或)小片段DNA的数量,进一步激活cGAS-STING通路和1型干扰素的表达。在临床前细胞与动物实验中,多个DNA修复及应激关键激酶的小分子抑制剂与放射治疗和免疫治疗联用取得了令人振奋的结果。譬如,ATM蛋白激酶抑制剂KU60019、ATR激酶抑制剂AZD6738、PARP抑制剂olaparib和WEE1激酶抑制剂AZD1775[40, 50-54]。更为重要的是,这些研究将有助于相关临床试验的设计和开展,使其在未来可能成为一种非常有希望的联合治疗方法。然而,如何安全有效地增强抗肿瘤免疫的效果,同时又减少免疫抑制作用,以及联用中药和放疗的剂量与时间顺序方面,仍需开展相应的工作以探索最佳的联合效应。我们期待利用放疗及DNA修复与应激关键激酶的小分子抑制剂增加免疫治疗的有效率和持久性,为肿瘤治疗提供新的思路,让更多的肿瘤患者获益。
Competing interests: The authors declare that they have no competing interests.作者贡献张强:撰写论文吴邵雅、张靖:修改论文 -
图 1 放疗诱发肿瘤基因片段化与微核形成,从而激活细胞质DNA/RNA核酸感受器,上调1型干扰素及促炎性细胞因子的表达
Figure 1 Radiation can induce tumor gene fragmentation and micronuclei formation, which then activates cytoplasmic DNA/RNA nucleic acid receptor and subsequently up-regulates the expression of type 1 interferon and proinflammatory cytokines
-
[1] Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma[J]. J Natl Cancer Inst, 1979, 63(5): 1229-1235. doi: 10.1002/jso.2930110412
[2] Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade[J]. Nat Med, 2018, 24(12): 1845-1851. doi: 10.1038/s41591-018-0232-2
[3] Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage Ⅲ NSCLC[J]. N Engl J Med, 2018, 379(24): 2342-2350. doi: 10.1056/NEJMoa1809697
[4] Harding SM, Benci JL, Irianto J, et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei[J]. Nature, 2017, 548(7668): 466-470. doi: 10.1038/nature23470
[5] Mackenzie KJ, Carroll P, Martin CA, et al. cGAS surveillance of micronuclei links genome instability to innate immunity[J]. Nature, 2017, 548(7668): 461-465. doi: 10.1038/nature23449
[6] Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease[J]. Nat Rev Immunol, 2016, 16(1): 35-50. doi: 10.1038/nri.2015.8
[7] Deng LF, Liang H, Xu M, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced Type Ⅰ Interferon-dependent antitumor immunity in immunogenic tumors[J]. Immunity, 2014, 41(5): 843-852. doi: 10.1016/j.immuni.2014.10.019
[8] Vance RE. Cytosolic DNA sensing: The field narrows[J]. Immunity, 2016, 45(2): 227-228. doi: 10.1016/j.immuni.2016.08.006
[9] Sun L, Wu J, Du F, et al. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the typeⅠinterferon pathway[J]. Science, 2013, 339(6121): 786-791. doi: 10.1126/science.1232458
[10] Wu J, Sun L, Chen X, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA[J]. Science, 2013, 339(6121): 826-830. doi: 10.1126/science.1229963
[11] Francica BJ, Ghasemzadeh A, Desbien AL, et al. TNFalpha and radioresistant stromal cells are essential for therapeutic efficacy of cyclic dinucleotide STING agonists in nonimmunogenic tumors[J]. Cancer Immunol Res, 2018, 6(4): 422-433. doi: 10.1158/2326-6066.CIR-17-0263
[12] Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity[J]. Nat Commun, 2017, 8: 15618. doi: 10.1038/ncomms15618
[13] Corrales L, Glickman LH, McWhirter SM, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity[J]. Cell Rep, 2015, 11(7): 1018-1030. doi: 10.1016/j.celrep.2015.04.031
[14] Sen T, Rodriguez BL, Chen L, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer[J]. Cancer Discov, 2019, 9(5): 646-661. doi: 10.1158/2159-8290.CD-18-1020
[15] Marcus A, Mao AJ, Lensink-Vasan M, et al. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response[J]. Immunity, 2018, 49(4): 754-763. e4. doi: 10.1016/j.immuni.2018.09.016
[16] Diamond JM, Vanpouille-Box C, Spada S, et al. Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs[J]. Cancer Immunol Res, 2018, 6(8): 910-920. doi: 10.1158/2326-6066.CIR-17-0581
[17] Li L, Yin Q, Kuss P, et al. Hydrolysis of 2'3'-cGAMP by ENPP1 and design of nonhydrolyzable analogs[J]. Nat Chem Biol, 2014, 10(12): 1043-1048. doi: 10.1038/nchembio.1661
[18] Luteijn RD, Zaver SA, Gowen BG, et al. SLC19A1 transports immunoreactive cyclic dinucleotides[J]. Nature, 2019, 573(7774): 434-438. doi: 10.1038/s41586-019-1553-0
[19] Ritchie C, Cordova AF, Hess GT, et al. SLC19A1 is an importer of the immunotransmitter cGAMP[J]. Mol Cell, 2019, 75(2): 372-381. doi: 10.1016/j.molcel.2019.05.006
[20] Li X, Deng M, Petrucelli AS, et al. Viral DNA binding to NLRC3, an inhibitory nucleic acid sensor, unleashes STING, a cyclic dinucleotide receptor that activates typeⅠ interferon[J]. Immunity, 2019, 50(3): 591-599. e6. doi: 10.1016/j.immuni.2019.02.009
[21] Unterholzner L, Keating SE, Baran M, et al. IFI16 is an innate immune sensor for intracellular DNA[J]. Nat Immunol, 2010, 11(11): 997-1004. doi: 10.1038/ni.1932
[22] Gray EE, Winship D, Snyder JM, et al. The AIM2-like receptors are dispensable for the interferon response to intracellular DNA[J]. Immunity, 2016, 45(2): 255-266. doi: 10.1016/j.immuni.2016.06.015
[23] Almine JF, O'Hare CAJ, Dunphy G, et al. IFI16 and cGAS cooperate in the activation of STING during DNA sensing in human keratinocytes[J]. Nat Commun, 2017, 8: 14392. doi: 10.1038/ncomms14392
[24] Dunphy G, Flannery SM, Almine JF, et al. Non-canonical activation of the DNA sensing adaptor STING by ATM and IFI16 mediates NF-kappaB signaling after Nuclear DNA Damage[J]. Mol Cell, 2018, 71(5): 745-760. e5. doi: 10.1016/j.molcel.2018.07.034
[25] Zhang ZQ, Yuan B, Bao MS, et al. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells[J]. Nat Immunol, 2011, 12(10): 959-965. doi: 10.1038/ni.2091
[26] Lee KG, Kim SSY, Kui L, et al. Bruton's tyrosine kinase phosphorylates DDX41 and activates its binding of dsDNA and STING to initiate type 1 interferon response[J]. Cell Rep, 2015, 10(7): 1055-1065. doi: 10.1016/j.celrep.2015.01.039
[27] Dillon MT, Bergerhoff KF, Pedersen M, et al. ATR inhibition potentiates the radiation-induced inflammatory tumor microenvironment[J]. Clin Cancer Res, 2019, 25(11): 3392-3403. doi: 10.1158/1078-0432.CCR-18-1821
[28] Widau RC, Parekh AD, Ranck MC, et al. RIG-I-like receptor LGP2 protects tumor cells from ionizing radiation[J]. Proc Natl Acad Sci U S A, 2014, 111(4): E484-E491. doi: 10.1073/pnas.1323253111
[29] Ranoa DR, Parekh AD, Pitroda SP, et al. Cancer therapies activate RIG-I-like receptor pathway through endogenous non-coding RNAs[J]. Oncotarget, 2016, 7(18): 26496-26515. doi: 10.18632/oncotarget.8420
[30] Willan J, Cleasby AJ, Flores-Rodriguez N, et al. ESCRT-Ⅲ is necessary for the integrity of the nuclear envelope in micronuclei but is aberrant at ruptured micronuclear envelopes generating damage[J]. Oncogenesis, 2019, 8(5): 29. doi: 10.1038/s41389-019-0136-0
[31] Liu H, Zhang HP, Wu XY, et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis[J]. Nature, 2018, 563(7729): 131-136. doi: 10.1038/s41586-018-0629-6
[32] Jiang H, Xue XY, Panda S, et al. Chromatin-bound cGAS is an inhibitor of DNA repair and hence accelerates genome destabilization and cell death[J]. EMBO J, 2019, 38(21): e102718. doi: 10.15252/embj.2019102718
[33] Parkes EE, Walker SM, Taggart LE, et al. Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer[J]. J Natl Cancer Inst, 2016, 109(1): djw199. doi: 10.1093/jnci/djw199
[34] Rudqvist NP, Pilones KA, Lhuillier C, et al. Radiotherapy and CTLA-4 blockade shape the TCR repertoire of tumor-infiltrating T cells[J]. Cancer Immunol Res, 2018, 6(2): 139-150. doi: 10.1158/2326-6066.CIR-17-0134
[35] Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer[J]. Nature, 2015, 520(7547): 373-377. doi: 10.1038/nature14292
[36] Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen[J]. Cancer Immunol Res, 2015, 3(4): 345-355. doi: 10.1158/2326-6066.CIR-14-0196
[37] Golden EB, Frances D, Pellicciotta I, et al. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death[J]. Oncoimmunology, 2014, 3: e28518. doi: 10.4161/onci.28518
[38] Matsumura S, Wang BM, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells[J]. J Immunol, 2008, 181(5): 3099-3107. doi: 10.4049/jimmunol.181.5.3099
[39] Ruocco MG, Pilones KA, Kawashima N, et al. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects[J]. J Clin Invest, 2012, 122(10): 3718-3730. doi: 10.1172/JCI61931
[40] McLaughlin M, Patin EC, Pedersen M, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy[J]. Nat Rev Cancer, 2020, 20(4): 203-217. doi: 10.1038/s41568-020-0246-1
[41] Mondini M, Loyher PL, Hamon P, et al. CCR2-dependent recruitment of tregs and monocytes following radiotherapy is associated with TNFalpha-mediated resistance[J]. Cancer Immunol Res, 2019, 7(3): 376-387. doi: 10.1158/2326-6066.CIR-18-0633
[42] Bos PD, Plitas G, Rudra D, et al. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy[J]. J Exp Med, 2013, 210(11): 2435-2466. doi: 10.1084/jem.20130762
[43] Connolly KA, Belt BA, Figueroa NM, et al. Increasing the efficacy of radiotherapy by modulating the CCR2/CCR5 chemokine axes[J]. Oncotarget, 2016, 7(52): 86522-86535. doi: 10.18632/oncotarget.13287
[44] Liang H, Deng LF, Hou YZ, et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance[J]. Nat Commun, 2017, 8(1): 1736. doi: 10.1038/s41467-017-01566-5
[45] Kalbasi A, Komar C, Tooker GM, et al. Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma[J]. Clin Cancer Res, 2017, 23(1): 137-148. doi: 10.1158/1078-0432.CCR-16-0870
[46] Marciscano AE, Ghasemzadeh A, Nirschl TR, et al. Elective nodal irradiation aqttenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy[J]. Clin Cancer Res, 2018, 24(20): 5058-5071. doi: 10.1158/1078-0432.CCR-17-3427
[47] Dovedi SJ, Cheadle EJ, Popple AL, et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade[J]. Clin Cancer Res, 2017, 23(18): 5514-5526. doi: 10.1158/1078-0432.CCR-16-1673
[48] Vanpouille-Box C, Diamond JM, Pilones KA, et al. TGFbeta is a master regulator of radiation therapy-induced antitumor immunity[J]. Cancer Res, 2015, 75(11): 2232-2242. doi: 10.1158/0008-5472.CAN-14-3511
[49] Rodriguez-Ruiz ME, Rodríguez I, Mayorga L, et al. TGFbeta blockade enhances radiotherapy abscopal efficacy effects in combination with anti-PD1 and anti-CD137 immunostimulatory monoclonal antibodies[J]. Mol Cancer Ther, 2019, 18(3): 621-631. doi: 10.1158/1535-7163.MCT-18-0558
[50] Zhang Q, Green MD, Lang X, et al. Inhibition of ATM increases interferon signaling and sensitizes pancreatic cancer to immune checkpoint blockade therapy[J]. Cancer Res, 2019, 79(15): 3940-3951. doi: 10.1158/0008-5472.CAN-19-0761
[51] Sheng H, Huang Y, Xiao Y, et al. ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma[J]. J Immunother Cancer, 2020, 8(1): e000340. doi: 10.1136/jitc-2019-000340
[52] Vendetti FP, Karukonda P, Clump DA, et al. ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumor activity following radiation[J]. J Clin Invest, 2018, 128(9): 3926-3940. doi: 10.1172/JCI96519
[53] Wang B, Sun L, Yuan Z, et al. Wee1 kinase inhibitor AZD1775 potentiates CD8+ T cell-dependent antitumour activity via dendritic cell activation following a single high dose of irradiation[J]. Med Oncol, 2020, 37(8): 66. doi: 10.1007/s12032-020-01390-w
[54] Patel P, Sun L, Robbins Y, et al. Enhancing direct cytotoxicity and response to immune checkpoint blockade following ionizing radiation with Wee1 kinase inhibition[J]. Oncoimmunology, 2019, 8(11): e1638207. doi: 10.1080/2162402X.2019.1638207
-
期刊类型引用(10)
1. 李强虎,辛勇. GSTP1和ERCC1基因多态性与食管癌患者放疗敏感性及预后的关系. 北华大学学报(自然科学版). 2024(01): 60-65 .  百度学术
百度学术
2. 李艳娇,鲁志兵,魏文秀. 食物增稠剂在放疗后吞咽困难患者中的应用研究. 当代护士(上旬刊). 2024(03): 64-67 .  百度学术
百度学术
3. 李家祥,严明基,张念华,匡云凤,张冰,陈俊宇. TOMO SBRT联合靶免治疗对不可手术切除肝癌患者临床疗效、肿瘤标志物的影响. 影像科学与光化学. 2024(06): 685-693 .  百度学术
百度学术
4. 陈颖秀,卢仁泉,郭林,郑慧. 直肠癌患者外周血淋巴细胞亚群与新辅助治疗效果的关系. 中华检验医学杂志. 2024(12): 1419-1425 .  百度学术
百度学术
5. 宋闽,朱志鹏,毛国华,周子杰,陈敏. cGAS-STING信号通路在脑肿瘤中的研究进展. 中华神经医学杂志. 2023(04): 405-409 .  百度学术
百度学术
6. 蔡丽,陈伟伟,张小曼,杨慧敏,周欠南. 吞咽肌群训练预防鼻咽癌放疗后病人吞咽障碍的作用及其对张口、吞咽功能及营养状况的影响. 全科护理. 2022(14): 1945-1947 .  百度学术
百度学术
7. 李瑞晓,唐启胜,马善金,张波,孙振业,李雪莲. 慢病毒介导NDRG2基因过表达抑制膀胱癌细胞的放射抗性. 中国医师杂志. 2021(07): 992-995, 1000 .  百度学术
百度学术
8. 于金明,颜薇薇,陈大卫. 肺癌放射免疫新实践. 山东大学学报(医学版). 2021(09): 1-8 .  百度学术
百度学术
9. 王茵,吴茜茜,刘来昱,官键. 肿瘤相关巨噬细胞在放疗中的研究进展. 国际放射医学核医学杂志. 2021(08): 532-538 .  百度学术
百度学术
10. 张佳秀,邓林锋,石建邦,吴健卫. 非小细胞肺癌患者肿瘤微环境中TAMs及PD-1表达水平及与临床预后的关系. 中国当代医药. 2021(32): 4-8+241 .  百度学术
百度学术
其他类型引用(7)



 下载:
下载:

