-
摘要:目的
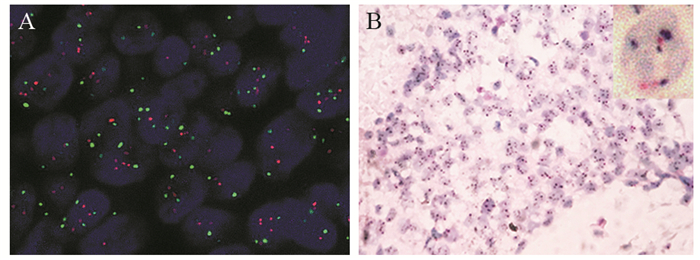
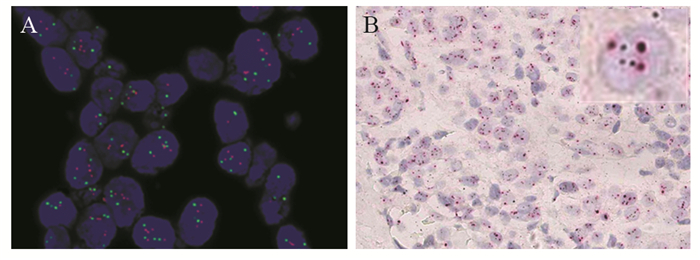
比较2013版及2018版美国临床肿瘤学会/美国病理医师学院(ASCO/CAP)乳腺癌HER2检测指南,对免疫组织化学HER2不确定(2+)乳腺癌FISH判读结果的影响及其临床意义。通过与FISH对比,分析DISH的准确性及存在问题。
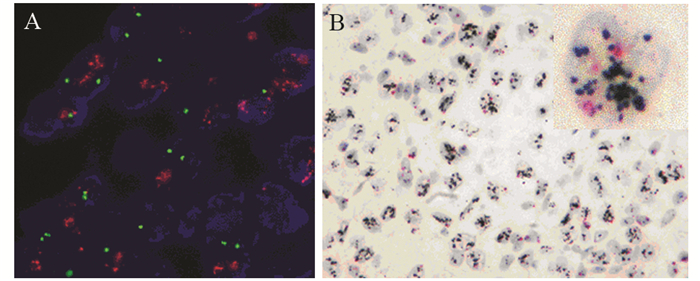
方法选取568例免疫组织化学HER2不确定浸润性乳腺癌石蜡包埋标本,使用FISH技术进行HER2基因状态检测,分别采用2013及2018版ASCO/CAP乳腺癌HER2检测指南进行结果判读。随机选取60例样本进行DISH检测,并与FISH对比分析。
结果依据2013版指南,HER2阳性183例(32.22%)、阴性365例(64.26%)、不确定20例(3.52%)。采用新指南判读标准,HER2阳性率减少了4.58%,阴性率无差别,两种判读标准差异具有统计学意义(P < 0.01)。对60例肿瘤样本的FISH和DISH检测结果比较,两者总符合率为95.0%,阳性符合率为94.7%;阴性符合率为100%,两种方法的检测结果显著相关(r=0.636, P < 0.01)。
结论2018版ASCO/CAP HER2检测指南与2013版相比,判读标准更明确、分类更精细,对临床用药更具指导作用。DISH技术具有亮视野的优点,与FISH有同样的临床诊断价值。
-
关键词:
- 荧光原位杂交 /
- 双色银增强原位杂交 /
- 人表皮生长因子受体2 /
- 乳腺癌 /
- 曲妥珠单抗
Abstract:ObjectiveTo compare the differences between 2013 and 2018 ASCO/CAP HER2 testing guidelines in 568 immunohistochemical HER2 equivocal breast cancer patients and to analyze the accuracy and deficiency of dual-color silver enhanced in-situ hybridization (DISH) by comparing with FISH.
MethodsWe selected 568 cases of paraffin-embedded invasive breast cancer specimen which were proved as HER2 equivocal by immunohistochemical (IHC). The HER2 gene amplification status was evaluated by FISH and comparatively analyzed according to both the 2013 and 2018 ASCO/CAP HER2 Testing guidelines respectively. Sixty specimens were randomly selected for DISH detection and compared with FISH.
ResultsAccording to the 2013 guideline, the number of HER2 positive, negative and equivocal cases were 183 (32.22%), 365 (64.26%) and 20 (53.52%), respectively. Compared with the 2013 guideline, HER2 positive rate decreased by 4.58% according to the revised 2018 guideline, and the negative rates were same; the difference of positive rates was statistically significant (P < 0.01). Comparison between FISH and DISH findings in 60 samples, the total, positive and negative coincidence rates were 95.0%, 94.7% and 100%, and the results of the two methods are significantly correlated (r=0.636, P < 0.01).
ConclusionCompared with 2013 ASCO/CAP guideline, the revised 2018 guidelines is more accurate and instructive for clinical medication. DISH could achieve similar precise results as compared to FISH. Bright field detection is the irreplaceable advantage of DISH.
-
作者贡献蒋依娜: 实验设计、部分FISH及DISH实验操作、数据整理及分析、论文撰写及修改王博: 统计学分析周灿: 临床资料整理及分析王鸿雁: 实验及论文指导
-
表 1 568例免疫组织化学不确定的浸润性乳腺癌HER2 FISH检测结果
Table 1 FISH results of HER2 in 568 cases of immunohistochemical HER2 equivocal invasive breast cancer

表 2 FISH和DISH方法检测HER2基因状态的比较
Table 2 Comparison of HER2 gene status between FISH and DISH test

-
[1] Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update[J]. J Clin Oncol, 2013, 31(31): 3997-4013. doi: 10.1200/JCO.2013.50.9984
[2] Baselga J, Perez EA, Pienkowski T, et al. Adjuvant Trastuzumab A Milestone in the Treatment of HER-2-Positive Early Breast Cancer[J]. Oncologist, 2006, 11Suppl 1: 4-12. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=Open J-Gate000000180483
[3] Press MF, Sauter G, Buyse M, et al. HER2 Gene Amplification Testing by Fluorescent In Situ Hybridization (FISH): Comparison of the ASCO-College of American Pathologists Guidelines With FISH Scores Used for Enrollment in Breast Cancer International Research Group Clinical Trials[J]. J Clin Oncol, 2016, 34(29): 3518-3528. doi: 10.1200/JCO.2016.66.6693
[4] von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer[J]. N Engl J Med, 2017, 377(2): 122-131. doi: 10.1056/NEJMoa1703643
[5] Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update[J]. J Clin Oncol, 2018, 36(20): 2105-2122. doi: 10.1200/JCO.2018.77.8738
[6] Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer correlation of relapse and survival with amplification of the HER-2 neu oncogene[J]. Science, 1987, 235(4785): 177-182. doi: 10.1126/science.3798106
[7] Schmidt M, Lewark B, Kohlschmidt N. Long-term prognostic significance of HER-2 neu in untreated node-negative breast cancer depends on the method of testing[J]. Breast Cancer Res, 2005, 7(2): R256-R266. doi: 10.1186/bcr991
[8] Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer[J]. N Engl J Med, 2005, 353(16): 1673-1684. doi: 10.1056/NEJMoa052122
[9] Zhao B, Zhao H. Impact of clinicopathological characteristics on the efficacy of neoadjuvant therapy in patients with human epidermal growth factor receptor-2-positive breast cancer[J]. Int J Cancer, 2018, 142(4): 844-853. doi: 10.1002/ijc.31097
[10] Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer[J]. J Clin Oncol, 2007, 25(1): 118-145. http://cn.bing.com/academic/profile?id=d6e2a4e0ba49fdc1c2c8878676f5fe6d&encoded=0&v=paper_preview&mkt=zh-cn
[11] Varga Z, Tubbs RR, Wang Z, et al. Co-amplification of the HER2 gene and chromosome 17 centromere: a potential diagnostic pitfall in HER2 testing in breast cancer[J]. Breast Cancer Res Treat, 2012, 132(3): 925-935. doi: 10.1007/s10549-011-1642-8
[12] 《乳腺癌HER2检测指南》编写组.乳腺癌HER2检测指南(2019版)[J].中华病理学杂志, 2019, 48(3): 169-175. doi: 10.3760/cma.j.issn.0529-5807.2019.03.001 Writing group of guide for the detection of HER-2 in breast cancer. Guide for the detection of HER-2 in breast cancer (2019 edition)[J]. Zhonghua Bing Li Xue Za Zhi, 2019, 48(3): 169-175. doi: 10.3760/cma.j.issn.0529-5807.2019.03.001
[13] Hanna WM, Barnes PJ, Chang MC, et al. Human epidermal growth factor receptor 2 testing in primary breast cancer in the era of standardized testing: a Canadian prospective study[J]. J Clin Oncol, 2014, 32(35): 3967-3973. doi: 10.1200/JCO.2014.55.6092
[14] 《乳腺癌HER2检测指南》编写组.乳腺癌HER2检测指南(2014版)[J].中华病理学杂志, 2014, 43(4): 262-267. doi: 10.3760/cma.j.issn.0529-5807.2014.04.012 Writing group of guide for the detection of HER-2 in breast cancer. Guide for the detection of HER-2 in breast cancer (2014) edition[J]. Zhonghua Bing Li Xue Za Zhi, 2014, 43(4): 262-267. doi: 10.3760/cma.j.issn.0529-5807.2014.04.012
[15] Tanner M, Gancberg D, Di Leo A, et al. Chromogenic in situ hybridization a practical alternative for fluorescence in situ hybridization to detect HER-2 neu oncogene amplification in archival breast cancer samples[J]. Am J Pathol, 2000, 157(5): 1467-1472. doi: 10.1016/S0002-9440(10)64785-2
[16] Lim SJ, Cantillep A, Carpenter PM, et al. Validation and workflow optimization of human epidermal growth factor receptor 2 testing using INFORM HER2 dual-color in situ hybridization[J]. Hum Pathol, 2013, 44(11): 2590-2596. doi: 10.1016/j.humpath.2013.07.005
[17] Lee Y, Ryu Y, Jeong H, et al. Effectiveness of silver-enhanced in situ hybridization for evaluating HER2 gene status in invasive breast carcinoma: a comparative study[J]. Arch Med Res, 2012, 43(2): 139-144. doi: 10.1016/j.arcmed.2012.03.010
[18] Garcia-Caballero T, Prieto O, Vazquez-Boquete A, et al. Dual-colour CISH is a reliable alternative to FISH for assessment of topoisomerase 2-alpha amplification in breast carcinomas[J]. Breast Cancer Res Treat, 2014, 143(1): 81-89. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ea04a983e1662d113dc56fe232606785



 下载:
下载: