-
摘要:目的
探讨光动力治疗对胆道恶性梗阻的疗效和安全性。
方法回顾性分析我中心接受经皮光动力治疗的胆管恶性梗阻患者临床资料,根据是否联合介入、靶向或免疫治疗分为光动力组和联合组。观察治疗后肝功能变化、胆道通畅时间及术后1月内并发症。
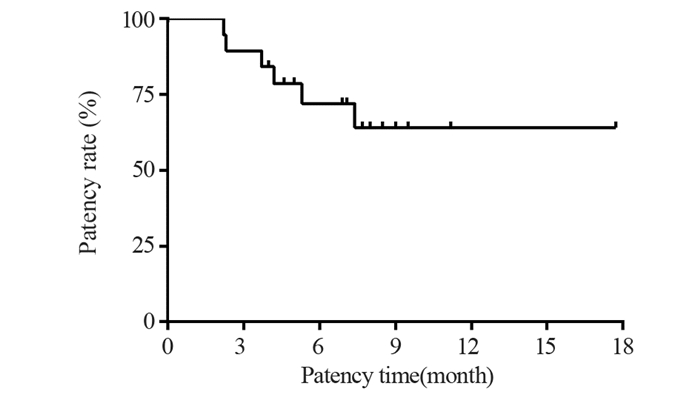
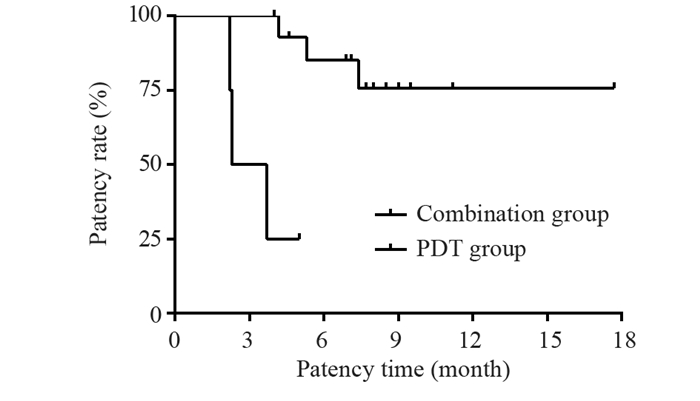
结果共入组19位患者,光动力治疗成功率达100%。术后1月未观察到肝功能下降。最长随访时间17.7月,术后1、3、6和12月胆道通畅率分别为100%、89.5%、72%和64%,平均胆道通畅时间约6.9±0.8月(95%CI: 5.2~8.7月)。Bismuth Ⅲ型胆道通畅时间7.5±1.1月,Bismuth Ⅳ型胆道通畅时间6.1±1.3月。单纯光动力治疗组胆道通畅时间约3.3±0.7月,联合治疗组患者胆道通畅时间约7.9±0.9月,两组差异具有统计学意义(P=0.017)。
结论光动力治疗Bismuth Ⅲ-Ⅳ型胆道恶性梗阻安全有效,联合全身治疗可使胆道通畅时间显著延长。
Abstract:ObjectiveTo investigate the safety and efficacy of photodynamic therapy (PDT) for malignant obstruction of the biliary tract.
MethodsWe retrospectively analyzed the clinical data of patients with malignant biliary obstruction treated by PDT in our medical center. On the basis of different treatment plans, the patients were categorized into the photodynamic only group and the combined treatment group, in which additional interventional operations, targeted therapy, or immunotherapy were arranged. The alterations in liver function, duration of biliary patency, and postoperative complications that occurred within one month were closely monitored in both groups.
ResultsA total number of 19 patients were enrolled in this study. The technical success rate of PDT was 100%. The deterioration of liver function was not observed in any patients within one month after PDT. Within a maximum of 17.7 months follow-up, the patency rates of the biliary tract were 100.0%, 89.5%, 72%, and 64% at 1, 3, 6, and 12 months after the procedure, respectively. The mean biliary patency time was 6.9±0.8 months (95%CI: 5.2-8.7 months). Specifically, the biliary patency times for Bismuth type Ⅲ and Ⅳ were 7.5±1.1 and 6.1±1.3 months, respectively. The biliary patency time was around 3.3±0.7 months in the photodynamic only group and 7.9±0.9 months in the combined treatment group (P=0.017).
ConclusionPDT for Bismuth Ⅲ-Ⅳ malignant biliary obstruction is safe and effective. Moreover, the period of biliary patency is greatly extended when PDT is combined with systemic therapy.
-
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:尉建安:数据收集与统计分析、图表制作及论文撰写苏天昊、金龙:参与手术,指导论文撰写及数据分析栗荐、杨思维:数据收集及图表制作魏建:参与手术,随访患者
-
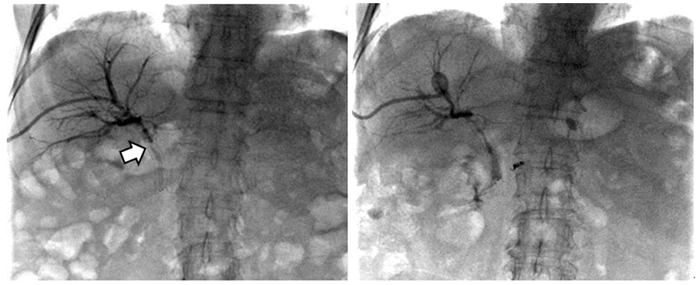
图 1 患者老年女性,胆道恶性肿瘤合并胆道狭窄,支架后再次出现皮肤巩膜黄染,PDT术前胆道造影见支架内充盈缺损(箭头处),对比剂无法顺利通过(左图);PDT治疗1月后再次造影见支架恢复通畅,对比剂可顺利通过并进入肠道内(右图)
Figure 1 An elderly female patient with biliary malignancy complicated with biliary stricture, presented with yellow staining of skin and sclera after stenting. Biliary angiography before photodynamic therapy (PDT) showed a filling defect in the stent (arrow), and the contrast agent could not pass smoothly (left). One month after PDT, the stent was unobstructed again and the contrast agent could pass through and enter the intestine smoothly (right)
-
[1] Xiang S, Lau WY, Chen XP, et al. Hilar cholangiocarcinoma: Controversies on the extent of surgical resection aiming at cure[J]. Int J Colorectal Dis, 2015, 30(2): 159-171. doi: 10.1007/s00384-014-2063-z
[2] Wang HW, Li XJ, Li SJ, et al. Biliary stent combined with iodine-125 seed strand implantation in malignant obstructive jaundice[J]. World J Clin Cases, 2021, 9(4): 801-811. doi: 10.12998/wjcc.v9.i4.801
[3] Zhang X, Mo R, Zhao H, et al. WITHDRAWN: A comparative effectiveness meta-analysis of photodynamic therapy and stent drainage for unresectable cholangiocarcinoma[J]. Photodiagnosis Photodyn Ther, 2018, S1572-1000(18)30085-1. Online ahead of print.
[4] Dolak W, Schwaighofer H, Hellmich B, et al. Photodynamic therapy with polyhematoporphyrin for malignant biliary obstruction: A nationwide retrospective study of 150 consecutive applications[J]. United European Gastroenterol J, 2017, 5(1): 104-110. doi: 10.1177/2050640616654037
[5] 丁瑜, 李伟, 李彬, 等. 光动力疗法与食管支架置入术改善中晚期食管癌所致吞咽困难的对比分析[J]. 中华医学杂志, 2020, 100(5): 378-381. Ding Y, Li W, Li B, et al. Comparison between photodynamic therapy and interventional esophageal stent implantation in dysphagia caused by advanced esophageal cancer[J]. Zhonghua Yi Xue Za Zhi, 2020, 100(5): 378-381.
[6] 陈士明, 娄玥, 方煊, 等. 光动力疗法联合支架引流与单独支架引流治疗不可切除胆管癌疗效的Meta分析[J]. 中华肝胆外科杂志, 2018, 24(9): 616-621. Chen SM, Lou Y, Fang X, et al. Photodynamic therapy plus biliary stenting versus biliary stenting alone to treat nonresectable ductal cholangiocarcinoma: a Meta-analysis[J]. Zhonghua Gan Dan Wai Ke Za Zhi, 2018, 24(9): 616-621.
[7] Moole H, Tathireddy H, Dharmapuri S, et al. Success of photodynamic therapy in palliating patients with nonresectable cholangiocarcinoma: A systematic review and meta-analysis[J]. World J Gastroenterol, 2017, 23(7): 1278-1288. doi: 10.3748/wjg.v23.i7.1278
[8] Blechacz B. Cholangiocarcinoma: Current Knowledge and New Developments[J]. Gut Liver, 2017, 11(1): 13-26. doi: 10.5009/gnl15568
[9] Olek M, Machorowska-Pieniążek A, Olek K, et al. Photodynamic therapy in the treatment of oral squamous cell carcinoma-The state of the art in preclinical research on the animal model[J]. Photodiagnosis Photodyn Ther, 2021, 34: 102236. doi: 10.1016/j.pdpdt.2021.102236
[10] Galiardi-Campoy AEB, Machado FC, Carvalho T, et al. Effects of photodynamic therapy mediated by emodin in cervical carcinoma cells[J]. Photodiagnosis Photodyn Ther, 2021, 35: 102394. doi: 10.1016/j.pdpdt.2021.102394
[11] Floriano BF, Carvalho T, Lopes TZ, et al. Effect of berberine nanoemulsion Photodynamic therapy on cervical carcinoma cell line[J]. Photodiagnosis Photodyn Ther, 2021, 33: 102174. doi: 10.1016/j.pdpdt.2020.102174
[12] Beltrán Hernández I, Yu Y, Ossendorp F, et al. Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations[J]. J Clin Med, 2020, 9(2): 333. doi: 10.3390/jcm9020333
[13] Mohan BP, Chandan S, Khan SR, et al. Photodynamic Therapy (PDT), Radiofrequency Ablation (RFA) With Biliary Stents in Palliative Treatment of Unresectable Extrahepatic Cholangiocarcinoma: A Systematic Review and Meta-analysis[J]. J Clin Gastroenterol, 2022, 56(2): e153-e160. doi: 10.1097/MCG.0000000000001524
[14] Chen P, Yang T, Shi P, et al. Benefits and safety of photodynamic therapy in patients with hilar cholangiocarcinoma: A meta-analysis[J]. Photodiagnosis Photodyn Ther, 2022, 37: 102712. doi: 10.1016/j.pdpdt.2022.102712
[15] Li Z, Jiang X, Xiao H, et al. Long-term results of ERCP- or PTCS-directed photodynamic therapy for unresectable hilar cholangiocarcinoma[J]. Surg Endosc, 2021, 35(10): 5655-5664. doi: 10.1007/s00464-020-08095-1
[16] Tan EK, Taner T, Heimbach JK, et al. Liver Transplantation for Peri-hilar Cholangiocarcinoma[J]. J Gastrointest Surg, 2020, 24(11): 2679-2685. doi: 10.1007/s11605-020-04721-4
[17] Inchingolo R, Acquafredda F, Ferraro V, et al. Non-surgical treatment of hilar cholangiocarcinoma[J]. World J Gastrointest Oncol, 2021, 13(11): 1696-1708. doi: 10.4251/wjgo.v13.i11.1696
[18] Inchingolo R, Acquafredda F, Ferraro V, et al. Non-surgical treatment of hilar cholangiocarcinoma[J]. World J Gastrointest Oncol, 2021, 13(11): 1696-1708. doi: 10.4251/wjgo.v13.i11.1696
[19] Shin DW, Kim MJ, Lee JC, et al. Gemcitabine Plus Cisplatin Chemotherapy Prolongs the Survival in Advanced Hilar Cholangiocarcinoma: A Large Multicenter Study[J]. Am J Clin Oncol, 2020, 43(6): 422-427. doi: 10.1097/COC.0000000000000682
[20] Zeng FL, Chen JF. Application of Immune Checkpoint Inhibitors in the Treatment of Cholangiocarcinoma[J]. Technol Cancer Res Treat, 2021, 20: 15330338211039952.
[21] Gonzalez-Carmona MA, Bolch M, Jansen C, et al. Combined photodynamic therapy with systemic chemotherapy for unresectable cholangiocarcinoma[J]. Aliment Pharmacol Ther, 2019, 49(4): 437-447. doi: 10.1111/apt.15050
[22] 刘朝莲, 吴宏磊, 徐可. 光动力疗法与抗肿瘤免疫治疗在肿瘤治疗中的应用[J]. 中国肿瘤临床, 2021, 48(1): 35-39. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGZL202101012.htm Liu CL, WU HL, Xu K. Photodynamic therapy and the application of anti-tumor immunotherapy for tumor treatment[J]. Zhongguo Zhong Liu Lin Chuang, 2021, 48(1): 35-39. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGZL202101012.htm
[23] Lobo ACS, Gomes-da-Silva LC, Rodrigues-Santos P, et al. Immune Responses after Vascular Photodynamic Therapy with Redaporfin[J]. J Clin Med, 2019, 9(1): 104. doi: 10.3390/jcm9010104
[24] Luz AFS, Pucelik B, Pereira MM, et al. Translating phototherapeutic indices from in vitro to in vivo photodynamic therapy with bacteriochlorins[J]. Lasers Surg Med, 2018, 50(5): 451-459. doi: 10.1002/lsm.22931



 下载:
下载: