Correlation of Tumor Markers with Clinical Efficacy of Advanced Lung Adenocarcinoma Patients with EGFR Mutation
-
摘要:目的
探讨治疗前血清肿瘤标志物的表达水平与EGFR阳性的晚期肺腺癌患者的突变率和疗效之间的关系。
方法选取经分子病理确诊为EGFR阳性的晚期肺腺癌患者143例,均采用一代EGFR-TKIs进行一线治疗。按治疗前CEA、CYFRA21-1、NSE的表达水平分为高水平组和正常组,回顾分析其无进展生存期(PFS),观察治疗前肿瘤标志物表达水平与EGFR-TKIs药物一线治疗非小细胞肺癌的疗效之间的关系。
结果CEA高表达组一线应用EGFR-TKIs的疗效显著优于CEA正常组(P < 0.05);CYFRA21-1正常组一线应用EGFR-TKIs治疗的疗效优于CYFRA21-1高表达组(P < 0.05);NSE正常组的中位PFS略优于NSE高表达组(P > 0.05)。亚组分析:ECOG PS评分 < 2组的中位PFS优于PS评分≥2组(P < 0.05);无吸烟史组一线EGFR-TKIs的疗效优于有吸烟史组(P < 0.05)。性别、年龄、分期、突变类型、靶向药物等因素差异均无统计学意义。多因素生存分析显示:一线应用EGFR-TKIs药物治疗的中位PFS与ECOG PS评分、有无吸烟史及CYFRA21-1的状态无关,CEA高表达为一线应用EGFR-TKIs治疗的正性独立预后因素。
结论CEA升高与EGFR突变状态具有相关性,是评价一代靶向药物作为一线治疗疗效的正性独立预后因素。
Abstract:ObjectiveTo explore the correlation of serum tumor markers expression with the mutation rate and efficacy of the patients with EGFR-positive advanced lung adenocarcinoma.
MethodsWe selected 143 patients who were molecular pathologically diagnosed as positive EGFR mutation had received epidermal growth factor receptor tyrosine kinase inhibitors(EGFR-TKIs) treatment. According to CYFRA21-1, CEA and NSE expression levels before treatment, the patients were randomly divided into high level group and normal level group, respectively. We retrospectively analyzed progression-free survival(PFS) after the targeted therapy to explore the relationship between tumor markers expression before treatment and the efficacy of EGFR-TKIs as the first-line treatment on non-small cell lung cancer patients.
ResultsThe efficacy of high CEA group was significantly better than that of normal CEA group(P < 0.05). The curative effect of normal CYFRA21-1 group was longer than that of high CYFRA21-1 group(P < 0.05). The median PFS had no significant difference between high NSE group and normal NSE group(P > 0.05). In subgroup analysis, ECOG PS < 2 group was better than PS≥2 group in PFS(P < 0.05). Non-smoking history group was better than smoking history group in curative effect(P < 0.05). Gender, age, TNM stage, mutation type and targeted drug had no significant differences in statistics. Multivariate survival analysis showed that median PFS was not related with ECOG PS score, smoking history or the state of CYFRA21-1, and the high expression of CEA was an independent prognostic factor for EGFR-TKIs as the first-line treatment.
ConclusionHigh CEA has a certain correlation with EGFR mutation status. It is a positive independent prognostic factor for the evaluation of targeted therapy.
-
Key words:
- Lung adenocarcinoma /
- Tumor markers /
- EGFR mutation /
- PFS
-
0 引言
肺癌是最常见的恶性肿瘤之一,死亡率占恶性肿瘤首位,其中有85%为非小细胞肺癌[1]。随着分子靶向治疗及免疫治疗的迅速发展,表皮生长因子受体(epidermal growth factor receptor, EGFR)、ALK等基因检测成为非小细胞肺癌的常规检测,对于EGFR敏感突变阳性的患者,吉非替尼、厄洛替尼等推荐作为优于一线化疗的首选治疗[2-3],既往数据显示EGFR-TKIs(epidermal growth factor receptor tyrosine kinase inhibitors)治疗的无进展生存期(progression-free survival, PFS)优于标准方案化疗,在总生存期(overall survival, OS)上无明显优势。临床上常将肿瘤标志物作为恶性肿瘤诊断及疗效评价的辅助方法[4],已有研究表明血清中CYFRA21-1、CEA的高水平表达提示非小细胞肺癌患者预后较差,两者综合判断结果更加可靠[5-6],但对于肿瘤标志物的表达水平与EGFR阳性的患者对于EGFR-TKIs药物的敏感度和疗效有无差异仍存在争议。本研究为探讨治疗前血清肿瘤标志物的表达水平与EGFR阳性的晚期肺腺癌患者的突变率和疗效的关系,以加深对肿瘤标志物的认识,探寻可靠、便捷、廉价的方式来判断晚期肺腺癌患者的预后。
1 资料与方法
1.1 临床资料及方法
回顾分析郑州大学第一附属医院2013年6月至2014年6月经分子病理确诊为EGFR阳性的晚期肺腺癌患者143例,所有患者一线治疗均应用一代EGFR-TKIs药物,按治疗前CEA(carcinoembryonic antigen)、CYFRA21-1(cytokeratin 19 fragments)、NSE(neuron-specific enolase)的表达水平分为高表达组和正常组,分析其PFS,探究治疗前肿瘤标志物表达水平与EGFR突变率及EGFR-TKIs药物疗效的关系。EGFR基因检测由郑州大学第一附属医院病理科应用实时荧光PCR及DNA测序法,采用ABI Step One/ABI Sequence Analyzer仪器测得。肿瘤标志物的检测采用电化学发光免疫法,按郑州大学第一附属医院肿瘤实验室的标准将CEA≤5 ng/ml、CYFRA21-1≤3.3 ng/ml、NSE≤25 ng/ml纳入正常组,CEA > 5 ng/ml、CYFRA21-1 > 3.3 ng/ml、NSE > 25 ng/ml纳入高表达组。体能状况评价采用ECOG PS(eastern cooperative oncology group performance status)评分标准。口服靶向药物的方法:吉非替尼250 mg,1次/天;厄洛替尼150 mg,1次/天;埃克替尼125 mg,3次/天。本研究经过郑州大学第一附属医院医学伦理委员会批准,且临床资料和数据收集均已获得患者知情同意。
1.2 疗效评价方法
所有纳入研究的患者均依据实体瘤疗效评价标准1.1版(response evaluation criteria in solid tumor, RECIST),分为完全缓解(CR)、部分缓解(PR)、稳定(SD)和疾病进展(PD),每2周期复查一次CT,评价疗效。PFS:指患者从接受靶向治疗开始,到观察到疾病进展或发生严重不良事件终止治疗的时间。对于在截至随访日期仍无进展的病例,视为删失数据处理。
1.3 统计学方法
采用SPSS 17.0统计软件进行统计学分析。分类变量表示为率(%)。单因素生存分析用Kaplan-Meier法并行Log rank检验,多因素生存分析采用Cox比例风险模型。所有统计检验均为双侧概率检验,P < 0.05为差异有统计学意义。
2 结果
2.1 主要结果的单因素生存分析
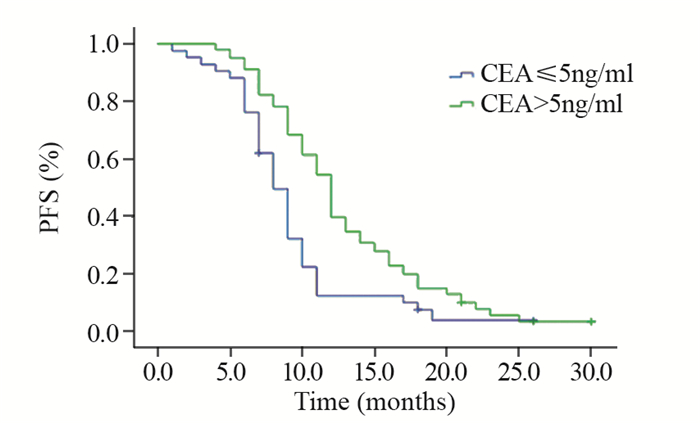
CEA高表达组有101例,占EGFR阳性患者的70.6%(101/143),其一线应用EGFR-TKIs治疗的中位PFS显著优于CEA正常组,差异有统计学意义(P < 0.0003);CYFRA21-1高表达组有61例,一线EGFR-TKIs治疗的中位PFS,CYFRA21-1正常组优于高表达组,且两组间差异有统计学意义(P=0.031);NSE高表达组有114例,NSE正常组的中位PFS略优于NSE高表达组,但差异无统计学意义(P=0.488),见表 1。
表 1 143例患者临床及病理特征的单因素分析Table 1 Univariate survival analysis of 143 cases in clinical and pathology features
2.2 亚组的单因素生存分析
ECOG PS评分低组( < 2分)的中位PFS优于PS评分高组(≥2分),差异有统计学意义;无吸烟史组的中位PFS优于有吸烟史组,两组间差异有统计学意义;男性患者和年龄 < 60岁组患者的中位PFS分别略高于女性患者和高龄组,但差异无统计学意义;口服吉非替尼组97例,厄洛替尼组19例,埃克替尼组27例,埃克替尼组的中位PFS略优于吉非替尼组和厄洛替尼组,但三组间差异无统计学意义;Ⅳ期患者的中位PFS优于ⅢB期患者,但差异无统计学意义;19 del突变组76例,21 L858R突变组62例,其他突变组5例,19 del突变组的中位PFS优于21 L858R组及其他突变组,三组间差异无统计学意义。亚组分析提示:性别、年龄、分期、突变类型、NSE水平、口服靶向药物类型的差异均无统计学意义,见表 1。
2.3 多因素生存分析
将CEA、CYFRA21-1、吸烟史、ECOG PS评分纳入多因素分析研究对象,提示一线应用EGFR-TKIs药物治疗的中位PFS与ECOG PS评分、有无吸烟史及CYFRA21-1的状态无关,CEA高表达为一线应用EGFR-TKIs药物治疗疗效的正性独立预后因素(HR=0.595, 95%CI: 0.396~0.893, P=0.012),见表 2、图 1。
表 2 一代EGFR-TKIs药物的PFS相关因素的多因素生存分析Table 2 Multivariate survival analysis of related factors with EGFR-TKIs in PFS
3 讨论
血清肿瘤标志物是在肿瘤发生发展过程中产生的特异或非特异细胞因子,随着患者肿瘤的进展其浓度增大,经过治疗后随着症状的改善,肿瘤负荷减小,患者血清中肿瘤标志物随之下降, ,当肿瘤复发时,肿瘤标志物还会再次升高[7]。CEA是一种参与细胞黏附的糖蛋白,正常可于胚胎期产生,在许多恶性肿瘤中高表达,包括非小细胞肺癌[5],然而CEA在吸烟者、良性肿瘤及一些炎性反应中亦可高表达[8]。CYFRA21-1是角蛋白19(Keratin 19)片段的标志物,CYFRA21-1的升高不仅是因为细胞裂解或坏死增多所致,肿瘤细胞中活化的蛋白酶所产生的角蛋白丝也可使血清中CYFRA21-1升高,CYFRA21-1对于非小细胞肺癌,尤其是鳞状细胞癌的诊断具有较高的敏感度和特异性[8-9]。既往认为,NSE是参与糖酵解途径的烯醇化酶的一种,存在于神经组织和神经内分泌组织中,NSE的变化与小细胞肺癌病情的变化密切相关[10-11],NSE的升高可能与肿瘤脑转移引起的脑组织损伤有关[12],NSE高表达提示预后差。
本研究的143例患者,CEA高表达组占70.6%,有假说认为CEA与EGFR基因突变率之间具有一定的相关性[13],这可能与EGFR基因突变导致其下游信号分子过度激活,抗凋亡作用增强,癌细胞增殖加速,继而CEA过度表达有关[14-15]。CEA高表达组靶向治疗的PFS明显优于CEA正常组。Cui等[16]报道了208例接受EGFR-TKIs治疗的晚期肺腺癌患者,分析年龄、吸烟史、EGFR状态、分化程度、肿瘤标志物的表达情况、手术史等因素对PFS的影响,认为无吸烟史、CEA高表达及一线应用EGFR-TKIs是获得更长PFS的独立预后因素,而本研究结果未显示吸烟史是影响EGFR-TKIs疗效的一个预后因素,分析原因可能如下:(1)Cui等报道的人群为有EGFR阳性、阴性或突变状态未知的非小细胞肺癌人群,部分人群既往有手术史,且并非全部一线应用EGFR-TKIs治疗,与本研究的研究对象不完全相符;(2)本研究的样本数量较小;(3)两个研究均有少量的失访数据,上述原因均可使试验结果产生偏倚。此外,在EGFR基因检测为阴性的中晚期非小细胞肺癌人群中,CEA高表达患者的中位PFS较CEA正常组显著缩短,差异有统计学意义,且无论CEA处于何种水平的EGFR阴性患者,其中位PFS均较EGFR阳性患者缩短[11],提示CEA升高是晚期非小细胞肺癌预后不良的因素,但CEA增高的患者对EGFR-TKIs具有更高的反应率和更好的疗效,尤其对于突变状态未知的非小细胞肺癌患者具有一定的临床指导价值[17-18]。
无吸烟史组的疗效显著优于有吸烟史组,有研究认为吸烟患者对靶向治疗的低反应性可能与EGFR下游信号分子ERK1/2及AKT处于持续活化状态,EGFR-TKI无法将上述通路阻断,且香烟烟雾长期暴露可诱导上皮间质转化(epithelial-mesenchymal transition, EMT),易引起获得性耐药[19]。PS评分较低组的中位PFS显著优于PS评分高组,两者差异有统计学意义。口服吉非替尼、埃克替尼、厄洛替尼三组患者的PFS无显著差异,与既往数据相符[20]。19 del组和21 L858R组治疗后的中位PFS优于其他罕见突变组,与相关文献报道相符[16, 21]。
综上所述,CEA高表达与EGFR突变状态具有一定相关性,CEA高表达为评价一代靶向药物作为一线治疗疗效的正性独立预后因素,可预测一代靶向药物治疗的临床疗效,提示EGFR阳性且CEA升高的患者应用一代靶向药物可能有更大的获益。同时,本研究为回顾性的单中心研究,仍存在很多不足之处,这一研究结果也需要更多的前瞻性研究加以证实。
-
表 1 143例患者临床及病理特征的单因素分析
Table 1 Univariate survival analysis of 143 cases in clinical and pathology features

表 2 一代EGFR-TKIs药物的PFS相关因素的多因素生存分析
Table 2 Multivariate survival analysis of related factors with EGFR-TKIs in PFS

-
[1] Jemal A, Siegel R, Ward E. Cancer statistics, 2008[J]. CA Cancer J Clin, 2008, 58(2): 71-96. doi: 10.3322/CA.2007.0010
[2] 薛洪省, 周少华, 卢万鹏, 等.非小细胞肺癌靶向治疗的靶点研究进展[J].中国肺癌杂志, 2013, 16(2): 107-13. doi: 10.3779/j.issn.1009-3419.2013.02.09 Xue HS, Zhou SH, Lu WP, et al. The targets research of non-small cell lung cancer targeted therapy[J]. Zhongguo Fei Ai Za Zhi, 2013, 16(2): 107-13. doi: 10.3779/j.issn.1009-3419.2013.02.09
[3] Ettinger DS, Wood DE, Akerley W, et al. NCCN guidelines insights: non-small cell lung cancer, Version 4. 2016[J]. J Natl Compr Canc Netw, 2016, 14(3): 255-64. doi: 10.6004/jnccn.2016.0031
[4] Dong Y, Zheng X, Yang Z, et al. Serum carcinoembryonic antigen, neuron-specific enolase as biomarkers for diagnosis of non-small cell lung cancer[J]. J Cancer Res Ther, 2016, 12(supplement): 34-6. http://www.medscape.com/medline/abstract/27721249
[5] Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer[J]. Lung cancer, 2012, 76(2): 138-43. doi: 10.1016/j.lungcan.2011.11.012
[6] Cedrés S, Nuñez I, Longo M, et al. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer(NSCLC)[J]. Clin Lung Cancer, 2011, 12(3): 172-9. doi: 10.1016/j.cllc.2011.03.019
[7] 张艳, 刘晶, 杨梦迪.非小细胞肺癌晚期靶向药物治疗中血清肿瘤标志物预测疗效的临床意义[J].临床药物治疗杂志, 2014, 12(5): 35-9. http://www.cnki.com.cn/Article/CJFDTOTAL-MDJB201605069.htm Zhang Y, Liu J, Yang MD. Clinical significance of serum tumor markers in predicting outcomes of targeted therapy in advanced non-small cell lung cancer[J]. Lin Chuang Yao Wu Zhi Liao Za Zhi, 2014, 12(5): 35-9. http://www.cnki.com.cn/Article/CJFDTOTAL-MDJB201605069.htm
[8] Ferrigno D, Buccheri G, Giordano C. Neuron-specific enolase is an effective tumor marker in non-small cell lung cancer(NSCLC)[J]. Lung Cancer, 2003, 41(3): 311-20. doi: 10.1016/S0169-5002(03)00232-0
[9] Sharma SK, Bhat S, Chandel V, et al. Diagnostic utility of serum and pleural fluid carcinoembryonic antigen, and cytokeratin 19 fragments in patients with effusion from nonsmall cell lung cancer[J]. J Carcinoq, 2015, 14: 7. doi: 10.4103/1477-3163.170662
[10] Tanaka K, Hata A, Kaji R, et al. Cytokeratin 19 fragment predicts the efficacy of epidermal growth factor receptor-tyrosine kinase inhibitor in non-small-cell lung cancer harboring EGFR mutation[J]. J Thorac Oncol, 2013, 8(7): 892-8. doi: 10.1097/JTO.0b013e31828c3929
[11] 杜军华, 乔洪源, 尹宜发.血清CEA、CA125及Cyfra21-1水平对中晚期非小细胞肺癌患者预后的影响[J].肿瘤防治研究, 2016, 43(2): 137-40. doi: 10.3971/j.issn.1000-8578.2016.02.009 Du JH, Qiao HY, Yin YF. Prognostic value of serum CEA, CA125 and Cyfra21-1 in patients with advanced non-small cell lung cancer[J]. Zhong Liu Fang Zhi Yan Jiu, 2016, 43(2): 137-40. doi: 10.3971/j.issn.1000-8578.2016.02.009
[12] 陈燕, 彭伟, 黄艳芳, 等.治疗前血清神经元特异性烯醇化酶水平在预测晚期非小细胞肺癌脑转移及预后中的意义[J].中国肺癌杂志, 2015, 37(7): 508-11. http://cdmd.cnki.com.cn/Article/CDMD-10459-1014392841.htm Chen Y, Peng W, Huang YF, et al. Significance of serum neuron-secific enolase before treatment in predicting brain metastases and prognosis of advanced non-small cell lung cancer[J]. Zhongguo Fei Ai Za Zhi, 2015, 37(7): 508-11. http://cdmd.cnki.com.cn/Article/CDMD-10459-1014392841.htm
[13] Shoji F, Yonshino I, Yano T, et al. Serum carcinoembryonic antigen level is associated with epidermal growth factor receptor mutations in recurrent lung ademocarcinomas[J]. Cancer, 2007, 110(12): 2793-8. doi: 10.1002/cncr.v110:12
[14] Jung M, Kim SH, Hong S, et al. Prognostic and predictive value of carcinoembryonic antigen and cytokeratin-19 fragments levels in advanced non-small cell lung cancer patients treated with gefitinib or erlotinib[J]. Yonsei Med J, 2012, 53(5): 931-9. doi: 10.3349/ymj.2012.53.5.931
[15] Qin HF, Qu LL, Liu H, et al. Serum CEA level change and its significance before and after Gefitinib therapy on patients with advanced non-small cell lung cancer[J]. Asian Pac J Cancer Prev, 2013, 14(7): 4205-8. doi: 10.7314/APJCP.2013.14.7.4205
[16] Cui S, Xiong L, Lou Y, et al. Factors that predict progression-free survival in Chinese lung adenocarcinoma patients treated with epidermal growth factor receptor tyrosine kinase inhibitors[J]. J Thorac Dis, 2016, 8(1): 68-78. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4740173/
[17] 谢亚琳, 梁继珍, 苏宁.吉非替尼与厄洛替尼在EGFR基因敏感突变晚期NSCLC患者一线治疗中的疗效比较[J].南方医科大学学报, 2015, 35(3): 446-9. http://www.cnki.com.cn/Article/CJFDTOTAL-DYJD201503028.htm Xie YL, Liang JZ, Su N. Gefitinib versus Erlotinib as fist-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer[J]. Nanfang Yi Ke Da Xue Xue Bao, 2015, 35(3): 446-9. http://www.cnki.com.cn/Article/CJFDTOTAL-DYJD201503028.htm
[18] Okamoto T, Nakamura T, Ikeda J, et al. Serum carcinoembryonic antigen as a predictive marker for sensitivity to gefitinib in advanced non-small cell lung cancer[J]. Eur J Cancer, 2005, 41(9): 1286-90. doi: 10.1016/j.ejca.2005.03.011
[19] Liu M, Zhou C, Zheng J. Cigarette smoking impairs the response of EGFR-TKIs therapy in lung adenocarcinoma patients by promoting EGFR signaling and epithelial-mesenchymal transition[J]. Am J Transl Res, 2015, 7(10): 2026-35. https://www.researchgate.net/publication/286293726_Cigarette_smoking_impairs_the_response_of_EGFR-TKIs_therapy_in_lung_adenocarcinoma_patients_by_promoting_EGFR_signaling_and_epithelial-mesenchymal_transition
[20] Eigenmann MJ, Frances N, Hoffmann G, et al. Combining nonclinical experiments with translational PKPD modeling to differentiate erlotinib and gefitinib[J]. Mol Cancer Ther, 2016, 15(12): 3110-9. doi: 10.1158/1535-7163.MCT-16-0076
[21] 柳菁菁, 张爽, 吴春娇, 等.中国不同表皮生长因子受体敏感突变类型非小细胞肺癌患者接受表皮生长因子受体酪氨酸激酶抑制剂一线治疗的临床疗效比较[J].中华肿瘤杂志, 2016, 38(3): 211-7. http://cdmd.cnki.com.cn/Article/CDMD-10246-1015428797.htm Liu JJ, Zhang S, Wu CJ, et al. Comparison of clinical outcoms of patients with non-small cell cancer harboring different types of epidermal growth factor receptor sensitive mutations after first-line EGFR-TKI treatment[J]. Zhonghua Zhong Liu Za Zhi, 2016, 38(3): 211-7. http://cdmd.cnki.com.cn/Article/CDMD-10246-1015428797.htm



 下载:
下载: