Prediction of Responses of Patients with Hepatocellular Carcinoma to Anti-PD-1 Immunotherapy by T-cell Invigoration to Tumour Burden Ratio
-
摘要:目的
探讨抗PD-1治疗后肝细胞癌患者外周血T细胞活化及其与肿瘤负荷比值对免疫治疗疗效的预测价值。
方法分析85例接受抗PD-1治疗的肝细胞癌(HCC)患者治疗前后的血清样本,通过流式细胞仪分析其外周血细胞亚群、T细胞活化,结合影像检查X-tile软件选取截断值,生存分析评估患者预后情况。
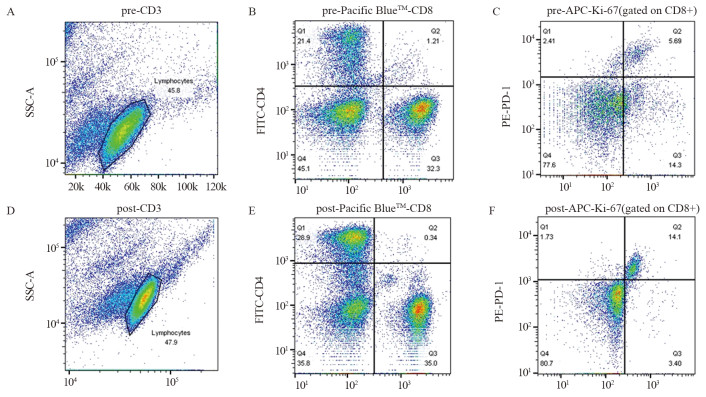
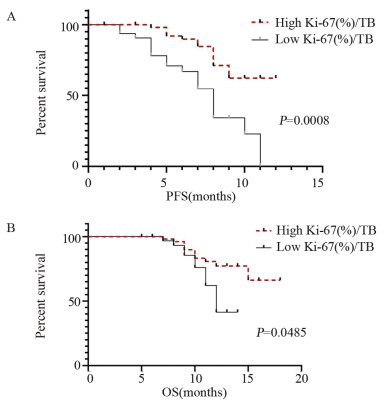
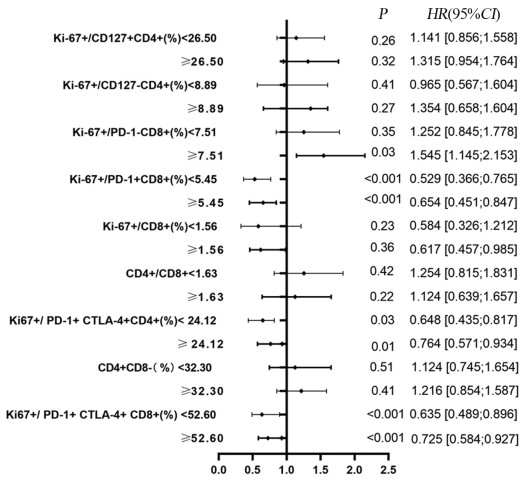
结果治疗周期中Ki-67+/PD-1+/CD8+ T细胞的最大倍数变化和通过影像确定的肿瘤负荷与免疫治疗的生存获益相关。在抗PD-1免疫治疗的第一个周期,T细胞Ki-67+/PD-1+/CD8+表达与肿瘤负荷比大于0.6与PFS和OS的改善相关(P < 0.05)。
结论免疫治疗后外周血T细胞的活化与肿瘤负荷比可能与肝细胞癌抗PD-1免疫治疗的临床疗效相关。
Abstract:ObjectiveTo explore the predictive value of T cell activation in peripheral blood of patients with hepatocellular carcinoma(HCC) after anti PD-1 therapy and its ratio to tumor burden on the efficacy of immunotherapy.
MethodsSerum specimens were obtained before and after treatment from 85 patients with HCC who received anti-PD-1 treatment. Indicators such as cell subpopulations and T cell activation were detected by flow cytometry. Combined with imaging analysis, cutoff value was obtained by X-tile software. Survival analysis was used to evaluate patients' outcomes.
ResultsThe maximum fold change of Ki-67+/PD-1+/CD8+ T cells in treatment cycles and the tumor burden determined by imaging were associated with prognoses. The ratio of T cell Ki-67+/PD-1+/CD8+ expression to tumor burden ratio greater than 0.6 at the first cycle of anti-PD-1 immunotherapy was associated with improvements in progression-free survival and overall survival (P < 0.05).
ConclusionThe ratio of activationa in T cells in peripheral blood after immunotherapy to the tumor burden may be related to the clinical efficacy of anti-PD-1 immunotherapy for HCC.
-
Key words:
- Immunotherapy /
- Peripheral blood biomarkers /
- Tumour burden /
- Hepatocellular carcinoma
-
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:吴慧:实验设计与实施、数据分析、论文撰写及修改孙丽娜:数据收集、统计学分析及流式细胞检测潘璋驰:实验设计、临床数据筛选施纯玫:课题设计、数据审核及指导论文撰写
-
表 1 85例肝细胞癌患者T细胞活化与肿瘤负荷比与临床病理特征的关系
Table 1 Relationship between T-cell invigoration to tumour burden ratio and clinicopathological features in 85 cases of HCC

-
[1] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[2] Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. doi: 10.3322/caac.21338
[3] Ma W, Gilligan BM, Yuan J, et al. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy[J]. J Hematol Oncol, 2016, 9(1): 47. doi: 10.1186/s13045-016-0277-y
[4] Sharma P, Allison JP. The future of immune checkpoint therapy[J]. Science, 2015, 348(6230): 56-61. doi: 10.1126/science.aaa8172
[5] Liu Z, Meng X, Tang X, et al. Intratumoral tertiary lymphoid structures promote patient survival and immunotherapy response in head neck squamous cell carcinoma[J]. Cancer Immunol Immunother, 2023, 72(6): 1505-1521. doi: 10.1007/s00262-022-03310-5
[6] Dolina JS, Van Braeckel-Budimir N, Thomas GD, et al. CD8(+) T Cell Exhaustion in Cancer[J]. Front Immunol, 2021, 12: 715234. doi: 10.3389/fimmu.2021.715234
[7] 沈仕俊, 王巧丽, 杨金江, 等. 肿瘤突变负荷对PD-1/PD-L1抑制剂治疗非小细胞肺癌临床疗效预测的Meta分析[J]. 肿瘤防治研究, 2021, 48(3): 281-287. doi: 10.3971/j.issn.1000-8578.2021.20.0765 Shen SJ, Wang QL, Yang JJ, et al. Predictive Value of Tumor Mutation Burden for PD-1/PD-L1 Inhibitors Treatment on Non-small Cell Lung Cancer: A Meta-analysis[J]. Zhong Liu Fang Zhi Yan Jiu, 2021, 48(3): 281-287. doi: 10.3971/j.issn.1000-8578.2021.20.0765
[8] 许晶, 金顺花. 循环肿瘤DNA在消化系统肿瘤中的应用进展[J]. 医学研究生学报, 2022, 35(8): 883-888. https://www.cnki.com.cn/Article/CJFDTOTAL-JLYB202208017.htm Xu J, Jin SH. Advances in the application of circulating tumor DNA in digestive system tumors[J]. Yi Xue Yan Jiu Sheng Xue Bao, 2022, 35(8): 883-888. https://www.cnki.com.cn/Article/CJFDTOTAL-JLYB202208017.htm
[9] 王亚东, 杨笑盈, 贾梓淇, 等. 循环肿瘤细胞PD-L1表达在非小细胞肺癌免疫治疗中的应用[J]. 中国胸心血管外科临床杂志, 2021, 28(1): 110-115. https://www.cnki.com.cn/Article/CJFDTOTAL-ZXYX202101021.htm Wang YD, Yang XY, Jia ZQ, et al. Clinical utility of PD-L1 expression in circulating tumor cells in non-small cell lung cancer patients treated with immunotherapy[J]. Zhongguo Xiong Xin Xue Guan Wai Ke Lin Chuang Za Zhi, 2021, 28(1): 110-115. https://www.cnki.com.cn/Article/CJFDTOTAL-ZXYX202101021.htm
[10] Pinter M, Jain RK, Duda DG. The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma: A Review[J]. JAMA Oncol, 2021, 7(1): 113-123. doi: 10.1001/jamaoncol.2020.3381
[11] Khagi Y, Goodman AM, Daniels GA, et al. Hypermutated Circulating Tumor DNA: Correlation with Response to Checkpoint Inhibitor-Based Immunotherapy[J]. Clin Cancer Res, 2017, 23(19): 5729-5736. doi: 10.1158/1078-0432.CCR-17-1439
[12] Yue C, Jiang Y, Li P, et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy[J]. Oncoimmunology, 2018, 7(7): e1438111. doi: 10.1080/2162402X.2018.1438111
[13] Bullwinkel J, Baron-Lühr B, Lüdemann A, et al. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells[J]. J Cell Physiol, 2006, 206(3): 624-635. doi: 10.1002/jcp.20494
[14] Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection[J]. Nat Immunol, 2009, 10(1): 29-37. doi: 10.1038/ni.1679
[15] Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer[J]. Nature, 2015, 520(7547): 373-377. doi: 10.1038/nature14292
[16] Marei HE, Hasan A, Pozzoli G, et al. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired[J]. Cancer Cell Int, 2023, 23(1): 64. doi: 10.1186/s12935-023-02902-0
[17] Kim KH, Cho J, Ku BM, et al. The First-week Proliferative Response of Peripheral Blood PD-1+CD8+ T Cells Predicts the Response to Anti-PD-1 Therapy in Solid Tumors[J]. Clin Cancer Res, 2019, 25(7): 2144-2154. doi: 10.1158/1078-0432.CCR-18-1449
[18] Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential[J]. Cell, 2015, 161(2): 205-214. doi: 10.1016/j.cell.2015.03.030
[19] Hack SP, Zhu AX, Wang Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities[J]. Front Immunol, 2020, 11: 598877. doi: 10.3389/fimmu.2020.598877
[20] Wang W, Green M, Choi JE, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy[J]. Nature, 2019, 569(7755): 270-274. doi: 10.1038/s41586-019-1170-y
[21] Yi L, Huang P, Zou X, et al. Integrative stemness characteristics associated with prognosis and the immune microenvironment in esophageal cancer[J]. Pharmacol Res, 2020, 161: 105144. doi: 10.1016/j.phrs.2020.105144
[22] Guo W, Tan F, Huai Q, et al. Comprehensive Analysis of PD-L1 Expression, Immune Infiltrates, and m6A RNA Methylation Regulators in Esophageal Squamous Cell Carcinoma[J]. Front Immunol, 2021, 12: 669750. doi: 10.3389/fimmu.2021.669750
[23] Chi H, Zhao S, Yang J, et al. T-cell exhaustion signatures characterize the immune landscape and predict HCC prognosis via integrating single-cell RNA-seq and bulk RNA-sequencing[J]. Front Immunol, 2023, 14: 1137025. doi: 10.3389/fimmu.2023.1137025
[24] Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response[J]. Nature, 2017, 545(7652): 60-65. doi: 10.1038/nature22079
[25] Bengsch B, Seigel B, Ruhl M, et al. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation[J]. PLoS Pathog, 2010, 6(6): e1000947. doi: 10.1371/journal.ppat.1000947



 下载:
下载: