Construction and Validation of A Predictive Model Including TCM Pathogenic Syndrome for Short-term Efficacy of PD-1 Inhibitors in Advanced Non-small Cell Lung Cancer
-
摘要:目的
评估PD-1抑制剂治疗非小细胞肺癌(NSCLC)近期疗效的预测因素,构建预测模型。
方法前瞻性纳入2019年10月—2021年11月间,符合入组标准、应用PD-1抑制剂的晚期NSCLC患者221例,2021年5月1日前入组的为建模组(n=149例),之后的为验证组(n=72例)。采集患者的一般临床资料及中医四诊信息,并进行中医证素辨别。使用R软件4.0.4版本构建客观缓解率的列线图临床预测模型,通过受试者工作特征曲线及校准曲线来评价该模型的预测能力和区分度,并通过验证组进行外部验证。
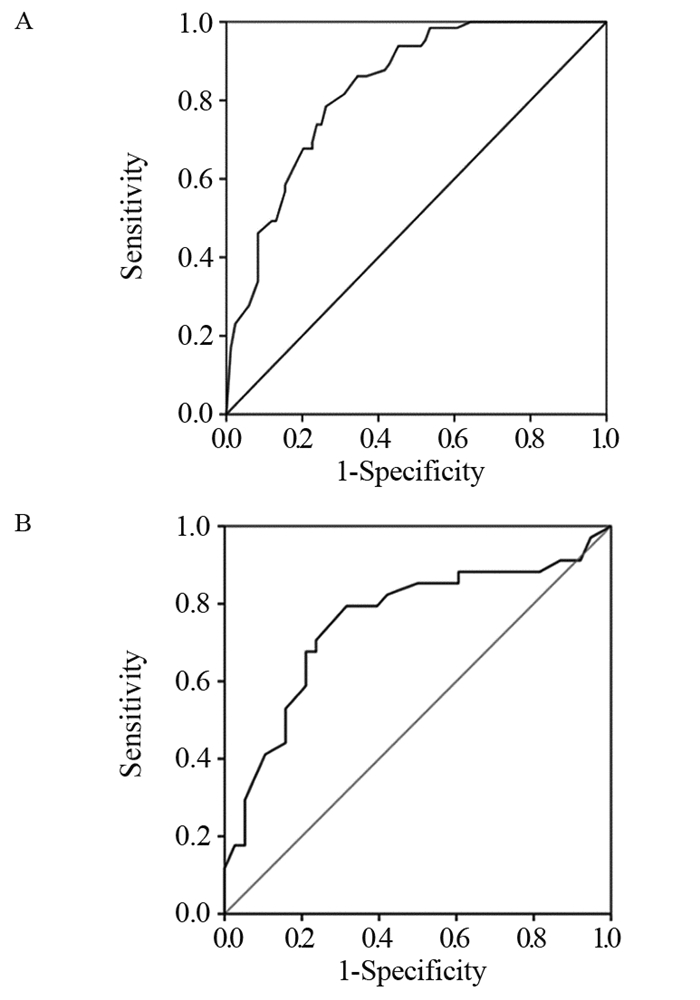
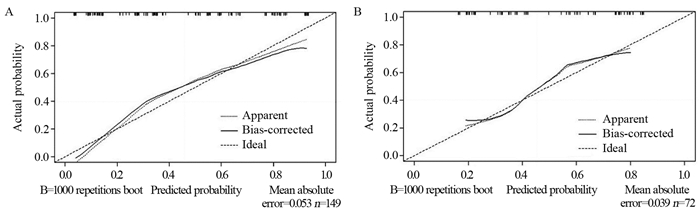
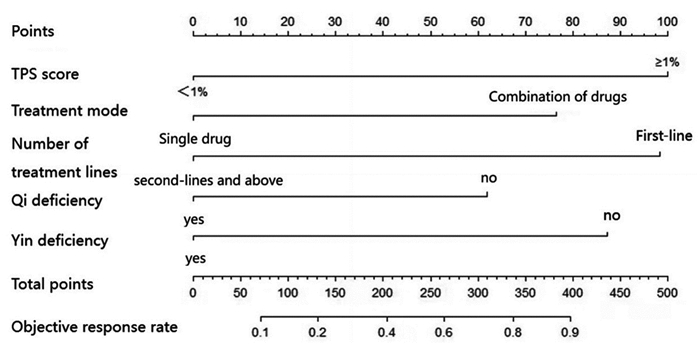
结果221例患者经PD-1抑制剂治疗2~4个周期后,总的客观缓解率为44.80%。建模组多因素Logistic回归分析发现,TPS评分(OR=0.261, P=0.001)、治疗线数(OR=3.749, P=0.002)、治疗模式(OR=2.796, P=0.019)、气虚病性证素(OR=2.296, P=0.043)、阴虚病性证素(OR=3.228, P=0.005)是PD-1抑制剂近期疗效的独立预测因素。基于以上5个独立预测因子构建PD-1抑制剂近期疗效的列线图预测模型,建模组和验证组的AUC值分别为0.8317和0.7535,两组校准曲线在预测值与真实值之间符合的平均绝对误差分别为0.053和0.039,显示出较高吻合度,表明该模型的预测性能良好。
结论基于中医气虚病性证素、阴虚病性证素以及TPS评分、治疗线数和治疗模式构建的列线图模型是预测晚期非小细胞肺癌PD-1抑制剂近期疗效的稳定有效工具。
Abstract:ObjectiveTo evaluate predictive factors affecting the short-term efficacy of PD-1 inhibitors in non-small cell lung cancer (NSCLC) and to construct a prediction model.
MethodsFrom October 2019 to November 2021, 221 patients with advanced NSCLC who met the inclusion criteria and were treated with PD-1 inhibitors were prospectively enrolled. Patients who were enrolled before May 1st, 2021 were included inthe modeling group (n=149), whereas those who enrolled thereafter were included in the validation group (n=72). The general clinical data of patients, information of the four TCM diagnoses were collected, and TCM syndrome elements were identified. R software version 4.0.4 was used in constructing a nomogram clinical prediction model of objective response rate. The predictive ability and discrimination of the model were evaluated and externally validated by using a validation group.
ResultsAfter two to four cycles of PD-1 inhibitor therapy in 221 patients, the overall objective response rate was 44.80%. Multivariate logistic regression analysis of the modeling group showed that the TPS score (OR=0.261, P=0.001), number of treatment lines (OR=3.749, P=0.002), treatment mode (OR=2.796, P=0.019), qi deficiency disease syndrome elements (OR=2.296, P=0.043), and syndrome elements of yin deficiency disease (OR=3.228, P=0.005) were the independent predictors of the short-term efficacy of PD-1 inhibitors. Based on the above five independent predictors, a nomogram prediction model for the short-term efficacy of PD-1 inhibitors was constructed. The AUC values of the modeling and validation groups were 0.8317 and 0.7535, respectively. The calibration curves of the two groups showed good agreement between the predicted and true values. The mean absolute errors were 0.053 and 0.039, indicating that the model has good predictive performance.
ConclusionThe nomogram model constructed on the basis of the syndrome elements of Qi-deficiency disease and Yin-deficiency syndrome of TCM, as well as TPS score, number of treatment lines and treatment mode, is a stable and effective tool for predicting the short-term efficacy of PD-1 inhibitors in advanced non-small cell lung cancer.
-
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:马军燕:实验实施,文章撰写吴琼:分析、解释数据董量、李春阳:采集、分析、解释数据、行政、技术及材料支持王志武:审阅文章、指导
-
表 1 221例晚期NSCLC患者的治疗方法
Table 1 Treatment of 221 patients with advanced NSCLC

表 2 221例晚期NSCLC患者的一般临床资料分布情况统计
Table 2 Distribution of general clinical data of 221 patients with advanced NSCLC

表 3 221例晚期NSCLC患者疗效评价分布情况统计
Table 3 Distribution of curative effect evaluation of 221 patients with advanced NSCLC

表 4 影响PD-1抑制剂近期疗效的患者一般临床资料单因素分析
Table 4 Univariate analysis of general clinical data on short-term efficacy of PD-1 inhibitors

表 5 建模组PD-1抑制剂近期疗效的多因素分析
Table 5 Multivariate analysis of short-term efficacy of PD-1 inhibitor in modeling group

-
[1] Socinski MA, Obasaju C, Gandara D, et al. Clinicopathologic Features of Advanced Squamous NSCLC[J]. J Thorac Oncol, 2016, 11(9): 1411-1422. doi: 10.1016/j.jtho.2016.05.024
[2] 宋鹏. 中国非小细胞肺癌患者免疫检查点抑制剂疗效预测模型建立及肠道菌群结构与代谢组特征分析与比较[D]. 中国医学科学院北京协和医学院, 2020. Song P. Establishment of predictive model of immune checkpoint inhibitors for patients with non-small cell lung cancer in China and analysis and comparison of intestinal flora structure and metabonomic characteristics[D]. Chinese Academy of Medical Sciences and Peking Union Medical College, 2020.
[3] Xia Q, Chen G, Ren Y, et al. Investigating efficacy of "microbiota modulation of the gut-lung Axis" combined with chemotherapy in patients with advanced NSCLC: study protocol for a multicenter, prospective, double blind, placebo controlled, randomized trial[J]. BMC Cancer, 2021, 21(1): 721. doi: 10.1186/s12885-021-08448-6
[4] Nix MG, Rowbottom CG, Vivekanandan S, et al. Chemoradiotherapy of locally advanced non-small cell lung cancer: Analysis of radiation dose-response, chemotherapy and survival-limiting toxicity effects indicates a low α/β ratio[J]. Radiother Oncol, 2020, 143(2): 58-65.
[5] Paessens BJ, von Schilling C, Berger K, et al. Health resource consumption and costs attributable to chemotherapy-induced toxicity in German routine hospital care in lymphoproliferative disorder and NSCLC patients[J]. Ann Oncol, 2011, 22(10): 2310-2319. doi: 10.1093/annonc/mdq759
[6] Chu TQ, Li R, Shao MH, et al. RAD18 polymorphisms are associated with platinum-based chemotherapy toxicity in Chinese patients with non-small cell lung cancer[J]. ActaPharmacol Sin, 2016, 37(11): 1490-1498.
[7] Deek MP, Kim S, Ahmed I, et al. Prognostic Impact of Missed Chemotherapy Doses During Chemoradiation Therapy for Non-Small Cell Lung Cancer[J]. Am J Clin Oncol, 2018, 41(4): 362-366. doi: 10.1097/COC.0000000000000293
[8] Greillier L, Tomasini P, Barlesi F. The clinical utility of tumor mutational burden in non-small cell lung cancer[J]. Transl Lung Cancer Res, 2018, 7(6): 639-646.
[9] Liang H, Wang M. Prospect of immunotherapy combined with anti-angiogenic agents in patients with advanced non-small cell lung cancer[J]. Cancer Manag Res, 2019, 11: 7707-7719. doi: 10.2147/CMAR.S212238
[10] 朱文锋. 证素辨证学[M]. 北京: 人民卫生出版社, 2008: 88-90. Zhu WF. Syndrome element Differentiation[M]. Beijing: People's Medical Publishing House, 2008: 88-90.
[11] 中华人民共和国卫生部. 中药新药临床研究指导原则(试行)[M]. 北京: 中国医药科技出版社, 2002: 216-224. Ministry of Health of the People's Republic of China. Guidelines for clinical research of New Chinese Medicine (Trial)[M]. Beijing: China Medical Science and Technology Press, 2002: 216-224.
[12] 宣立功, 肖琳, 姚蕾. 安罗替尼对晚期非小细胞肺癌患者中医证型及演变特征的影响[J]. 中国临床研究, 2021, 34(7): 950-953. doi: 10.13429/j.cnki.cjcr.2021.07.020 Xuan LG, Xiao L, Yao L. Effect of anlotinib on TCM syndrome types and evolution characteristics of patients with advanced non-small cell lung cancer[J]. Zhongguo Lin Chuang Yan Jiu, 2021, 34(7): 950-953. doi: 10.13429/j.cnki.cjcr.2021.07.020
[13] 王露, 冯贞贞, 张东, 等. 基于数据挖掘的非小细胞肺癌中医证素分布规律及其特征研究[J]. 时珍国医国药, 2021, 32(7): 1772-1775. https://www.cnki.com.cn/Article/CJFDTOTAL-SZGY202107069.htm Wang L, Feng ZZ, Zhang D, et al. Study on the distribution law and characteristics of TCM syndromes of non-small cell lung cancer based on data mining[J]. Shizhen Guo Yi Guo Yao, 2021, 32(7): 1772-1775. https://www.cnki.com.cn/Article/CJFDTOTAL-SZGY202107069.htm
[14] Merino DM, McShane LM, Fabrizio D, et al. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phaseⅠ of the Friends of Cancer Research TMB Harmonization Project[J]. J Immunother Cancer, 2020, 8(1): e00014.
[15] 凌徐心仪, 张瑶, 钟华. 非小细胞肺癌免疫治疗获益人群筛选的研究进展[J]. 上海交通大学学报(医学版), 2021, 41(8): 1114-1119. https://www.cnki.com.cn/Article/CJFDTOTAL-SHEY202108020.htm Ling XXY, Zhang Y, Zhong H. Research progress in screening non-small cell lung cancer patients who benefit from immunotherapy[J]. Shanghai Jiao Tong Da Xue Xue Bao (Yi Xue Ban), 2021, 41(8): 1114-1119. https://www.cnki.com.cn/Article/CJFDTOTAL-SHEY202108020.htm
[16] Wang Y, Tong Z, Zhang W, et al. FDA-Approved and Emerging Next Generation Predictive Biomarkers for Immune Checkpoint Inhibitors in Cancer Patients[J]. Front Oncol, 2021, 11(8): 68341.
[17] Bie F, Tian H, Sun N, et al. Research Progress of Anti-PD-1/PD-L1 Immunotherapy Related Mechanisms and Predictive Biomarkers in NSCLC[J]. Front Oncol, 2022, 12(2): 769124.
[18] Darabi S, Braxton DR, Eisenberg BL, et al. Predictive Biomarkers for Immunotherapy Response Beyond PD-1/PD-L1[J]. Oncology (Williston Park), 2020, 34(8): 321-327. doi: 10.46883/ONC.3408.321
[19] Han Q, Liu S, Cui Z, et al. Case Report and Literature Review: Diagnosis, Tailored Genetic Counseling and Cancer Prevention for a Locally Advanced dMMR/MSI-H/TMB-H Lung Cancer Patient With Concurrent Lynch Syndrome Mediated by a Rare PMS2 Splicing Variant (c. 1144+ 1G > A)[J]. Front Genet, 2022, 12: 799807. doi: 10.3389/fgene.2021.799807
[20] De Marchi P, Berardinelli GN, Cavagna R, et al. EP1.04-11 Frequency of Microsatellite Instability (MSI) in Brazilian TKI non-treatable Non-Small Cell Lung Cancer (NSCLC) patients[J]. J Thorac Oncol, 2019, 14(10): S973.
[21] Lantuejoul S, Sound-Tsao M, Cooper WA, et al. PD-L1 testing for lung cancer in 2019: perspective from the IASLC Pathology Committee[J]. J Thorac Oncol, 2020, 15(4): 499-519. doi: 10.1016/j.jtho.2019.12.107
[22] 王军委, 许昱, 魏素菊. 肺癌免疫治疗中肿瘤突变负荷(TMB)临床指导意义的研究进展[J]. 中国免疫学杂志, 2019, 35(14): 1784-1790. https://www.cnki.com.cn/Article/CJFDTOTAL-ZMXZ201914026.htm Wang JW, Xu Y, Wei SJ. Research progress of tumor mutation burden (TMB) in lung cancer immune-therapy[J]. Zhongguo Mian Yi Xue Za Zhi, 2019, 35(14): 1784-1790. https://www.cnki.com.cn/Article/CJFDTOTAL-ZMXZ201914026.htm
[23] Goodman AM, Kato S, Bazhenova L, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers[J]. Mol Cancer Ther, 2017, 16(11): 2598-2608. doi: 10.1158/1535-7163.MCT-17-0386
[24] Zheng Y, Yao M, Yang Y. Higher Tumor Mutation Burden Was a Predictor for Better Outcome for NSCLC Patients Treated with PD-1 Antibodies: A Systematic Review and Meta-analysis[J]. SLAS Technol, 2021, 26(6): 605-614. doi: 10.1177/24726303211024557
[25] 权阳. 非小细胞肺癌PD-L1表达状态与临床病理特征及免疫治疗疗效的相关性研究[D]. 西安医学院, 2020. Quan Y. Correlation between PD-L1 expression in non-small cell lung cancer and clinicopathological features and immunotherapy efficacy[D]. Xi'an Medical College, 2020.
[26] Zhou Y, Wang C, Jiang Y, et al. Clinical and molecular characteristics associated with survival among cancer patients receiving first-line anti-PD-1/PD-L1-based therapies[J]. Biomarkers, 2020, 25(6): 441-448. doi: 10.1080/1354750X.2020.1794042
[27] Yang F, Wang JF, Wang Y, et al. Comparative Analysis of Predictive Biomarkers for PD1/PD-L1 Inhibitors in Cancers: Developments and Challenges[J]. Cancers (Basel), 2021, 14(1): 109. doi: 10.3390/cancers14010109
[28] 陈鸿志, 周永慧, 李燕, 等. 免疫检查点抑制剂联合放化疗治疗非小细胞肺癌的疗效预测标志物研究进展[J]. 临床肺科杂志, 2022, 27(1): 109-113. https://www.cnki.com.cn/Article/CJFDTOTAL-LCFK202201024.htm Chen HZ, Zhou YH, Li Y, et al. Research progress on prognostic markers of immune checkpoint inhibitors combined with radiotherapy and chemotherapy for non-small cell lung cancer[J]. Lin Chuang Fei Ke Za Zhi, 2022, 27(1): 109-113. https://www.cnki.com.cn/Article/CJFDTOTAL-LCFK202201024.htm
[29] Shi Y, Lei Y, Liu L, et al. Integration of comprehensive genomic profiling, tumor mutational burden, and PD-L1 expression to identify novel biomarkers of immunotherapy in non-small cell lung cancer[J]. Cancer Med, 2021, 10(7): 2216-2231. doi: 10.1002/cam4.3649
[30] Li Y, Zhang Z, Hu Y, et al. Pretreatment Neutrophil-to-Lymphocyte Ratio (NLR) May Predict the Outcomes of Advanced Non-small-cell Lung Cancer (NSCLC) Patients Treated With Immune Checkpoint Inhibitors (ICIs)[J]. Front Oncol, 2020, 10(6): 654.
[31] Shirasawa M, Yoshida T, Shimoda Y, et al. Differential Immune-Related Microenvironment Determines Programmed Cell Death Protein-1/Programmed Death-Ligand 1 Blockade Efficacy in Patients With Advanced NSCLC[J]. J Thorac Oncol, 2021, 16(12): 2078-2090.
[32] 农靖颖, 顾艳斐, 张毅. 晚期非小细胞肺癌免疫治疗预测生物标志物的研究进展[J]. 中华肺部疾病杂志(电子版), 2021, 14(4): 536-538. https://www.cnki.com.cn/Article/CJFDTOTAL-ZFBD202104040.htm Nong JY, Gu YF, Zhang Y. Research progress on biomarkers for immunotherapy prediction of advanced non-small cell lung cancer[J]. Zhonghua Fei Bu Ji Bing Za Zhi (Dian Zi Ban), 2021, 14(4): 536-538. https://www.cnki.com.cn/Article/CJFDTOTAL-ZFBD202104040.htm
[33] 杨丽. 晚期非小细胞肺癌患者免疫细胞变化与中医证型的相关性研究[D]. 新疆医科大学, 2008. Yang L. Study on the correlation between immune cell changes and TCM syndromes in patients with advanced non-small cell lung cancer[D]. Xinjiang Medical University, 2008.
[34] Powell SF, Rodríguez-Abreu D, Langer CJ, et al. Outcomes With Pembrolizumab Plus Platinum-Based Chemotherapy for Patients With NSCLC and Stable Brain Metastases: Pooled Analysis of KEYN0TE-021, -189, and -407[J]. J Thorac Oncol, 2021, 16(11): 1883-1892.
[35] Rodríguez-Abreud D, Powell SF, Hochmair MJ, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189[J]. Ann Oncol, 2021, 32(7): 881-895.
-
期刊类型引用(7)
1. 秦浩然,朱红. 胰腺癌术后帕博利珠单抗免疫治疗联合化疗对预后的影响. 现代医学与健康研究电子杂志. 2023(08): 80-82 .  百度学术
百度学术
2. 罗旭,党会芬,王千千,王玉凤,田迎霞. 免疫、靶向及节拍化疗综合治疗晚期胰腺癌1例. 甘肃医药. 2023(04): 376-378+381 .  百度学术
百度学术
3. 段伯焘,赵学凯,高倩倩,逯永晋,周磊. 2020年版与2018年版中国胰腺癌综合诊治指南比较分析. 中国普通外科杂志. 2022(03): 287-294 .  百度学术
百度学术
4. 李政晓,薛彩强,赵君. 影像组学在肿瘤微环境评估中的研究进展. 兰州大学学报(医学版). 2022(08): 76-79 .  百度学术
百度学术
5. 雷洋洋,王小林. “精准医学”模式下胰腺癌防治的探索与进展. 复旦学报(医学版). 2021(02): 255-260 .  百度学术
百度学术
6. 董静,牛昆,庞丽然,江一帆. 免疫治疗相关单克隆抗体靶点在三阴性乳腺癌中的研究现状. 现代肿瘤医学. 2021(22): 4044-4049 .  百度学术
百度学术
7. 任刚,夏廷毅,王颖杰. 如何发挥放疗在胰腺癌治疗中的作用. 肿瘤防治研究. 2021(11): 989-993 .  本站查看
本站查看
其他类型引用(7)



 下载:
下载: