Inhibitory Effect of UHRF1 on Invasion and Migration of Colorectal Cancer Cells via WNT/MMP9 Signaling Pathway
-
摘要:目的
探索UHRF1基因与结直肠癌(CRC)患者临床病理特性的关系,慢病毒转染过表达和敲减UHRF1对CRC细胞增殖、侵袭及迁移的影响及可能的信号通路。
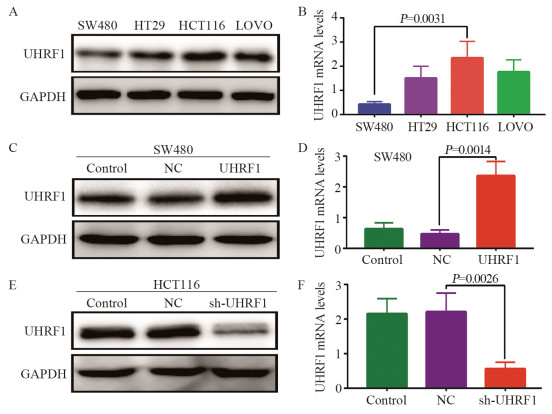
方法免疫组织化学和RT-PCR法检测112例CRC癌组织与癌旁组织UHRF1的表达。构建慢病毒转染过表达和敲减UHRF1载体,分别转染SW480和HCT116细胞株,RT-PCR和Western blot检测转染前后及IWP-2(WNT拮抗剂)和HLY78(WNT活化剂)干预前后的UHRF1、WNT信号通路关键分子和MMP9的表达。EDU检测细胞增殖能力;Transwell实验检测细胞迁移和侵袭能力。
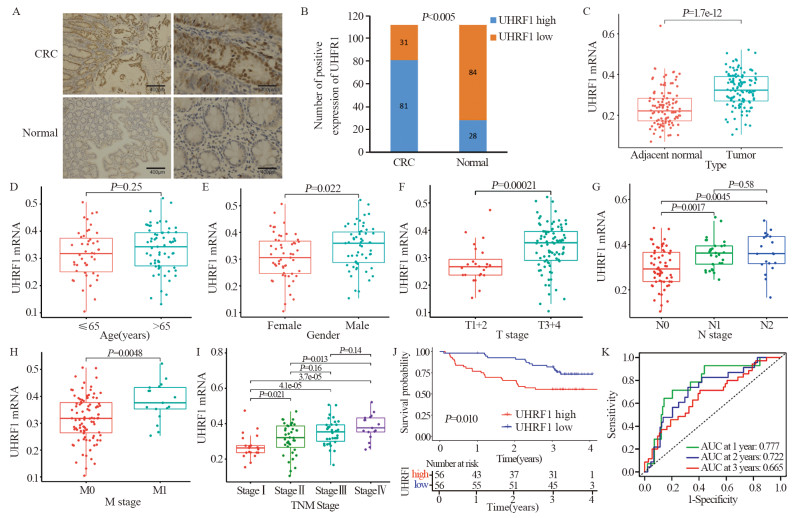
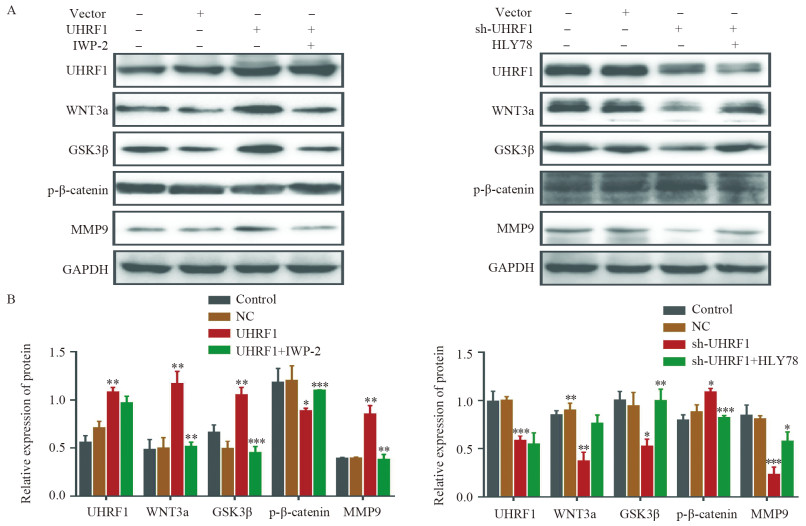
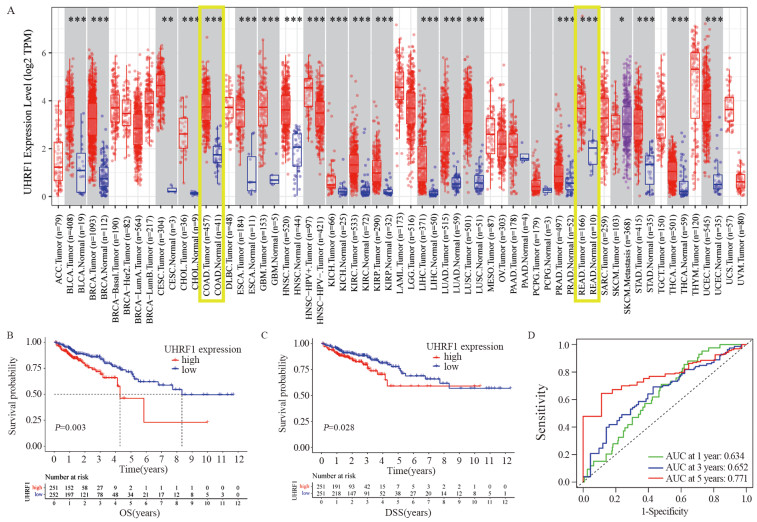
结果(1)TCGA数据库和临床数据中,癌组织中UHRF1 mRNA和蛋白表达均高于正常组织。UHRF1表达量与TNM分期、N和M分期密切相关。TCGA中UHRF1低表达患者有更长的5年OS和疾病相关存活时间(DSS),UHRF1预测1、3和5年OS的ROC曲线下面积(AUC)分别为0.634、0.652和0.771;在临床数据中3年OS也有相同生存获益,UHRF1高表达是CRC不良预后因素。(2)过表达UHRF1后,SW480中WNT3a、GSK3β和MMP9分子表达明显升高,p-β-catenin表达下降(P < 0.05);敲减UHRF1后,HCT116细胞中WNT3a、GSK3β、MMP9表达下降,而p-β-catenin表达升高(P < 0.05);分别用IWP-2和HLY78进行“拯救”实验,能够获得一致结果。(3)过表达组细胞增殖、迁移和侵袭能力明显高于对照组;IWP-2处理后,细胞增殖、迁移及侵袭能力则受到抑制。敲减实验呈现与过表达实验相反的结果。
结论UHRF1在CRC发生发展中可能具有重要作用,UHRF1高表达可能是CRC不良预后因素,UHRF1可能通过WNT/MMP9信号通路来影响CRC的增殖、迁移和侵袭。
-
关键词:
- 结直肠癌 /
- UHRF1 /
- WNT/MMP9信号通路 /
- 增殖 /
- 侵袭
Abstract:ObjectiveTo explore the relationship of UHRF1 with the clinicopathological characteristics of colorectal cancer (CRC) patients, as well as the effects of lentivirus transfection overexpression and knockdown of UHRF1 on the proliferation, invasion, and migration of CRC cells and the possible signaling pathways.
MethodsThe expression of UHRF1 mRNA and protein in CRC tissues and adjacent tissues was detected by immunohistochemical staining and RT-PCR. The effects of the constructed UHRF1 overexpression- and knockdown-group cells on the expression of UHRF1, related molecules in the WNT signaling pathway, and MMPR9 were examined by Western blot and RT-PCR. EDU and Transwell assays were used to detect changes in the proliferation, migration, and invasion of CRC cells.
Results(1) In the TCGA database and clinical data, the mRNA and protein expression levels of UHRF1 in CRC cancer tissues were significantly higher than those in adjacent normal tissues. UHRF1 expression was closely correlated with TNM stage, N stage, and M stage. Patients with low UHRF1 expression in TCGA had better 5-year OS and disease-specific survival. The area under the ROC curve of UHRF1 for predicting 1-, 3-, and 5-year OS were 0.634, 0.652, and 0.771, respectively. The 3-year OS in the clinical data also showed the same survival benefit. UHRF1 overexpression was a poor prognostic factor for CRC patients. (2) After UHRF1 overexpression, the expression of WNT3a, GSK3β, and MMP9 in SW480 cells significantly increased, whereas the expression of p-β-catenin decreased (P < 0.05). After UHRF1 knockdown, the expression of WNT3a, GSK3β, and MMP9 in HCT116 cells decreased, whereas the expression of p-β-catenin increased (P < 0.05). The "rescue" experiment with IWP-2 and HLY78 can produce consistent results. (3) Compared with the control group, the cell proliferation, migration, and invasion abilities of the UHRF1 overexpression group were enhanced. After IWP-2 treatment, the cell proliferation, migration, and invasion abilities were inhibited. Knockdown experiment exhibited the reverse results to overexpression experiment.
ConclusionUHRF1 may play an important role in the occurrence and development of CRC. UHRF1 overexpression may be a poor prognostic factor for CRC patients. UHRF1 may affect the proliferation, migration, and invasion of CRC cells through the WNT/MMP9 signaling pathway.
-
Key words:
- Colorectal cancer /
- UHRF1 /
- WNT/MMP9 signaling pathway /
- Proliferation /
- Invasion
-
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:陈志华:研究设计和实验实施、文章撰写郑艳:研究设计和实验实施林素勇:数据分析陈绍勤:文章审阅
-
表 1 UHRF1表达与CRC患者临床病理特征的关系
Table 1 Relationships between UHRF1 expression and clinicopathological characteristics in CRC patients

-
[1] Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023[J]. CA Cancer J Clin, 2023, 73(1): 17-48. doi: 10.3322/caac.21763
[2] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[3] Munro MJ, Wickremesekera SK, Peng L, et al. Cancer stem cells in colorectal cancer: a review[J]. J Clin Pathol, 2018, 71(2): 110-116. doi: 10.1136/jclinpath-2017-204739
[4] Katoh M, Katoh M. WNT signaling and cancer stemness[J]. Essays Biochem, 2022, 66(4): 319-331. doi: 10.1042/EBC20220016
[5] Birrer DL, Tschuor C, Reiner C, et al. Multimodal treatment strategies for colorectal liver metastases[J]. Swiss Med Wkly, 2021, 151: w20390. doi: 10.4414/smw.2021.20390
[6] Chen S, Chen Z, Lin S, et al. KISS1 methylation and expression as predictors of disease progression in colorectal cancer patients[J]. World J Gastroenterol, 2014, 20(29): 10071-10081. doi: 10.3748/wjg.v20.i29.10071
[7] Hopfner R, Mousli M, Jeltsch JM, et al. ICBP90, a novel human CCAAT binding protein, involved in the regulation of topoisomerase Ⅱalpha expression[J]. Cancer Res, 2000, 60(1): 121-128.
[8] Bronner C, Krifa M, Mousli M. Increasing role of UHRF1 in the reading and inheritance of the epigenetic code as well as in tumorogenesis[J]. Biochem Pharmacol, 2013, 86(12): 1643-1649. doi: 10.1016/j.bcp.2013.10.002
[9] Bronner C, Alhosin M, Hamiche A, et al. Coordinated Dialogue between UHRF1 and DNMT1 to Ensure Faithful Inheritance of Methylated DNA Patterns[J]. Genes (Basel), 2019, 10(1): 65. doi: 10.3390/genes10010065
[10] Hu Q, Qin Y, Ji S, et al. UHRF1 promotes aerobic glycolysis and proliferation via suppression of SIRT4 in pancreatic cancer[J]. Cancer Lett, 2019, 452: 226-236. doi: 10.1016/j.canlet.2019.03.024
[11] Xu X, Ding G, Liu C, et al. Nuclear UHRF1 is a gate-keeper of cellular AMPK activity and function[J]. Cell Res, 2022, 32(1): 54-71. doi: 10.1038/s41422-021-00565-y
[12] Pacaud R, Brocard E, Lalier L, et al. The DNMT1/PCNA/UHRF1 disruption induces tumorigenesis characterized by similar genetic and epigenetic signatures[J]. Sci Rep, 2014, 4: 4230. doi: 10.1038/srep04230
[13] Wang H, Cao D, Wu F. Long noncoding RNA UPAT promoted cell proliferation via increasing UHRF1 expression in non-small cell lung cancer[J]. Oncol Lett, 2018, 16(2): 1491-1498.
[14] Wang F, Yang YZ, Shi CZ, et al. UHRF1 promotes cell growth and metastasis through repression of p16(ink(4)a) in colorectal cancer[J]. Ann Surg Oncol, 2012, 19(8): 2753-2762. doi: 10.1245/s10434-011-2194-1
[15] Kong X, Chen J, Xie W, et al. Defining UHRF1 Domains that Support Maintenance of Human Colon Cancer DNA Methylation and Oncogenic Properties[J]. Cancer Cell, 2019, 35(4): 633-648. e7. doi: 10.1016/j.ccell.2019.03.003
[16] Lin Y, Chen Z, Lin S, et al. MiR-202 inhibits the proliferation and invasion of colorectal cancer by targeting UHRF1[J]. Acta Biochim Biophys Sin (Shanghai), 2019, 51(6): 598-606.
[17] Lin Y, Chen Z, Zheng Y, et al. MiR-506 Targets UHRF1 to Inhibit Colorectal Cancer Proliferation and Invasion via the KISS1/PI3K/NF-κB Signaling Axis[J]. Front Cell Dev Biol, 2019, 7: 266. doi: 10.3389/fcell.2019.00266
[18] Mancini M, Magnani E, Macchi F, et al. The multi-functionality of UHRF1: epigenome maintenance and preservation of genome integrity[J]. Nucleic Acids Res, 2021, 49(11): 6053-6068. doi: 10.1093/nar/gkab293
[19] Ren Y. Regulatory mechanism and biological function of UHRF1-DNMT1-mediated DNA methylation[J]. Funct Integr Genomics, 2022, 22(6): 1113-1126. doi: 10.1007/s10142-022-00918-9
[20] Kim JK, Kan G, Mao Y, et al. UHRF1 downmodulation enhances antitumor effects of histone deacetylase inhibitors in retinoblastoma by augmenting oxidative stress-mediated apoptosis[J]. Mol Oncol, 2019, 14(2): 329-346.
[21] Jiao D, Huan Y, Zheng J, et al. UHRF1 promotes renal cell carcinoma progression through epigenetic regulation of TXNIP[J]. Oncogene, 2019, 38(28): 5686-5699. doi: 10.1038/s41388-019-0822-6
[22] Lu NH, Wei CY, Qi FZ, et al. Hsa-let-7b suppresses cell proliferation by targeting UHRF1 in melanoma[J]. Cancer Invest, 2020, 38(1): 52-60. doi: 10.1080/07357907.2019.1709482
[23] Wang F, Yang Y, Shi C, et al. UHRF1 promotes cell growth and metastasis through repression of p16(ink4a) in colorectal cancer[J]. Ann Surg Oncol, 2012, 19(8): 2753-2762. doi: 10.1245/s10434-011-2194-1
[24] Niinuma T, Kitajima H, Kai M, et al. UHRF1 depletion and HDAC inhibition reactivate epigenetically silenced genes in colorectal cancer cells[J]. Clin Epigenetics, 2019, 11(1): 70. doi: 10.1186/s13148-019-0668-3
[25] Cui X, Cui Y, Du T, et al. SHMT2 Drives the Progression of Colorectal Cancer by Regulating UHRF1 Expression[J]. Can J Gastroenterol Hepatol, 2022, 2022: 3758697.
[26] Patel S, Alam A, Pant R, et al. Wnt Signaling and Its Significance Within the Tumor Microenvironment: Novel Therapeutic Insights[J]. Front Immunol, 2019, 10: 2872.
[27] Zhao H, Ming T, Tang S, et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target[J]. Mol Cancer, 2022, 21(1): 144.
[28] Parsons MJ, Tammela T, Dow LE. WNT as a Driver and Dependency in Cancer[J]. Cancer Discov, 2021, 11(10): 2413-2429.
[29] Yu F, Yu C, Li F, et al. Wnt/β-catenin signaling in cancers and targeted therapies[J]. Signal Transduct Target Ther, 2021, 6(1): 307.
[30] Zhou Y, Xu J, Luo H, et al. Wnt signaling pathway in cancer immunotherapy[J]. Cancer Lett, 2022, 525: 84-96.
[31] Han J, Chen X, Xu J, et al. Simultaneous silencing Aurora-A and UHRF1 inhibits colorectal cancer cell growth through regulating expression of DNMT1 and STAT1[J]. Int J Med Sci, 2021, 18(15): 3437-3451.



 下载:
下载: