Efficacy and Safety of PD-1/PD-L1 Inhibitor Versus Chemotherapy in First-line Treatment of Advanced Non-small Cell Lung Cancer: A Meta-analysis
-
摘要:目的
系统评价PD-1/PD-L1抑制剂对比化疗一线治疗晚期非小细胞肺癌的疗效及安全性。
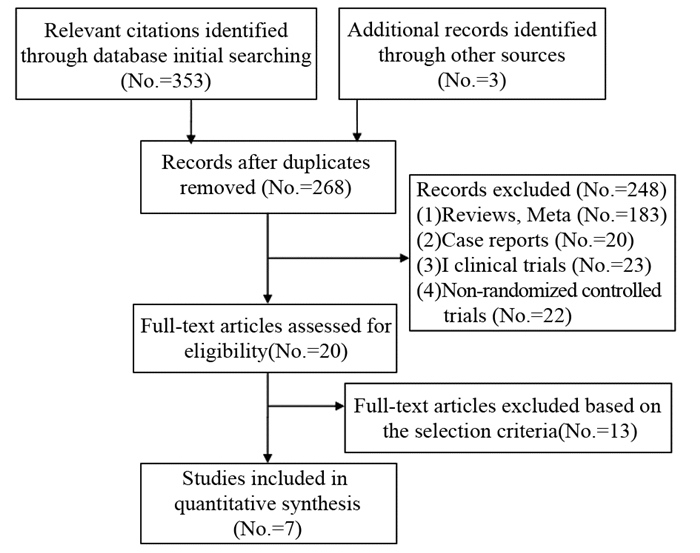
方法通过Web of science等国内外数据库,ASCO会议摘要及杂志筛选文献,进行Meta分析。
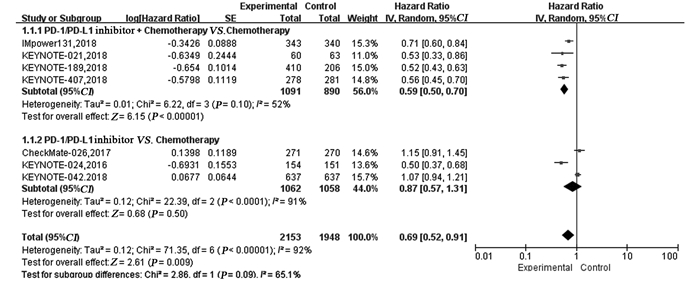
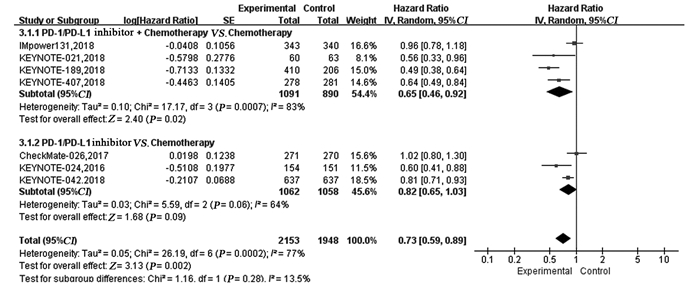
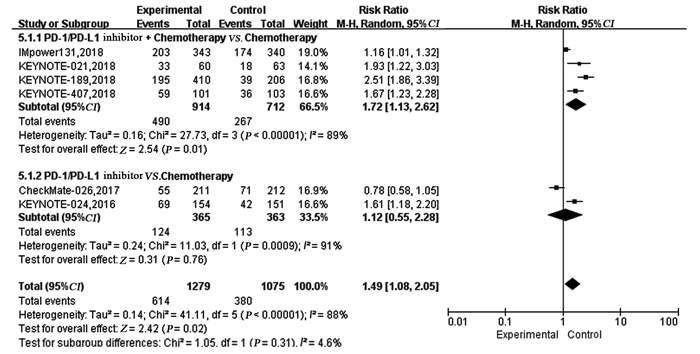
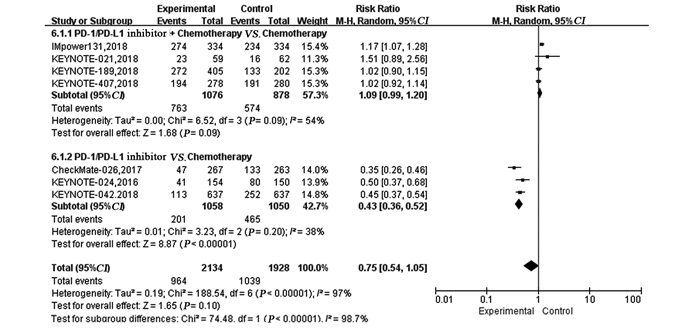
结果纳入7项RCT研究,4 101例患者,荟萃分析显示抑制剂联合化疗对比化疗可显著延长患者的PFS(HR=0.59, 95%CI: 0.50~0.70, P < 0.00001)、OS(HR=0.65, 95%CI: 0.46~0.92, P=0.02)及ORR(RR=1.72, 95%CI: 1.13~2.62, P=0.01)。亚组分析显示,抑制剂联合化疗可显著延长PFS及OS,且PD-L1表达程度越高,疗效获益越显著。而单药抑制剂对比化疗在延长晚期NSCLC患者的PFS(HR=0.87, 95%CI: 0.57~1.31, P=0.50)、OS(HR=0.82, 95%CI: 0.65~1.03, P=0.09)及提高ORR(RR=1.12, 95%CI: 0.55~2.28, P=0.76)方面两组差异无统计学意义。与化疗相比,单药抑制剂一线治疗PD-L1高表达的晚期NSCLC患者可显著延长OS,但在延长PFS方面未见明显优势。与化疗组相比,抑制剂联合化疗组3~4级不良反应发生率无明显改善(HR=1.09,95%CI: 0.99~1.20, P=0.09),而单药PD-1/PD-L1抑制剂组3~4级不良反应发生率低(RR=0.43, 95%CI: 0.36~0.52, P < 0.00001)。
结论PD-1/PD-L1抑制剂联合化疗一线治疗晚期NSCLC患者疗效优于化疗方案;PD-L1高表达者单药PD-1/PD-L1抑制剂可作为一线治疗的优先选择,且具有良好的安全性。
-
关键词:
- PD-1/PD-L1抑制剂 /
- 化疗 /
- 一线治疗 /
- 晚期非小细胞肺癌 /
- Meta分析
Abstract:ObjectiveTo review the effectiveness and safety of PD-1/PD-L1 inhibitor versus chemotherapy in the first-line treatment of advanced non-small cell lung cancer.
MethodsRelevant literatures were searched through Web of Science database, ASCO meeting abstract, journals, etc. for meta-analysis.
ResultsTotally 7 RCTs including 4101 patients were analyzed. The meta-analysis showed that compared with chemotherapy, PD-1/PD-L1 inhibitor combined with chemotherapy significantly prolonged PFS (HR=0.59, 95%CI: 0.59-0.70, P < 0.00001), OS (HR=0.65, 95%CI: 0.65-0.92, P=0.02) and ORR(RR=1.72, 95%CI: 1.13-2.62, P=0.01). Subgroup analysis showed PD-1/PD-L1 inhibitor combined with chemotherapy could significantly prolong PFS and OS, compared with chemotherapy. The higher PD-L1 expression was, the more significant the curative effect was. There was no statistical significance in prolonging PFS (HR=0.87, 95%CI: 0.57-1.31, P=0.50), OS (HR=0.82, 95%CI: 0.65-1.03, P=0.09) or increasing ORR (RR=1.12, 95%CI: 0.55-2.28, P=0.76) between single-agent PD-1/PD-L1 inhibitor and chemotherapy. Compared with chemotherapy, single-agent PD-1/PD-L1 inhibitor in the first-line treatment could significantly prolong the OS of advanced NSCLC patients with high PD-L1 expression. Grade 3-4 treatment-related adverse effect was not statistically significant different between PD-1/PD-L1 inhibitor combined with chemotherapy group and chemotherapy group (HR=1.09, 95%CI: 0.99-1.20, P=0.09), while that in single-agent PD-1/PD-L1 inhibitor group was lower (RR=0.43, 95%CI: 0.36-0.52, P < 0.00001).
ConclusionCompared with chemotherapy, PD-1/PD-L1 inhibitor combined with chemotherapy in the first-line treatment on advanced NSCLC patients is more effective; single-agent PD-1/PD-L1 inhibitor should be used as the first-line treatment preferentially on advanced NSCLC patient with high PD-L1 expression.
-
作者贡献魏 瑜: 文献筛选、数据分析、论文撰写张 莉: 文献筛选、论文指控及审核
-
表 1 文献基本特征
Table 1 Basic characteristics of included studies

表 2 PD-1/PD-L1抑制剂联合化疗组和单药PD-1/PD-L1抑制剂组在不同PD-L1表达程度PFS的亚组分析
Table 2 Subgroup analysis of PFS of advanced NSCLC patients with different PD-L1 expression in PD-1/PD-L1 inhibitor combined with chemotherapy group and singleagent PD-1/PD-L1 inhibitor group

表 3 PD-1/PD-L1抑制剂联合化疗组和单药PD-1/PD-L1抑制剂组在不同PD-L1表达程度总生存期(OS)的亚组分析
Table 3 Subgroup analysis of OS of advanced NSCLC patients with different PD-L1 expression in PD-1/PD-L1 inhibitor combined chemotherapy group and single-agent PD-1/PD-L1 inhibitor group

-
[1] Ricciuti B, Leonardi GC, Metro G, et al. Targeting the KRAS variant for treatment of non-small cell lung cancer: potential therapeutic applications[J]. Expert Rev Respir Med, 2016, 10(1): 53-68. doi: 10.1586/17476348.2016.1115349
[2] Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer[J]. J Clin Oncol, 2010, 28(2): 357-60. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3870288
[3] Blakely CM, Watkins TBK, Wu W, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers[J]. Nat Genet, 2017, 49(12): 1693-704. doi: 10.1038/ng.3990
[4] 魏瑜, 杨媚, 张建清, 等.含铂双药对比非铂单药二线治疗晚期非小细胞肺癌的系统评价[J].中华肿瘤防治杂志, 2015, 22(4): 239-99.[ Wei Y, Yang M, Zhang JQ, et al. Systematic Review: Comparison of Platinum-based doublet versus non-Platinum single-agent as second-line treatment of advanced non-small-cell lung cancer[J]. Zhonghua Zhong Liu Fang Zhi Za Zhi, 2015, 22(4): 239-99.]
[5] Kazandjian D, Suzman DL, Blumenthal G, et al. FAD Approveal Summary: Nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after Platinum-Based chemotherapy[J]. Oncologist, 2016, 21(5): 634-42. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4861371/
[6] Higgins J, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0(updatedmarch2011)[M]. Coehrane Colloboration, 2011: 79-87.
[7] Parmar MK, Torri V, Stewart L, et al. Extracting summary statistics to perform meta-analysis of the published literature for survival endpoints[J]. Stat Med, 1998, 17(24): 2815-34. doi: 10.1002/(ISSN)1097-0258
[8] Altman DG, Bland JM. Interaction revisited: the difference between two estimates[J]. BMJ, 2003, 326(7382): 219. doi: 10.1136/bmj.326.7382.219
[9] Carbone DP, Reck M, PazaAres L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer[J]. N Engl J Med, 2017, 376(25): 2415-26. doi: 10.1056/NEJMoa1613493
[10] Luis G, Paz-Ares, Alexander Luft, et al. Phase 3 study of carboplatin-paclitaxel/nab-paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients (Pts) with metastatic squamous (Sq) non-small cell lung cancer (NSCLC)[J]. J Clin Oncol, 2018, 36(suppl):105. http://cn.bing.com/academic/profile?id=896c1939a2d325d5bd00aa6edf5721cb&encoded=0&v=paper_preview&mkt=zh-cn
[11] Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer[J]. N Engl J Med, 2016, 375(19): 1823-33. doi: 10.1056/NEJMoa1606774
[12] Borghaei H, Langer CJ, Gadgeel S, et al. 24-month overall survival from KEYNOTE-021 cohort G: Pemetrexed-carboplatin plus pembrolizumab as first-line therapy for advanced nonsquamous NSCLC[J]. J Thorac Oncol, 2019, 14(1): 124-29.
[13] Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer[J]. N Engl J Med, 2018, 378(22): 2078-92. doi: 10.1056/NEJMoa1801005
[14] Lopes G, Wu YL, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥1%: Open-label, phase 3 KEYNOTE-042 study[J]. J Clin Oncol, 2018, 36(18-suppl): abstr LBA4.
[15] Socinski MA, Rittmeyer A, Shapovalov D, et al. IMpower131: Progression-free survival (PFS) and overall survival (OS) analysis of a randomised phase Ⅲ study of atezolizumab+carboplatin+paclitaxel or nab-paclitaxel vs. carboplatin+nab-paclitaxel in 1L advanced squamous NSCLC[J]. Ann Oncol, 2018, 29(suppl-8): abstr LBA65.
[16] Merelli B, Massi D, Cattaneo L, et al. Targeting the PD1/PD-Ll axis in melanoma: Biological rationale, clinical challenges and opportunities[J]. Crit Rev Oncol Hematol, 2014, 89(1): 140-65. doi: 10.1016/j.critrevonc.2013.08.002
[17] Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation oflymphocyte activation[J]. J Exp Med, 2000, 192(7): 1027-34. doi: 10.1084/jem.192.7.1027
[18] Topalian SL, Hodi FS, Brabmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer[J]. N Engl J Med, 2012, 366(26): 2443-54. doi: 10.1056/NEJMoa1200690
[19] Borghaei H, PazAres L, Hom L, et al. Nivolumab versus docetaxel in advanced non squamous non-small-cell lung cancer[J]. N Engl J Med, 2015, 373(17): 1627-39. doi: 10.1056/NEJMoa1507643
[20] Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous cell non-small-cell lung cancer[J]. N Engl J Med, 2015, 373(2): 123-35. doi: 10.1056/NEJMoa1504627



 下载:
下载: