Network Meta-Analysis of Effectiveness of First-Line Immunotherapy Treatments for Patients with Brain Metastases from Advanced Non-Small Cell Lung Cancer
-
摘要:目的
对晚期非小细胞肺癌(NSCLC)脑转移患者一线免疫治疗有效性进行网络荟萃分析。
方法计算机检索Pubmed、Embase、Cochrane等数据库的文献,由2名研究者筛选文献、提取资料并对纳入研究进行偏倚风险评估,使用R(4.1.3)软件对纳入临床试验进行统计分析。对于研究结局指标OS、PFS,从纳入研究中提取风险比(HR)和95%可信区间(CI),进行对数转换为效应分析统计量。
结果最终纳入6篇随机对照试验(RCT)文献,包括327例不可剔除NSCLC脑转移患者。网状荟萃分析提示:与传统化疗提升患者OS相比PD-1抑制剂+CTLA-4更具优势(HR: 0.13,95%CI: 0.03~0.71),其次是PD-L1抑制剂(HR: 0.17,95%CI: 0.04~0.74)和PD-1抑制剂+化疗(HR: 0.36,95%CI: 0.2~0.63)。与传统化疗提升患者PFS相比,PD-1抑制剂+CTLA-4最具优势(HR: 0.37,95%CI: 0.15~0.93),其次是PD-L1抑制剂+化疗(HR: 0.44,95%CI: 0.29~0.66)和PD-1抑制剂(HR: 0.48,95%CI: 0.27~0.86)。
结论免疫检查点抑制剂治疗可提高晚期NSCLC脑转移患者的生存期,尤其PD-1抑制剂与CTLA-4抑制剂的联合治疗显示出优异的生存获益。
Abstract:ObjectiveTo conduct a network meta-analysis on the effectiveness of first-line immunotherapy on patients with brain metastases from advanced non-small cell lung cancer (NSCLC).
MethodsTwo investigators conducted a computerized search of Pubmed, Embase, Cochrane, and other databases to screen the literature, extract the information, and assess the risk of bias of the included studies. The included clinical trials were statistically analyzed using R (4.1.3) software. For the study outcome indicators OS and PFS, the risk ratios (HRs), and the 95% confidence intervals (CIs) were extracted from the included studies and logarithmically transformed into effect analysis statistics.
ResultsSix randomized controlled trials were finally included, including 327 patients with non-excludable NSCLC brain metastases. Network meta-analysis suggested that PD-1 inhibitor + CTLA-4 was more advantageous than the conventional chemotherapy for enhancing patients’ OS (HR: 0.13, 95%CI: 0.03-0.71), followed by PD-L1 inhibitor (HR: 0.17, 95%CI: 0.04-0.74) and PD-1 inhibitor + chemotherapy (HR: 0.36, 95%CI: 0.2-0.63). PD-1 inhibitor + CTLA-4 was also more advantageous (HR: 0.37, 95%CI: 0.15-0.93) than the conventional chemotherapy for boosting patients’ PFS, followed by PD-L1 inhibitor + chemotherapy (HR: 0.44, 95%CI: 0.29-0.66) and PD-1 inhibitor (HR: 0.48, 95%CI: 0.27-0.86).
ConclusionImmune checkpoint inhibitor therapy improves the survival of patients with brain metastases from advanced NSCLC. In particular, the combination of PD-1 inhibitor and CTLA-4 inhibitor show excellent survival benefit.
-
Key words:
- Non-small cell lung cancer /
- Immunotherapy /
- Brain metastasis /
- Meta-analysis
-
0 引言
肺癌是当今世界癌症相关死亡的主要原因,其中80%为非小细胞肺癌(non-small cell lung cancer, NSCLC)[1-2]。晚期NSCLC预后较差,5年生存率仅为5%左右[3]。脑是肺癌最常见的转移部位,发生率可达40%,严重影响患者生存质量。脑转移可引起头痛、癫痫发作、步态障碍、认知功能下降等症状,如果不及时治疗,中枢神经系统症状可能快速进展,危及生命[4]。过去,晚期NSCLC脑转移的标准一线治疗为全脑放疗联合铂类为基础的化疗,但中位生存期仅为8~10个月[5-6]。化疗相关不良反应也可能进一步加重神经系统症状,导致患者依从性下降。
近年来,免疫检查点抑制剂作为一类新型抗肿瘤药物,可以阻断肿瘤细胞的免疫逃逸,从而显示出优异的抗肿瘤效果[7]。肿瘤细胞可通过多种途径逃避机体的免疫监视和杀伤,其中PD-1/PD-L1通路的激活在抑制T淋巴细胞功能中起关键作用[8]。PD-1抑制剂(如帕博利珠单抗)和PD-L1抑制剂(如阿特珠单抗)的应用显著改善了多种实体瘤尤其是NSCLC的预后[9-10]。CTLA-4作为另一重要的免疫检查点,其抑制剂伊匹木单抗可阻断CTLA-4与其配体的结合,增强T细胞活性,与PD-1/PD-L1抑制剂联合使用可产生协同增效作用[10-12]。尽管免疫治疗在晚期NSCLC治疗中已取得长足进展,但对于存在脑转移的患者群体,是否首选免疫疗法仍存在争议[13-14]。由于大部分临床试验拒绝纳入脑转移患者,直接证据较为有限。另外,血脑屏障也被认为是影响脑转移患者应用免疫疗法的关键因素之一[15-16]。尽管一部分研究结果支持免疫疗法在NSCLC脑转移患者中的应用,但样本量较小,存在选择性偏倚,且不同研究结果存在一定差异[17-20]。本研究采用Meta分析方法,系统评价不同免疫治疗方案与化疗在晚期NSCLC脑转移患者中的临床效果,为这一患者群体的临床决策提供更可靠的循证医学证据。
1 资料与方法
1.1 纳入排除标准
1.1.1 纳入标准
研究对象:经组织学/细胞学确诊为晚期不可切除NSCLC,并有脑转移/中枢神经系统转移。干预措施:至少有一个试验组使用免疫检查点抑制剂治疗(包含免疫检查点抑制剂单药、免疫检查点抑制剂双药、免疫检查点抑制剂联合化疗、免疫检查点抑制剂联合靶向治疗)。对照组:对照组至少包含一种系统治疗方式(免疫检查点抑制剂/化疗/靶向治疗)。结局指标:主要结局指标为无进展生存期(PFS)、总生存期(OS)。纳入研究类型:纳入文献均为随机对照研究。
1.1.2 排除标准
不符合晚期不可切除NSCLC脑转移诊断的患者;干预措施不包含免疫检查点抑制剂治疗的研究;综述、动物实验、meta分析、回顾性研究、系统评价等非RCT研究。
1.2 检索策略
检索Pubmed、Embase、Cochrane从建库至2023年2月文献,检索词包括“Non Small Cell Lung Carcinoma,Immune Checkpoint Inhibitors,PD-1 Inhibitor,PD-L1 Inhibitor,randomized controlled trial”等,根据不同数据库特征应用“AND”或“OR”对以上不同主题词及自由词进行检索。
1.3 资料提取与文献质量评价
将文献导入End Note X9文献管理器,删除重复的文献。由两名研究者独立完成整理、筛选、提取数据,提取内容包括纳入文献的题目、作者信息、年份、NCT编号、研究干预措施、基线数据、结局指标(OS、PFS)、95%置信区间(CI)及文献质量评价相关资料。有不一致的方面由彼此进行商讨或由第三位研究者介入决定。使用Cochrane Handbook 6.0推荐工具评估试验中的偏倚风险[20]。
1.4 统计学方法
使用R4.1.3软件对纳入临床试验进行统计分析。对于研究结局指标OS、PFS,从纳入研究中提取风险比(HR)和 95%CI,进行对数转换为效应分析统计量,根据研究数据异质性水平I2选择固定效应模型或随机效应模型(I2>50%,采用随机效应模型,否则采用固定效应模型)。使用优选概率排名曲线(surface under the cumulative ranking, SUCRA)[21]对干预措施进行排名,数值越大提示效果越好。绘制网状关系图,当存在闭合环时,采用点分法进行不一致性检测[22],检验水准α=0.05。
表 1 纳入研究基本特征Table 1 Basic characteristics of included studiesIncluded
studiesClinical trials/phasing Number of cases of
brain metastasisInterventions HR(95%CI) Experimental
groupControl
groupExperimental
groupControl
groupOS PFS Zhou, et al.
2021[23]SHR-1210-303
NCT03134872/stage Ⅲ10 5 Camrelizumab for
Injection +
chemotherapyChemotherapy NA 0.14
(0.01-0.88)Reck, et al.
2019[24]KEYNOTE-024
NCT02142738/stage Ⅲ18 10 Pembrolizumab Chemotherapy NA 0.55
(0.20-1.56)Gandhi, et al.
2018[25]KEYNOTE-189
NCT02578680/stage Ⅲ73 35 Pembrolizumab +
chemotherapyChemotherapy 0.36 (0.2-0.62) 0.42
(0.26-0.68)Boyer, et al.
2021[26]KEYNOTE-598
NCT03302234/stage Ⅲ31 29 Pembrolizumab +
ipilimumabPembrolizumab 0.79 (0.36-1.75) 0.78
(0.39-1.57)Sezer, et al.
2021[27]EMPOWER-Lung 1
NCT03088540/stage Ⅲ44 39 Cemiplimab Chemotherapy 0.17 (0.04-0.76) 0.45
(0.22-0.92)Zhang, et al.
2021[28]ORIENT-11
NCT03607539/stage Ⅲ19 14 Sintilimab +
chemotherapyChemotherapy NA 0.58
(0.24-1.18)Notes: OS: overall survival; PFS: progression-free survival; NA: not available. 2 结果
2.1 纳入研究基本特征
初步检索得到1 365篇文献,剔除192篇重复文献。阅读文章题目和摘要后从1 173篇文献排除1 153篇文献(剔除综述、动物实验、meta分析、回顾性研究、系统评价等文献),剩余20篇文献,阅读全文后剔除14篇文献(剔除非随机对照、干预措施不符、数据丢失、无法获取全文文献),最终纳入6篇文献。相关文献检索流程见图1。纳入研究基本特征见表1。
2.2 纳入研究基本特征及文献质量评价
共纳入6篇随机对照研究,共327例晚期不可切除NSCLC脑转移患者,治疗方式包括PD-1抑制剂、PD-1抑制剂联合化疗、PD-L1抑制剂联合CTLA-4抑制剂、化疗。纳入文献质量评价结果见图2,使用计算机生成随机序列、报告完整结果、无选择性偏倚的研究有6项,进行分配隐藏的研究有3项,未使用盲法的研究有1项。
2.3 晚期不可切除非小细胞肺癌脑转移患者人群网状Meta分析结果
2.3.1 总生存期
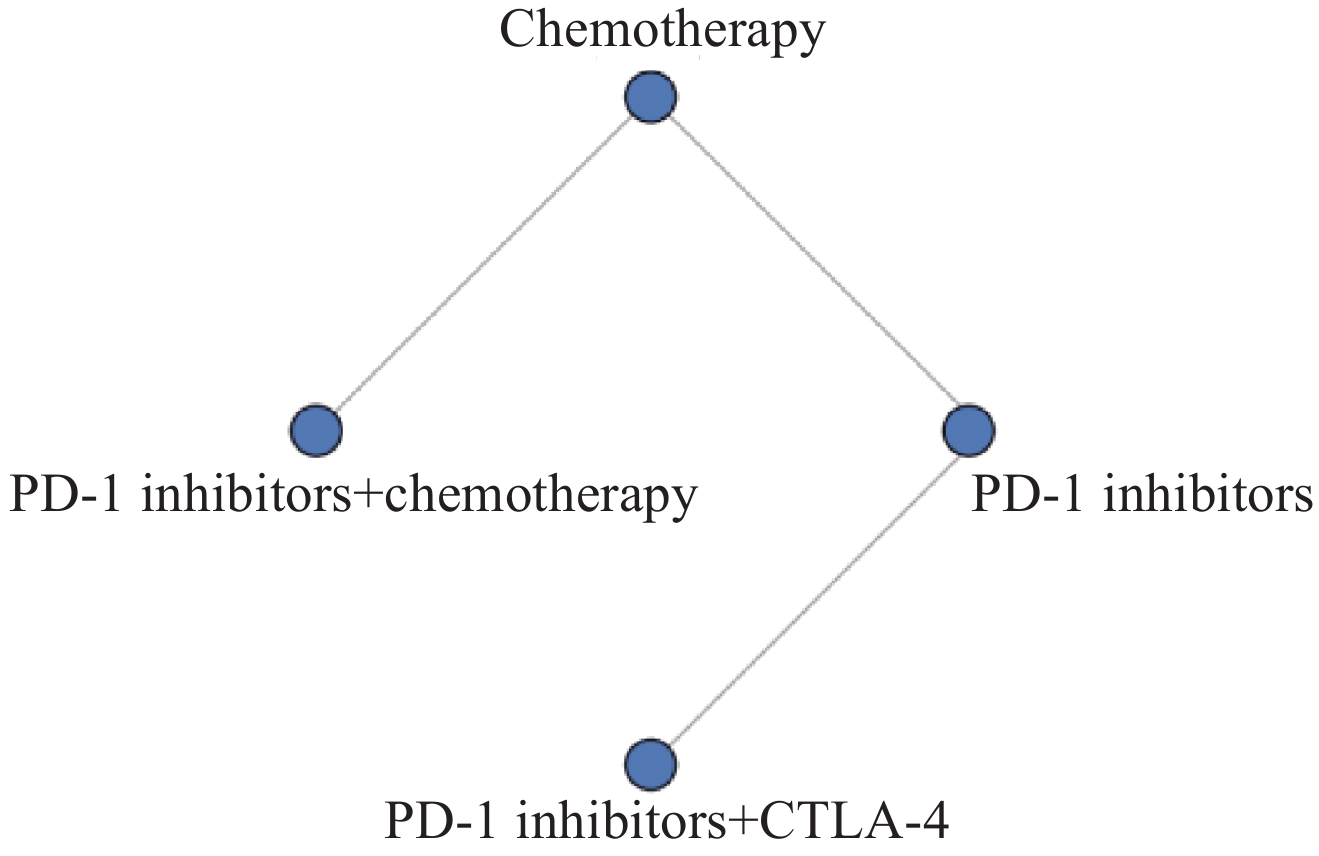
3项研究报告OS结果,共包括251例和4种治疗方案。其OS网状关系图见图3。
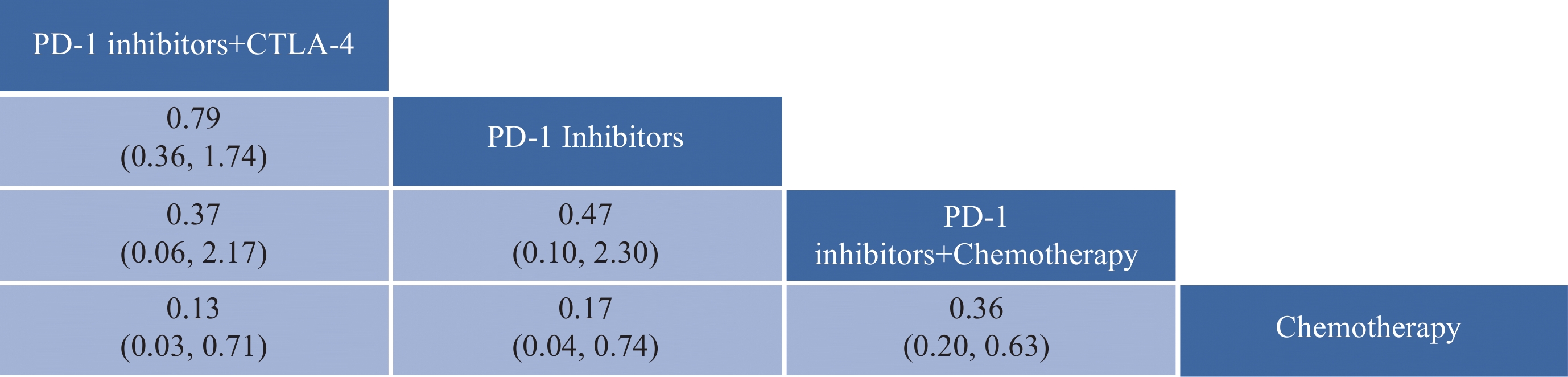
OS网状Meta分析结果见图4。PD-1抑制剂+CTLA-4、PD-1抑制剂、PD-1抑制剂+化疗均较化疗方案显著延长晚期不可切除NSCLC脑转移患者OS,其中PD-1抑制剂+CTLA-4最具优势(HR: 0.13,95%CI: 0.03~0.71),接着是PD-L1抑制剂(HR: 0.17,95%CI: 0.04~0.74)和PD-1抑制剂+化疗(HR: 0.36,95%CI: 0.20~0.63)。
![]() 图 4 OS网状Meta分析结果Figure 4 OS network meta-analysis resultsThe values within the cells represent the hazard ratio (HR) and the 95% confidence interval (CI) when comparing the treatment approach in the upper row against the one in the lower row. An HR greater than 1 indicates that the treatment approach in the lower row is superior in efficacy, whereas an HR less than 1 suggests that the treatment approach in the upper row is more effective. Bolded values in the cells denote results that are statistically significant.
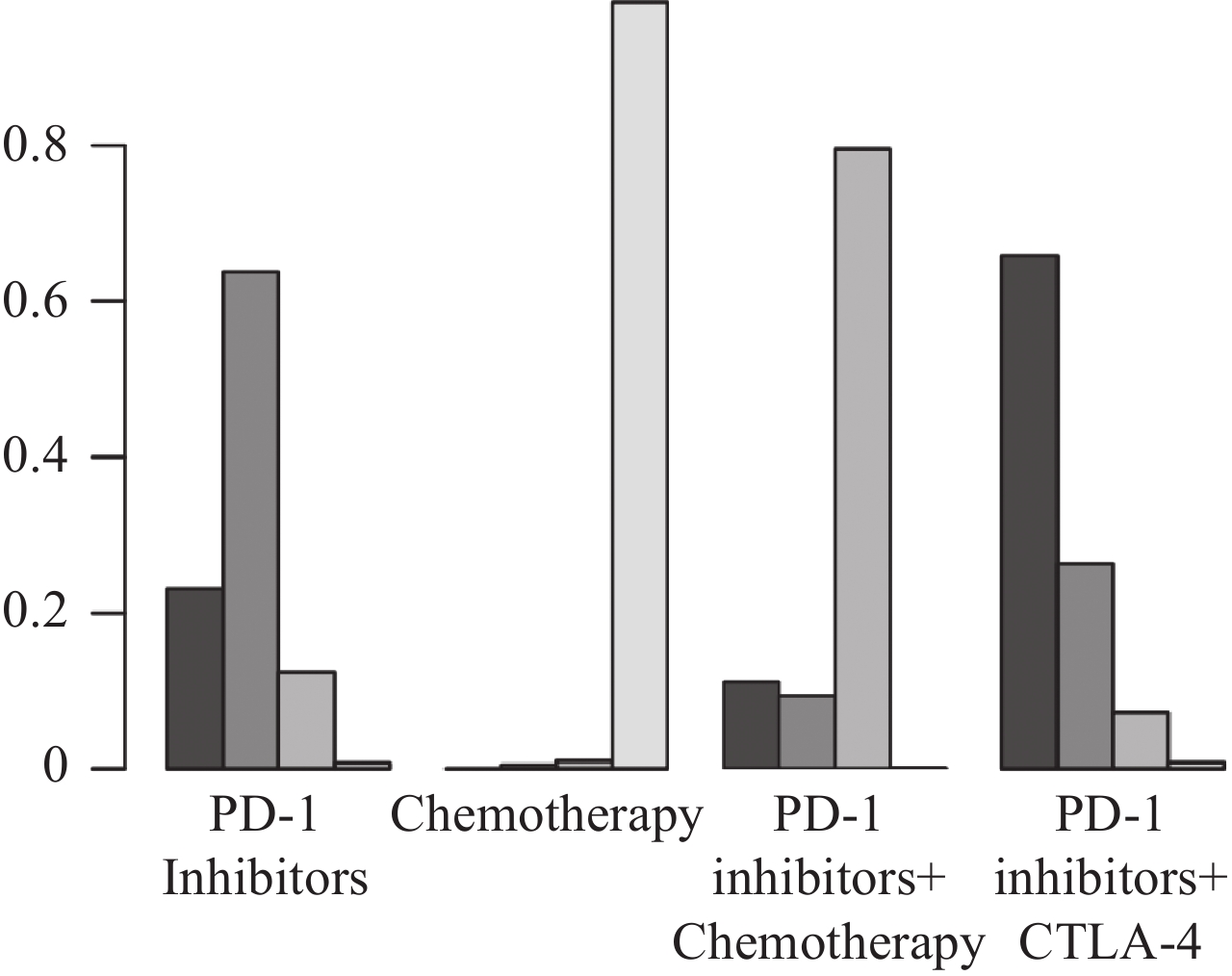
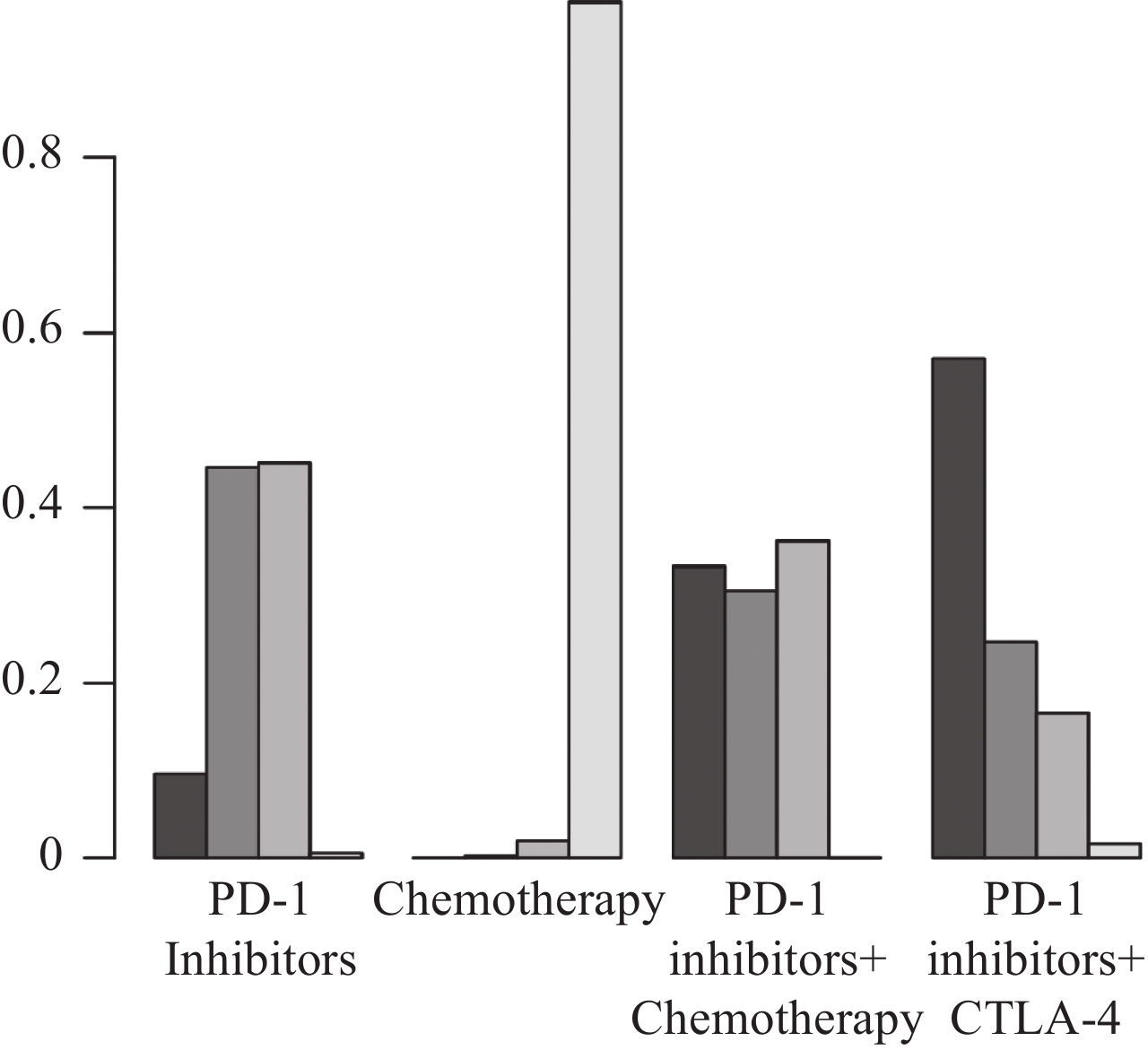
图 4 OS网状Meta分析结果Figure 4 OS network meta-analysis resultsThe values within the cells represent the hazard ratio (HR) and the 95% confidence interval (CI) when comparing the treatment approach in the upper row against the one in the lower row. An HR greater than 1 indicates that the treatment approach in the lower row is superior in efficacy, whereas an HR less than 1 suggests that the treatment approach in the upper row is more effective. Bolded values in the cells denote results that are statistically significant.根据SUCRA结果对4种治疗方案进行排名,从左往右依次为:PD-1抑制剂+CTLA-4、PD-L1抑制剂、PD-1抑制剂+化疗、化疗,见图5。结果表明:在延长OS方面,PD-1抑制剂+CTLA-4最具优势,其次是PD-L1抑制剂。
![]() 图 5 不同干预措施OS最优选概率排名曲线图Figure 5 Curve of probability ranking for optimal OS among different intervention measuresThe horizontal axis represents the various intervention measures, and the vertical axis indicates the probability of ranking for each intervention measure. Each bar graph shows the probability of different intervention measures being ranked from first position to fourth position.
图 5 不同干预措施OS最优选概率排名曲线图Figure 5 Curve of probability ranking for optimal OS among different intervention measuresThe horizontal axis represents the various intervention measures, and the vertical axis indicates the probability of ranking for each intervention measure. Each bar graph shows the probability of different intervention measures being ranked from first position to fourth position.2.3.2 无进展生存期
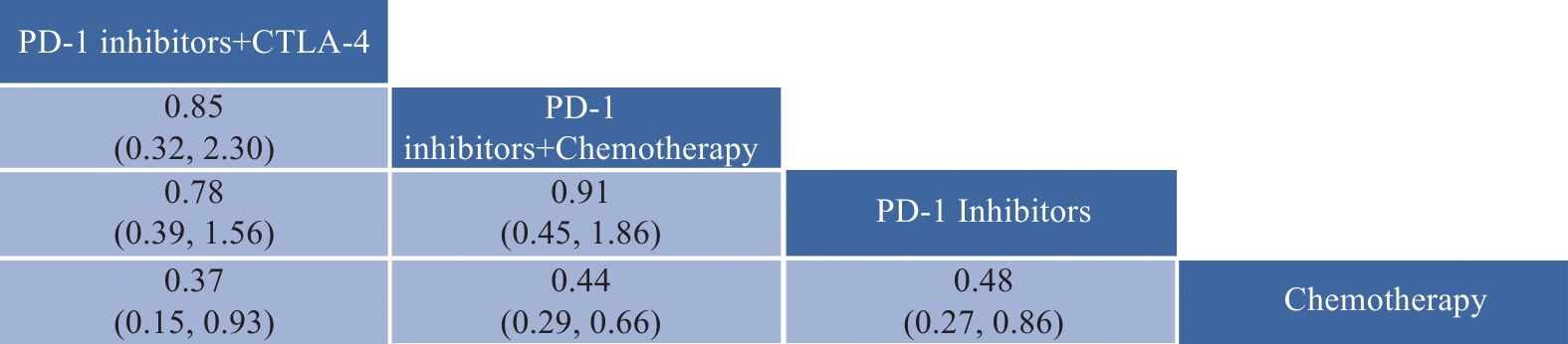
6项研究报告PFS结果,共包括327例和4种治疗方案。PFS网状关系与OS网状关系相似。PFS网状Meta分析结果见图6。PD-1抑制剂+CTLA-4、PD-1抑制剂、PD-1抑制剂+化疗均较化疗方案显著延长晚期不可切除NSCLC脑转移患者OS,其中PD-1抑制剂+CTLA-4最具优势(HR: 0.37,95%CI: 0.15~0.93),接着是PD-L1抑制剂+化疗(HR: 0.44,95%CI: 0.29~0.66)和PD-1抑制剂(HR: 0.48,95%CI: 0.27~0.86)。
![]() 图 6 PFS网状Meta分析结果Figure 6 PFS network meta-analysis resultsThe values in the cells represent the hazard ratio (HR) and the 95% confidence interval (CI) comparing the treatment option in the upper row to that in the lower row. An HR greater than 1 indicates that the treatment option in the lower row is more effective, while an HR less than 1 suggests that the treatment option in the upper row is more effective. Values in bold within the cells indicate statistically significant results.
图 6 PFS网状Meta分析结果Figure 6 PFS network meta-analysis resultsThe values in the cells represent the hazard ratio (HR) and the 95% confidence interval (CI) comparing the treatment option in the upper row to that in the lower row. An HR greater than 1 indicates that the treatment option in the lower row is more effective, while an HR less than 1 suggests that the treatment option in the upper row is more effective. Values in bold within the cells indicate statistically significant results.根据SUCRA结果对4种治疗方案进行排名,见图7,从左往右依次为:PD-1抑制剂+CTLA-4、PD-1抑制剂+化疗、PD-L1抑制剂、化疗。结果表明:在延长OS方面,PD-1抑制剂+CTLA-4最具优势,其次是PD-1抑制剂+化疗。
![]() 图 7 不同干预措施PFS最优选概率排名曲线图Figure 7 Curve of probability ranking for optimal PFS among different intervention measuresThe horizontal axis represents the various intervention measures, and the vertical axis indicates the probability of ranking for each intervention measure. Each bar graph shows the probability of different intervention measures being ranked from first position to fourth position.
图 7 不同干预措施PFS最优选概率排名曲线图Figure 7 Curve of probability ranking for optimal PFS among different intervention measuresThe horizontal axis represents the various intervention measures, and the vertical axis indicates the probability of ranking for each intervention measure. Each bar graph shows the probability of different intervention measures being ranked from first position to fourth position.3 讨论
本研究通过系统评价和Meta分析方法,对比了不同免疫治疗方案与化疗在晚期NSCLC脑转移患者中的疗效。结果显示,无论是OS还是PFS,PD-1抑制剂联合CTLA-4抑制剂组均优于化疗组。这表明免疫双联疗法可能是该患者人群治疗的优先选择。
PD-1和CTLA-4作为两种关键免疫检查点,其功能互补协同。PD-1主要在肿瘤微环境中发挥作用,而CTLA-4主要在淋巴结中发挥抑制T细胞活性的作用[29]。两种药物联合应用,可协同增强机体抗肿瘤免疫应答,产生协同增效作用[10]。CTLA-4抑制剂可上调肿瘤细胞表面肿瘤抗原表达,增加肿瘤免疫原性,而PD-1抑制剂可增强肿瘤微环境中的效应T细胞功能,最终增强对肿瘤细胞的杀伤作用[30-31]。临床研究也证实,相比单药治疗,联合用药可提高NSCLC患者的客观缓解率及延长无进展生存期[25,32-33]。
本研究结果还显示,PD-1抑制剂单药治疗组PFS优于化疗组,OS也有延长趋势,提示即使存在脑转移,PD-1抑制剂也可通过损伤的血脑屏障发挥一定疗效。这与最近研究结果一致:在脑转移NSCLC患者中,抗PD-1单抗可使约30%患者达到客观缓解[17]。抗PD-1抗体分子量较大,主要通过受损血脑屏障进入脑实质[34]。在脑转移存在时,血脑屏障功能受损,增加了PD-1抑制剂通过血脑屏障的机会。未来可开展动物实验,通过检测脑脊液或脑组织中抗体浓度,进一步验证这一假说。
值得注意的是,本研究共纳入6项RCTs,但样本量较小,存在一定的选择性偏倚。由于部分临床试验排除ECOG>1分的患者,因此实际临床中体力状态较差的患者人群,结果还需谨慎推广。另外,不同研究中干预措施并不完全一致,如化疗方案选择,这也可能对Meta分析产生一定影响。今后还需在大样本、多中心的临床研究进一步验证,设计的临床试验应尽可能完成治疗方案标准化,以减少可能的混杂因素对结果的影响。
综上所述,免疫检查点抑制剂治疗可提高晚期NSCLC脑转移患者的生存期,尤其PD-1抑制剂与CTLA-4抑制剂的联合治疗显示出优异的生存获益。这为该患者人群的临床决策提供了重要参考。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:贾牧原:文献搜集与选择、文章撰写、统计分析、结果解释张洪俊:文献检索策略制定、评估研究质量和偏倚、文章撰写与修订、统计分析李琳、吴剑慧、龚欢欢、任博文:文献搜索与筛选、纳入指标质量评估刘 涵:研究设计、方法学设计、质量控制 -
表 1 纳入研究基本特征
Table 1 Basic characteristics of included studies
Included
studiesClinical trials/phasing Number of cases of
brain metastasisInterventions HR(95%CI) Experimental
groupControl
groupExperimental
groupControl
groupOS PFS Zhou, et al.
2021[23]SHR-1210-303
NCT03134872/stage Ⅲ10 5 Camrelizumab for
Injection +
chemotherapyChemotherapy NA 0.14
(0.01-0.88)Reck, et al.
2019[24]KEYNOTE-024
NCT02142738/stage Ⅲ18 10 Pembrolizumab Chemotherapy NA 0.55
(0.20-1.56)Gandhi, et al.
2018[25]KEYNOTE-189
NCT02578680/stage Ⅲ73 35 Pembrolizumab +
chemotherapyChemotherapy 0.36 (0.2-0.62) 0.42
(0.26-0.68)Boyer, et al.
2021[26]KEYNOTE-598
NCT03302234/stage Ⅲ31 29 Pembrolizumab +
ipilimumabPembrolizumab 0.79 (0.36-1.75) 0.78
(0.39-1.57)Sezer, et al.
2021[27]EMPOWER-Lung 1
NCT03088540/stage Ⅲ44 39 Cemiplimab Chemotherapy 0.17 (0.04-0.76) 0.45
(0.22-0.92)Zhang, et al.
2021[28]ORIENT-11
NCT03607539/stage Ⅲ19 14 Sintilimab +
chemotherapyChemotherapy NA 0.58
(0.24-1.18)Notes: OS: overall survival; PFS: progression-free survival; NA: not available. -
[1] Wang R, Yamada T, Kita K, et al. Transient IGF-1R inhibition combined with osimertinib eradicates AXL-low expressing EGFR mutated lung cancer[J]. Nat Commun, 2020, 11(1): 4607. doi: 10.1038/s41467-020-18442-4
[2] Hai J, Liu S, Bufe L, et al. Synergy of WEE1 and mTOR Inhibition in Mutant KRAS-Driven Lung Cancers[J]. Clin Cancer Res, 2017, 23(22): 6993-7005. doi: 10.1158/1078-0432.CCR-17-1098
[3] Zhao B, Zhang W, Yu D, et al. The benefit and risk of nivolumab in non-small-cell lung cancer: a single-arm meta-analysis of noncomparative clinical studies and randomized controlled trials[J]. Cancer Med, 2018, 7(5): 1642-1659. doi: 10.1002/cam4.1387
[4] Kim H, Park S, Jung HA, et al. Long-term Survival in Non-Small Cell Lung Cancer Patients with Metachronous Brain-Only Oligorecurrence Who Underwent Definitive Treatment[J]. Cancer Res Treat, 2022, 54(1): 150-156. doi: 10.4143/crt.2021.306
[5] Argiris A, Lee JW, Stevenson J, et al. Phase Ⅱ randomized trial of carboplatin, paclitaxel, bevacizumab with or without cixutumumab (IMC-A12) in patients with advanced non-squamous, non-small-cell lung cancer: a trial of the ECOG-ACRIN Cancer Research Group (E3508)[J]. Ann Oncol, 2017, 28(12): 3037-3043. doi: 10.1093/annonc/mdx534
[6] Holleman MS, Al MJ, Zaim R, et al. Cost-effectiveness analysis of the first-line EGFR-TKIs in patients with non-small cell lung cancer harbouring EGFR mutations[J]. Eur J Health Econ, 2020, 21(1): 153-164. doi: 10.1007/s10198-019-01117-3
[7] Palicelli A, Croci S, Bisagni A, et al. What Do We Have to Know about PD-L1 Expression in Prostate Cancer? A Systematic Literature Review. Part 3: PD-L1, Intracellular Signaling Pathways and Tumor Microenvironment[J]. Int J Mol Sci, 2021, 22(22): 12330. doi: 10.3390/ijms222212330
[8] Alexander ET, Mariner K, Donnelly J, et al. Polyamine Blocking Therapy Decreases Survival of Tumor-Infiltrating Immunosuppressive Myeloid Cells and Enhances the Antitumor Efficacy of PD-1 Blockade[J]. Mol Cancer Ther, 2020, 19(10): 2012-2022. doi: 10.1158/1535-7163.MCT-19-1116
[9] Peters S, Gettinger S, Johnson ML, et al. Phase Ⅱ Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH)[J]. J Clin Oncol, 2017, 35(24): 2781-2789. doi: 10.1200/JCO.2016.71.9476
[10] Kidwell KM, Postow MA, Panageas KS. Sequential, Multiple Assignment, Randomized Trial Designs in Immuno-oncology Research[J]. Clin Cancer Res, 2018, 24(4): 730-736. doi: 10.1158/1078-0432.CCR-17-1355
[11] Xu F, Chen JX, Yang XB, et al. Analysis of Lung Adenocarcinoma Subtypes Based on Immune Signatures Identifies Clinical Implications for Cancer Therapy[J]. Mol Ther Oncolytics, 2020, 17: 241-249. doi: 10.1016/j.omto.2020.03.021
[12] Deng J, Wang ES, Jenkins RW, et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation[J]. Cancer Discov, 2018, 8(2): 216-233. doi: 10.1158/2159-8290.CD-17-0915
[13] Schoenfeld AJ, Arbour KC, Rizvi H, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib[J]. Ann Oncol, 2019, 30(5): 839-844. doi: 10.1093/annonc/mdz077
[14] Gray JE, Saltos A, Tanvetyanon T, et al. Phase Ⅰ/Ⅰb Study of Pembrolizumab Plus Vorinostat in Advanced/Metastatic Non-Small Cell Lung Cancer[J]. Clin Cancer Res, 2019, 25(22): 6623-6632. doi: 10.1158/1078-0432.CCR-19-1305
[15] Zhu H, Liu W, Fang H. Inflammation caused by peripheral immune cells across into injured mouse blood brain barrier can worsen postoperative cognitive dysfunction induced by isoflurane[J]. BMC Cell Biol, 2018, 19(1): 23. doi: 10.1186/s12860-018-0172-1
[16] Chen W, Jin D, Shi Y, et al. The underlying mechanisms of lorlatinib penetration across the blood-brain barrier and the distribution characteristics of lorlatinib in the brain[J]. Cancer Med, 2020, 9(12): 4350-4359. doi: 10.1002/cam4.3061
[17] Goldberg SB, Schalper KA, Gettinger SN, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial[J]. Lancet Oncol, 2020, 21(5): 655-663. doi: 10.1016/S1470-2045(20)30111-X
[18] Horinouchi H, Nogami N, Saka H, et al. Pembrolizumab plus pemetrexed-platinum for metastatic nonsquamous non-small-cell lung cancer: KEYNOTE-189 Japan Study[J]. Cancer Sci, 2021, 112(8): 3255-3265. doi: 10.1111/cas.14980
[19] Cortinovis D, Chiari R, Catino A, et al. Italian Cohort of the Nivolumab EAP in Squamous NSCLC: Efficacy and Safety in Patients With CNS Metastases[J]. Anticancer Res, 2019, 39(8): 4265-4271. doi: 10.21873/anticanres.13590
[20] Gadgeel SM, Lukas RV, Goldschmidt J, et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: Exploratory analyses of the phase Ⅲ OAK study[J]. Lung Cancer, 2019, 128: 105-112. doi: 10.1016/j.lungcan.2018.12.017
[21] Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2[J]. Value Health, 2011, 14(4): 429-437. doi: 10.1016/j.jval.2011.01.011
[22] van Valkenhoef G, Dias S, Ades AE, et al. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis[J]. Res Synth Methods, 2016, 7(1): 80-93. doi: 10.1002/jrsm.1167
[23] Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial[J]. Lancet Respir Med, 2021, 9(3): 305-314. doi: 10.1016/S2213-2600(20)30365-9
[24] Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer[J]. N Engl J Med, 2016, 375(19): 1823-1833. doi: 10.1056/NEJMoa1606774
[25] Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer[J]. N Engl J Med, 2018, 378(22): 2078-2092. doi: 10.1056/NEJMoa1801005
[26] Boyer M, Şendur MAN, Rodríguez-Abreu D, et al. Pembrolizumab Plus Ipilimumab or Placebo for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score 50%: Randomized, Double-Blind Phase Ⅲ KEYNOTE-598 Study[J]. J Clin Oncol, 2021, 39(21): 2327-2338. doi: 10.1200/JCO.20.03579
[27] Sezer A, Kilickap S, Gümüş M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial[J]. Lancet, 2021, 397(10274): 592-604. doi: 10.1016/S0140-6736(21)00228-2
[28] Zhang L, Wang Z, Fang J, et al. Final overall survival data of sintilimab plus pemetrexed and platinum as First-Line treatment for locally advanced or metastatic nonsquamous NSCLC in the Phase 3 ORIENT-11 study[J]. Lung Cancer, 2022, 171: 56-60. doi: 10.1016/j.lungcan.2022.07.013
[29] Yi JS, Ready N, Healy P, et al. Immune Activation in Early-Stage Non-Small Cell Lung Cancer Patients Receiving Neoadjuvant Chemotherapy Plus Ipilimumab[J]. Clin Cancer Res, 2017, 23(24): 7474-7482. doi: 10.1158/1078-0432.CCR-17-2005
[30] Yang K, Li J, Sun Z, et al. Retreatment with immune checkpoint inhibitors in solid tumors: a systematic review[J]. Ther Adv Med Oncol, 2020, 12: 1758835920975353.
[31] Sabari JK, Lok BH, Laird JH, et al. Unravelling the biology of SCLC: implications for therapy[J]. Nat Rev Clin Oncol, 2017, 14(9): 549-561. doi: 10.1038/nrclinonc.2017.71
[32] Wang YJ, Kathawala RJ, Zhang YK, et al. Motesanib (AMG706), a potent multikinase inhibitor, antagonizes multidrug resistance by inhibiting the efflux activity of the ABCB1[J]. Biochem Pharmacol, 2014, 90(4): 367-378. doi: 10.1016/j.bcp.2014.06.006
[33] Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer[J]. N Engl J Med, 2018, 379(21): 2040-2051. doi: 10.1056/NEJMoa1810865
[34] Fan LW, Carter K, Bhatt A, et al. Rapid transport of insulin to the brain following intranasal administration in rats[J]. Neural Regen Res, 2019, 14(6): 1046-1051. doi: 10.4103/1673-5374.250624
-
期刊类型引用(2)
1. 张淑香,殷宁. 替雷利珠单抗与卡铂及白蛋白紫杉醇联合治疗对非小细胞肺癌患者血清学指标的影响. 中华养生保健. 2025(07): 181-185 .  百度学术
百度学术
2. 秦毅,刘艺. 信迪利单抗联合白蛋白紫杉醇、顺铂化疗治疗Ⅳ期非小细胞肺癌的疗效及影响因素研究. 药品评价. 2024(11): 1410-1413 .  百度学术
百度学术
其他类型引用(0)



 下载:
下载: