Clinicopathologic Characteristics and Prognosis of Oligodendroglioma with IDH Mutation and 1p/19q Codeletion
-
摘要:目的
探讨IDH突变和1p/19q共缺失型少突胶质细胞瘤的临床病理特征及预后相关影响因素。
方法收集54例IDH突变和1p/19q共缺失型少突胶质细胞瘤病例, 分析其临床病理特点, 包括年龄、组织学分级和肿瘤部位等因素对无进展生存期和总生存期的影响。
结果54例患者中, 肿瘤发生于1个脑叶者46例, 发生于2个脑叶以上者8例。肿瘤组织学WHO分级2级12例, 3级42例。FISH检测显示54例均为1p/19q共缺失; 免疫组织化学检测显示Olig2均为弥漫强阳性; GFAP均为阳性; p53有6例强阳性; 48例患者ATRX未缺失; Ki-67增殖指数5%~60%。Sanger测序显示54例均发生IDH基因突变(40例为IDH1突变, 14例为IDH2突变), 33例发生TERT启动子突变。16例在治疗过程中发生复发及转移。单因素分析显示, 手术后复发转移间隔时间超过2年可以延长患者无进展生存和总生存期。54例患者平均无进展生存期33.5个月, 平均总生存期40.7个月。
结论IDH突变和1p/19q共缺失型少突胶质细胞瘤术后联合精准放化疗降低了进展风险, 手术后复发转移间隔时间与该型患者预后相关。
Abstract:ObjectiveTo analyze the clinicopathological characteristics and prognosis of oligodendroglioma with IDH mutation and 1p/19q codeletion.
MethodsWe collected the data of 54 oligodendroglioma patients with IDH mutation and 1p/19q codeletion.The patients'clinicopathological data, including age, histological grade, and tumor site, were analyzed for the effects on progression-free and overall survival.
ResultsAmong the 54 patients, 46 cases were with tumor sites in one lobe, and eight cases involved tumor sites in more than two lobes.A total of 12 and 42 cases had WHO grades 2 and 3 oligodendroglioma, respectively.Detection by fluorescence in situ hybridization showed 1p/19q co-deletion in all cases.Immunohistochemical tests revealed diffuse and strong positive results for Olig2.All glial fibrillary acidic proteins were positive.p53 was strongly positive in six cases.ATRX was expressed in all 48 cases.Ki-67 proliferation index ranged from 5% to 60%.Sanger sequencing showed that all 54 cases had IDH gene mutations (40 cases were IDH1 mutations, and 14 were IDH2 mutations), and 33 cases had telomerase reverse transcriptase promoter mutations.Relapse and metastasis occurred in 16 patients during treatment.Univariate analysis indicated that the postoperative recurrence and metastasis interval of more than two years can prolong the progression-free and overall survival of patients.All 54 patients had a mean progression-free survival of 33.5 months and the mean overall survival of 40.7 months.
ConclusionFor oligodendroglioma with IDH mutation and 1p/19q codeletion, precision chemoradiotherapy after surgery can reduce the risk of progression, and the postoperative recurrence and metastasis interval is associated with the prognosis.
-
Key words:
- Oligodendroglioma /
- Pathological features /
- Histological grading /
- Genetic testing /
- Prognosis
-
0 引言
2016年世界卫生组织(WHO)对中枢神经系统肿瘤的分类根据IDH和1p/19q基因的突变状态细分弥漫性胶质瘤[1]。2021年WHO中枢神经系统肿瘤分类中将成人型弥漫性胶质瘤细分为3种类型,IDH突变和1p/19q共缺失型少突胶质细胞瘤是其中一种亚型,其定义为无论组织学形态如何,只要出现IDH突变联合染色体1p/19q共缺失型的弥漫性生长的神经胶质瘤[2],均为以上亚型。这给形态学诊断带来巨大挑战。组织学和分子特征的整合诊断导致胶质瘤分类标准的改变,同时引起胶质瘤治疗反应和预后的差别,有研究指出,具有1p/19q共缺失的少突胶质细胞瘤通过术后积极的放化疗,可以使患者获得更长的生存时间[3]。由于IDH突变和1p/19q共缺失型少突胶质细胞瘤病例报道较少,其预后生存仍待进一步探讨。本文通过回顾性分析54例IDH突变和1p/19q共缺失型少突胶质细胞瘤的临床病理及分子病理特征,以提高临床与病理医师对该肿瘤的认识,进一步为该型肿瘤患者的诊治提供更多理论依据。
1 资料与方法
1.1 临床资料
收集2015年8月—2023年4月中山大学附属肿瘤医院分子诊断存档的54例IDH突变和1p/19q共缺失型少突胶质细胞瘤。其中男性32例,女性22例,年龄24~65岁,中位年龄45.5岁。依据WHO(2021)中枢神经系统分类标准,病理分级为WHO 2级12例,3级42例。
1.2 方法
1.2.1 免疫组织化学染色
所有手术或活检标本经10%中性缓冲甲醛溶液固定,常规脱水、石蜡包埋,制备3 μm组织切片,HE染色,光学显微镜观察。所有病例均由病理科两位经验丰富的高年资医师阅片诊断。采用EnVision两步法染色,抗体GFAP购自北京中杉金桥生物技术有限公司,Olig2及ATRX购自福州迈新生物技术开发有限公司,Ki-67购自丹麦Dako公司,p53购自苏州百道医疗科技有限公司。采用罗氏公司BenchMark XT全自动染色仪进行染色。具体操作步骤按试剂盒说明书进行。
1.2.2 Sanger测序
对IDH1(R132)及IDH2(R172)及TERT(启动子第228和250位点)进行基因突变检测。(1)基因组DNA提取:切取3 μm厚组织切片10张,置于试剂条中,采用恺硕生物Concert48全自动核酸纯化仪提取基因组DNA。(2)PCR扩增:使用PCR仪扩增IDH1、IDH2及TERT突变位点目的片段,引物由上海生工生物公司合成。按25 μl PCR体系进行扩增。IDH1及IDH2 PCR反应程序分别为:94℃预变性5 min;94℃变性30 s,56℃/62℃退火30 s,72℃延伸30 s,循环32次;72℃延伸5 min,12℃保温。TERT PCR(采用巢式PCR)反应程序为:94℃预变性5 min,98℃变性10 s,75℃退火1 min,循环30次;98℃变性10 s,58℃退火15 s,68℃延伸1 min,循环35次;72℃延伸5 min,12℃保温。(3)目的基因测序:使用美国应用生物系统公司ABI3500XL型测序分析仪进行测序,结果使用Chromas软件与基因组DNA序列进行比对。
1.2.3 荧光原位杂交(fluorescence in situ hybridization, FISH)
组织切片3 μm厚2张,65℃烘烤2 h,二甲苯及梯度乙醇脱蜡,复水,高温修复,蛋白酶K消化,2×SSC溶液洗涤,梯度乙醇脱水晾干,滴加1p36/1q21和19q13/19p13探针,37℃杂交孵育过夜。第二天将组织切片放入2×SSC及0.1% NP-40/2×SSC进行漂洗,梯度乙醇脱水晾干。DAPI对比染色,于暗处放置20 min后,荧光显微镜下观察信号。判读标准:缺失信号表现的细胞数占总细胞数比值达到20%或以上;或所选区域总红色信号/总绿色信号小于75%,即为阳性共缺失。
1.3 治疗情况
术后根据患者情况4~6周开始放疗。放疗剂量为(50~60)Gy/(25~30)次。放疗期间同步口服替莫唑胺(TMZ)胶囊,剂量按照75 mg/(m2·d),连续6周。同步放疗结束后休息1个月,继续辅助化疗,根据患者的耐受情况,TMZ剂量按照(120~300)mg/(m2·d),d1~5口服,28天/周期,建议至少服用6周期以上。
1.4 随访
根据患者术后病情,部分患者接受放疗及同步口服替莫唑胺(TMZ)胶囊进行化疗。通过医院随访系统或电话对54例患者进行随访,询问患者的治疗情况、无进展生存期(PFS)及总生存期(OS)。无进展生存期定义为肿瘤手术之日至临床诊断复发或转移为终点的时间,总生存期定义为从治疗开始至患者因疾病死亡或末次随访时间。末次随访日期为2023年10月30日。
1.5 统计学方法
使用SPSS25.0软件评估患者的期PFS和OS。Kaplan-Meier法进行单因素生存分析,Tarone-Ware检验比较不同组间的生存差异,P < 0.05为差异有统计学意义。
2 结果
2.1 临床特征
54例患者中,40例以“头痛、头晕”为首发症状,另14例表现为肢体乏力和四肢抽搐。肿瘤位于左侧大脑半球29例,右侧大脑半球25例;46例肿瘤发生于1个脑叶(其中额叶41例、顶叶1例、颞叶4例),8例肿瘤侵及多个脑叶(其中顶枕叶1例、额岛叶2例、额颞叶2例、颞顶叶1例、额颞岛叶1例、额叶及胼胝体1例)。
2.2 组织学特征
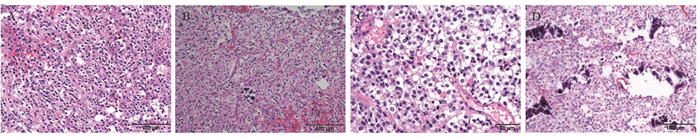
患者术后送检均为灰红、灰白色鱼肉状不规则碎组织块,最大径0.1~6.0 cm,质地可软、可硬。镜下可见肿瘤细胞呈弥漫片状分布,可见胶质细胞增生,形态学均呈少突胶质细胞瘤表型。肿瘤细胞胞质透明,呈细胞核周空晕所致的“煎蛋状”表现,见图 1A,典型少突胶质细胞瘤病例可见伴毛细血管增生构成的特征性“鸡爪状”血管网结构,见图 1B。瘤细胞核呈圆形或卵圆形,高级别少突胶质细胞瘤细胞核分裂相易见,伴微囊状排列、血管内皮增生、钙化及坏死,见图 1C~1D。
![]() 图 1 IDH突变和1p 19q共缺失型少突胶质细胞瘤的组织形态学特征(HE染色)Figure 1 Histomorphologic characteristics of oligodendroglioma with IDH mutation and 1p19q codeletion (HE staining)A: oligodendroglioma: IDH mutation and 1p19q co-deletion type. The tumor cells were diffuse and densely arranged, and some nuclear halos showed a "fried egg" appearance; B: tumor nucleus was round or oval, cell density in some areas was increased, the nucleus was enlarged, tumor size was inconsistent, and capillary proliferation was "chicken wire"; C: the tumor cells were arranged in plates, the cytoplasm was rich and transparent, the nucleus was oval, the nucleolus was visible, and the mitotic image was clear; interstitial blood vessels were abundant, and vascular endothelial hyperplasia was observed; D: the tumor cells were diffusely distributed with round or oval nuclei, with increased calcification and increased number of microsacs.
图 1 IDH突变和1p 19q共缺失型少突胶质细胞瘤的组织形态学特征(HE染色)Figure 1 Histomorphologic characteristics of oligodendroglioma with IDH mutation and 1p19q codeletion (HE staining)A: oligodendroglioma: IDH mutation and 1p19q co-deletion type. The tumor cells were diffuse and densely arranged, and some nuclear halos showed a "fried egg" appearance; B: tumor nucleus was round or oval, cell density in some areas was increased, the nucleus was enlarged, tumor size was inconsistent, and capillary proliferation was "chicken wire"; C: the tumor cells were arranged in plates, the cytoplasm was rich and transparent, the nucleus was oval, the nucleolus was visible, and the mitotic image was clear; interstitial blood vessels were abundant, and vascular endothelial hyperplasia was observed; D: the tumor cells were diffusely distributed with round or oval nuclei, with increased calcification and increased number of microsacs.2.3 免疫表型与分子检测
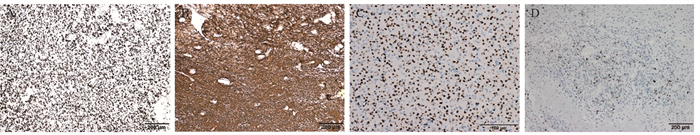
免疫组织化学检测:48例患者肿瘤组织进行了ATRX检测,均(100%)表达正常,未缺失;52例进行了p53检测,有6例(11.5%)强阳性,见图 2A;54例患者均进行了GFAP和Olig⁃2检测,结果显示54例患者肿瘤组织GFAP均呈阳性表达,Olig⁃2均呈弥漫强阳性表达,见图 2B、2C;Ki-67增殖指数为5%~60%,见图 2D。
![]() 图 2 IDH突变和1p 19q共缺失型少突胶质细胞瘤免疫组织化学检测结果(EnVision法)Figure 2 Immunohistochemical test results of oligodendroglioma with IDH mutation and 1p19q codeletion (EnVision method)A: positive expression of p53 in tumor cells; B: positive expression of GFAP in tumor cells; C: Olig-2 was diffusely positive in tumor cells; D: about 15% of tumor cells were Ki⁃67 positive.
图 2 IDH突变和1p 19q共缺失型少突胶质细胞瘤免疫组织化学检测结果(EnVision法)Figure 2 Immunohistochemical test results of oligodendroglioma with IDH mutation and 1p19q codeletion (EnVision method)A: positive expression of p53 in tumor cells; B: positive expression of GFAP in tumor cells; C: Olig-2 was diffusely positive in tumor cells; D: about 15% of tumor cells were Ki⁃67 positive.FISH检测显示54例患者肿瘤均为1p/19q共缺失阳性。本组54例患者肿瘤组织均经Sanger测序检出IDH突变,其中40例为IDH1 4号外显子NM_005896.4:c.395G > A(p.R132H)突变,14例为IDH2突变,其中12例为4号外显子NM_002168.4:c.515G > A(p.R172K)突变,2例为NM_002168.4:c.514A > T(p.R172W)突变,33例同时检出TERT基因启动子C228T位点或C250T位点突变(图略,图请扫描本文OSID码)。
2.4 随访及生存分析
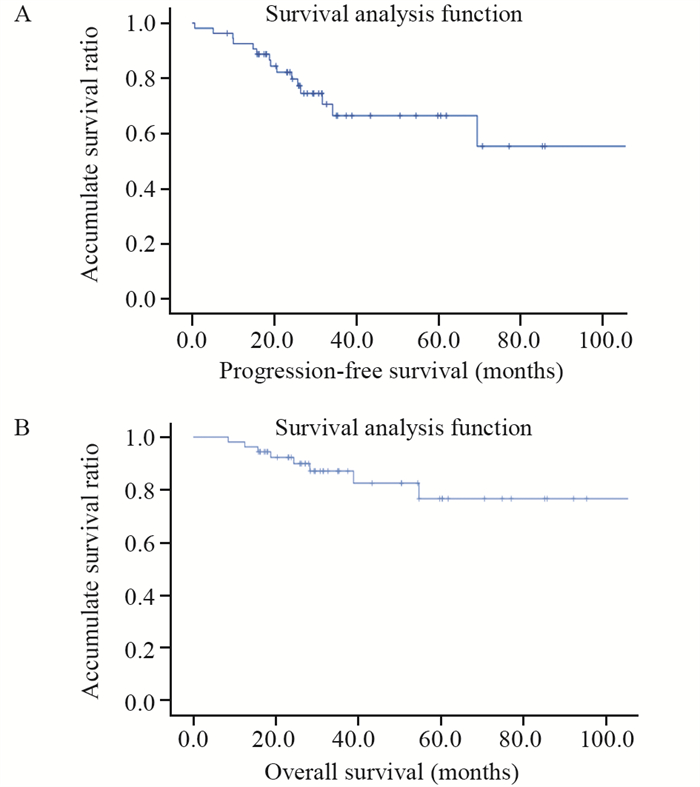
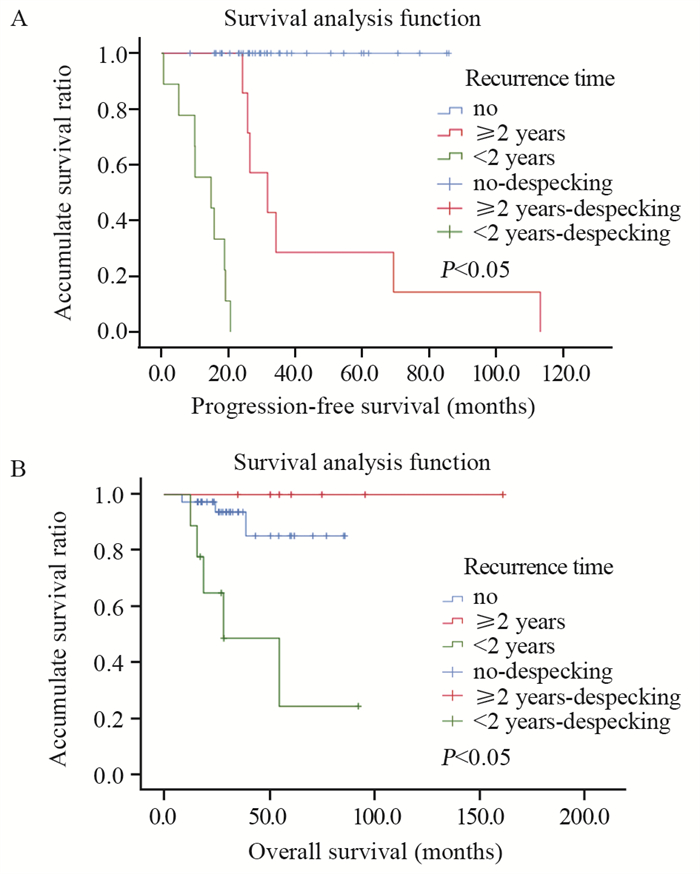
所有患者均于术后随访,40例患者进行了术后标准辅助放化疗。截至随访结束,46例患者存活,平均无进展生存期为33.5个月(0.6~113.3个月),平均总生存期为40.7个月(8.0~153.9个月)。1、3和5年无进展生存率分别为82%、67%和56%,见图 3A。1、3和5年总生存率分别为92%、81%和76%,见图 3B。8例患者死亡,另有16例复发转移,其中14例生存时间超过2年。单因素生存分析显示,肿瘤患者术后2年以上才发生复发转移的无进展生存时间及总生存时间均明显长于2年以内发生复发转移的患者,差异有统计学意义(P < 0.05)。但患者的年龄、性别、肿瘤组织学分级、是否放化疗和部位与预后并无相关性,见表 1,图 4。
表 1 少突胶质细胞瘤患者生存期的单因素相关分析Table 1 Univariate correlation analysis of survival of patients with oligodendroglioma
3 讨论
根据WHO(2021)中枢神经系统肿瘤最新的分类指南,少突胶质细胞瘤是一种异质性肿瘤,其特征是IDH突变以及染色体臂1p和19q共缺失[2],少突胶质细胞瘤占原发性中枢神经系统肿瘤的4%~8%,根据组织学和分子综合特征可分为WHO 2级和3级两组[4]。该肿瘤在成人弥漫性胶质瘤中预后相对较好[5]。其侵袭性较小,对放疗和化疗敏感,但仍然存在致命性[6]。本研究中复发转移与手术间隔超过2年的患者与2年以内发生复发转移的患者相比有更长的无进展生存期及总生存期,而没有发生复发转移的患者由于多是当年的病例,随访时间较短,导致无进展生存期及总生存期较复发转移与手术间隔超过2年的患者差。以上原因是由少突胶质细胞瘤较好的预后及本研究病例随访时间短导致的,有待于后期纳入更多研究对象及更长的随访时间来完善。以往文献报道IDH野生型胶质母细胞瘤的总生存期为12.6个月,IDH突变型星形细胞瘤的总生存期为26.4个月,儿童型高级别胶质瘤的总生存期为35.8个月,2~3级少突胶质细胞瘤的总生存期为53.4个月[7]。本组结果显示,少突胶质细胞瘤的总生存期为40.7个月,稍短于以上文献报道,可能是本研究患者纳入数量较少及随访时间较短引起的,但是这些结果也验证了基于组织学和分子标志物的WHO组织分子分类对弥漫性胶质瘤的预后具有较高的价值。
IDH突变是成人弥漫性低级别胶质瘤的分子标志物,具有重要的诊断预后和意义。约70%的2~3级胶质瘤携带IDH突变[8]。IDH的正常功能是将异柠檬酸转化为α-酮戊二酸(α-KG)[9]。IDH突变是酶活性位点精氨酸残基的错义突变,IDH1为R132、IDH2为R172[8]。这些突变改变了原来的酶活性,导致α-酮戊二酸(α-KG)产生肿瘤代谢物d-2-羟基戊二酸(2-HG),最终导致细胞分化受损,引起致癌基因和抑癌基因失调[10]。文献报道,IDH1发生于70%以上的低级别胶质瘤和20%以上高级别胶质瘤中;约4%的胶质瘤中发现了IDH2突变[11]。本组有74.1%患者携带IDH1突变,25.9%患者携带IDH2突变,与以上研究基本一致。因此,IDH突变也是治疗胶质瘤的重要靶点。IDH已成功应用于其他携带IDH突变的恶性肿瘤。目前,FDA已经批准两种IDH突变体抑制剂ivosidenib(被批准用于IDH1突变急性髓性白血病和胆管癌)和enasidenib(被批准用于IDH2突变的成人复发或难治性急性髓系白血病)[10, 12-13]。针对ivosidenib和vorasidenib对IDH突变型胶质瘤的疗效试验结果近期已发表[14-16]。研究[16]证明,ivosidenib和enasidenib两种靶向药均可降低2HG水平,总有效率为30.8%,56.7%的患者达到疾病稳定(SD),且中位无进展生存期为3.6个月。一项单中心回顾性研究显示:有IDH突变的神经胶质瘤患者接受奥拉帕尼和替莫唑胺联合治疗后,8例患者中有4例2级或3级患者完全或部分缓解,表明奥拉帕尼联合用药对有IDH突变的神经胶质瘤有一定疗效,但其回顾性设计受到条件限制,仍需要进一步验证[17]。
少突胶质细胞瘤可发生在任何年龄组,常见于成人患者(40~60岁),儿童和年轻人比较少见。文献报道,IDH突变型、1p19q共缺失少突胶质细胞瘤患者的中位年龄为45.4岁[5]。本组IDH突变型、1p19q共缺失少突胶质细胞瘤患者的中位年龄为45.5岁,与文献报道一致。以往文献报道年龄是重要的预后因素,且年龄40岁以下被认为是积极的预后因素,它可以成为指导手术治疗决策的参考指标[18-19]。本组单因素分析中,年龄并不是影响少突胶质细胞瘤患者预后的重要因素,可能与标本量少及年龄分布不均有关。胶质瘤的风险和预后存在性别差异[20-21]。男性胶质瘤患者的生存率明显低于女性患者[22],女性胶质瘤患者对手术、放疗和替莫唑胺标准治疗的反应优于男性[23]。本组女性患者的生存期略高于男性,但两者差异无统计学意义,与上述研究基本一致。本组样本量较小,具体影响情况还待后续积累样本进一步分析。
少突胶质细胞瘤多表现为质地柔软,钙化,边界不清,肿瘤黏附紧靠下颅神经和小脑半球脑干,这使其难以完整切除导致预后差,肿瘤复发时间短。因此,需要及时放疗和化疗,以延缓肿瘤的生长速度。Bell等[24]研究显示,术后化疗和放疗可以改善弥漫性低级别胶质瘤患者的总生存期。RTOG 0424试验比较单用替莫唑胺或放疗联合替莫唑胺治疗的弥漫性低级别胶质瘤,结果显示,联合方案比单独放疗具有更好的总生存期和无进展生存期[24]。本组单因素分析表明,术后行放化疗的患者与仅手术的患者相比具有更长的生存时间,提示术后规范的放化疗可提高患者的总生存期。然而,也有临床试验发现,与低剂量放疗相比,高剂量放疗对患者没有明显益处[25]。未来,弥漫性低级别胶质瘤的最佳放疗时间和剂量值得进一步研究。
ATRX基因是α地中海贫血伴智力低下综合征的致病基因,位于Xq21.1染色体上,编码参与DNA重组、修复和转录调控的280 kDa核蛋白[26]。ATRX基因突变存在于至少15种人类肿瘤中[26]。ATRX基因的截断性突变导致神经胶质瘤[27]中ATRX蛋白失表达或形成截短蛋白,并且与IDH突变相关,同时与1p/19q共缺失相互排斥。Ebrahimi等[27]的研究表明,53%的2级和3级星形细胞瘤患者肿瘤组织显示ATRX表达缺失。罕见发现1例伴有ATRX缺失,而组织学表现为少突胶质细胞瘤的患者。本研究中,48例患者进行了ATRX免疫组织化学检测,结果显示ATRX均未缺失。在本研究纳入的早期病例中,有两例患者ATRX免疫组织化学显示蛋白缺失,其分子检测显示有IDH1突变和1p/19q共缺失情况。鉴于ATRX蛋白缺失总是与1p/19q共缺失相互排斥,且有研究显示[28],FISH技术在检测1p/19q共缺失的情况中,由于无法区分染色体1p和19q的全臂缺失和部分缺失,存在一定的假阳性结果,在可疑的情况下,需要使用诊断准确性较高的高通量检测技术进行验证。因此本研究对两例ATRX蛋白缺失的患者进行高通量验证,结果显示1p为全臂缺失,19q为部分缺失,并不符合少突胶质细胞瘤的诊断要求,最终将其排除在外。
解剖位置也是影响胶质瘤预后的因素之一,一些研究发现右侧大脑半球比左侧大脑半球有更高发生肿瘤的概率,胶质瘤更偏好位于额叶和颞叶[29]。也有文献表明[30],低级别胶质瘤好发于额叶,发病于单一脑叶占78.38%。本组41例(75.9%)少突胶质细胞瘤患者发生于额叶,4例(7.4%)发生于颞叶,1例(1.9%)发生于顶叶,肿瘤侵袭单一脑叶占多数(46, 85.2%),少突胶质细胞瘤患者出现癫痫的其中一个原因是病变常累及皮层,但罕见于脊髓、脑干和小脑[31]。
本研究存在以下局限:首先,本研究为单中心研究,样本量较小,存在一定局限性,期望未来进行临床多中心研究,以便更好探索IDH突变和1p/19q共缺失型少突胶质细胞瘤的预后因素;其次,本研究随访时间较短,无法获得无进展生存期和总生存期的可靠结果,可能影响结论的客观性,期望后期进一步随访,以确切评估IDH突变和1p/19q共缺失型少突胶质细胞瘤的预后指标,从而更好地指导临床。
综上所述,IDH突变和1p/19q共缺失型少突胶质细胞瘤主要发生于成人,男性多见,好发于额叶,其次为颞叶。其病理特征及预后表现为年龄是患者预后较好的影响因素,术后联合放、化疗降低了进展风险,ATRX表达缺失与1p/19q共缺失常常相互排斥。IDH突变对于指导少突胶质细胞瘤未来临床靶向用药及预后评估具有重要意义。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:吴小延:选题和实验设计,资料收集,数据统计分析及解释,论文撰写王苏杰:资料收集,数据统计分析及解释王芳:数据汇总录入及实验实施,英文翻译杜紫明:统计分析及解释,英文翻译邓玲:选题和实验设计,数据汇总录入及实验实施 -
表 1 少突胶质细胞瘤患者生存期的单因素相关分析
Table 1 Univariate correlation analysis of survival of patients with oligodendroglioma

-
[1] Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary[J]. Acta Neuropathol, 2016, 131(6): 803-820. doi: 10.1007/s00401-016-1545-1
[2] Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary[J]. Neuro Oncol, 2021, 23(8): 1231-1251. doi: 10.1093/neuonc/noab106
[3] Wijnenga MMJ, Mattni T, French PJ, et al. Does early resection of presumed low-grade glioma improve survival? A clinical perspective[J]. J Neurooncol, 2017, 133(1): 137-146. doi: 10.1007/s11060-017-2418-8
[4] Bou Zerdan M, Assi HI. Oligodendroglioma: A Review of Management and Pathways[J]. Front Mol Neurosci, 2021, 14: 722396. doi: 10.3389/fnmol.2021.722396
[5] Cancer Genome Atlas Research Network, Brat DJ, Verhaak RG, et al. Comprehensive, integrative genomic analysis of diffuse lowergrade gliomas[J]. N Engl J Med, 2015, 372(26): 2481-2498. doi: 10.1056/NEJMoa1402121
[6] So J, Mamatjan Y, Zadeh G, et al. Transcription factor networks of oligodendrogliomas treated with adjuvant radiotherapy or observation inform prognosis[J]. Neuro Oncol, 2021, 23(5): 795-802. doi: 10.1093/neuonc/noaa300
[7] Brown NF, Ottaviani D, Tazare J, et al. Survival outcomes and prognostic factors in glioblastoma[J]. Cancers(Basel), 2022, 14(13): 3161.
[8] Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas[J]. N Engl J Med, 2009, 360(8): 765-773. doi: 10.1056/NEJMoa0808710
[9] Flavahan WA, Drier Y, Liau BB, et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas[J]. Nature, 2016, 529(7584): 110-114. doi: 10.1038/nature16490
[10] Stein EM, Fathi AT, DiNardo CD, et al. Enasidenib in patients with mutant IDH2 myelodysplastic syndromes: a phase 1 subgroup analysis of the multicentre, AG221-C-001 trial[J]. Lancet Haematol, 2020, 7(4): e309-e319. doi: 10.1016/S2352-3026(19)30284-4
[11] Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate[J]. Nature, 2009, 462(7274): 739-744. doi: 10.1038/nature08617
[12] DiNardo CD, Stein EM, de Botton S, et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML[J]. N Engl J Med, 2018, 378(25): 2386-2398. doi: 10.1056/NEJMoa1716984
[13] Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study[J]. Lancet Oncol, 2020, 21(6): 796-807. doi: 10.1016/S1470-2045(20)30157-1
[14] Mellinghoff IK, Penas-Prado M, Peters KB, et al. Vorasidenib, a dual inhibitor of mutant IDH1/2, in recurrent or progressive glioma; results of a first-in-human phase I trial[J]. Clin Cancer Res, 2021, 27(16): 4491-4499. doi: 10.1158/1078-0432.CCR-21-0611
[15] Mellinghoff IK, Peters KB, Cloughesy TF, et al. Vorasidenib (VOR; AG-881), an inhibitor of mutant IDH1 and IDH2, in patients (pts) with recurrent/progressive glioma: updated results from the phase I non-enhancing glioma population[J]. J Clin Oncol, 2020 38(15_ suppl): 2504.
[16] Mellinghoff IK, Wen PY, Taylor JW, et al. PL3.1 A phase 1, open-label, perioperative study of ivosidenib (AG-120) and vorasidenib (AG-881) in recurrent, IDH1-mutant, low-grade glioma: results from cohort 1[J]. Neuro Oncol, 2019, (Suppl_3): iii2.
[17] Schaff LR, Kushnirsky M, Lin AL, et al. Combination olaparib and temozolomide for the treatment of glioma: aretrospective case series[J]. Neurology, 2022, 99(17): 750-755. doi: 10.1212/WNL.0000000000201203
[18] Shaw EG, Wang M, Coons SW, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adultlow-grade glioma: initial results of RTOG 9802[J]. J Clin Oncol, 2012, 30(25): 3065-3070. doi: 10.1200/JCO.2011.35.8598
[19] Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versusradiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study[J]. Lancet Oncol, 2016, 17(11): 1521-1532. doi: 10.1016/S1470-2045(16)30313-8
[20] Claus EB, Cannataro VL, Gaffney SG, et al. Environmental and sexspecific molecular signatures of glioma causation[J]. Neuro Oncol, 2022, 24(1): 29-36. doi: 10.1093/neuonc/noab103
[21] Khan MT, Prajapati B, Lakhina S, et al. Identification of gender-specific molecular Differences in glioblastoma (GBM) and lowgrade glioma (LGG) by the Analysis of Large transcriptomic and epigenomic datasets[J]. Front Oncol, 2021, 11: 699594. doi: 10.3389/fonc.2021.699594
[22] Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the united states in 2012-2016[J]. Neuro Oncol, 2019, 21(Suppl 5): v1-v100.
[23] Yang W, Warrington NM, Taylor SJ, et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data[J]. Sci Trans Med, 2019, 11(473): eaao5253. doi: 10.1126/scitranslmed.aao5253
[24] Bell EH, Zhang P, Fisher BJ, et al. Association of MGMT Promoter methylation status With survival outcomes in patients with high-risk glioma treated With radiotherapy and temozolomide: An analysis From the NRG Oncology/RTOG 0424 Trial[J]. JAMA Oncol, 2018, 4(10): 1405-1409. doi: 10.1001/jamaoncol.2018.1977
[25] Breen WG, Anderson SK, Carrero XW, et al. Final report from intergroup NCCTG 86-72-51 (Alliance): A phase Ⅲ randomized clinical trial of high-dose versus low-dose radiation for adult low-grade glioma[J]. Neuro Oncol, 2020, 22(6): 830-837. doi: 10.1093/neuonc/noaa021
[26] Liu J, Zhang X, Yan X, et al. Significance of TERT and ATRX mutations in glioma[J]. Oncol Lett, 2019, 17(1): 95-102.
[27] Ebrahimi A, Skardelly M, Bonzheim I, et al. ATRX immunostaining predicts IDH and H3F3A status in gliomas[J]. Acta Neuoropathol Com, 2016, 4(1): 60. doi: 10.1186/s40478-016-0331-6
[28] Alnahhas I, Rayi A, Thomas D, et al. False-positive 1p/19q Testing Results in Gliomas: Clinical and Research Consequences[J]. Am J Clin Oncol, 43(11): 802-805.
[29] Larjavaara S, Mäntylä R, Salminen T, et al. Incidence of gliomas by anatomic location[J]. Neuro Oncol, 2007, 9(3): 319-325. doi: 10.1215/15228517-2007-016
[30] 孙彩红, 孔东生, 刘若愚, 等. 少突胶质细胞瘤与星形胶质细胞瘤的临床特征及预后比较[J]. 解放军医学院学报, 2022, 43(5): 536-539. https://www.cnki.com.cn/Article/CJFDTOTAL-JYJX202205008.htm Sun CH, Kong DS, Liu RY, et al. Comparison of clinical features and prognosis of oligodendroglioma versus astrocytoma[J]. Jie Fang Jun Yi Xue Yuan Xue Bao, 2022, 43(5): 536-539. https://www.cnki.com.cn/Article/CJFDTOTAL-JYJX202205008.htm
[31] 李芝, 邹琼, 粟占三. 罕见巨大钙化的砂粒体型少突胶质细胞瘤1例[J]. 临床与实验病理学杂志, 2015, 31(11): 1317-1318. https://www.cnki.com.cn/Article/CJFDTOTAL-LSBL201511043.htm Li Z, Zou Q, Su ZS. A rare case of sand like oligodendroglioma with huge calcification[J]. Lin Chuang Yu Shi Yan Bing Li Xue Za Zhi, 2015, 31(11): 1317-1318. https://www.cnki.com.cn/Article/CJFDTOTAL-LSBL201511043.htm



 下载:
下载: