Current Status and Exploration of Digestive Tract Reconstruction After Totally Laparoscopic Radical Gastrectomy for Gastric Cancer
-
摘要:
消化道重建是胃癌手术的关键操作之一,其质量直接影响术后相关并发症的发生及远期的营养状况及生活质量,合理选择完全腹腔镜下胃癌根治术后消化道重建的方式对降低术后并发症及改善术后营养状况及生活质量具有积极意义。本文通过对完全腹腔镜下远端胃切除术、全胃切除术常用的消化道吻合方式的优势及不足进行论述,探索目前可能的最优吻合方式,阐述完全腹腔镜近端胃切除术吻合方式的进展,并对生理学、生物力学重建理论的进展进行介绍。
Abstract:Digestive tract reconstruction is one of the key operations of gastric cancer surgery. Its quality directly affects the occurrence of postoperative complications and long-term nutritional status and quality of life. Reasonable selection of digestive tract reconstruction in totally laparoscopic radical gastrectomy for gastric cancer can significantly reduce postoperative complications and improve postoperative nutritional status and quality of life. This paper discusses the advantages and disadvantages of digestive tract anastomosis used in total laparoscopic distal gastrectomy and total gastrectomy, explores the best possible anastomosis at present, describes the progress of anastomosis in complete laparoscopic proximal gastrectomy, and introduces the progress of physiology and biomechanical reconstruction theory.
-
Key words:
- Radical gastrectomy /
- Digestive tract reconstruction /
- Total laparoscope
-
0 引言
第五版日本《胃癌治疗指南》[1]将胃癌手术划分为标准手术与非标准手术,标准手术是以治愈为目的按照标准所实施的胃切除法,切除2/3以上的胃并实施D2淋巴结清扫,对于cN(+)或T2以上的胃癌,依据肿瘤的部位,通常选择远端胃切除术(distal gastrectomy, DG)或全胃切除术(total gastrectomy, TG)。消化道重建作为胃癌根治术中的关键操作,已衍生出多种多样的重建方式,其质量直接影响术后相关并发症的发生及远期的生活质量。对于远端胃切除术,指南推荐的重建方式有BillrothⅠ式、BillrothⅡ式、Roux-en-Y吻合、空肠间置法;对于全胃切除术,推荐的重建方式有Roux-en-Y吻合、空肠间置法、双通道吻合法,加做空肠储袋的治疗效果仍处于研究之中。随着腔镜技术的发展,腹腔镜胃切除术及D2淋巴结清扫的技术日趋成熟,手术方式也逐渐由腹腔镜辅助向完全腹腔镜发展[2],使得腔镜下的消化道重建方法也日益创新。对于全腹腔镜远端胃切除的消化道重建,进入21世纪以来各国学者先后报道了Delta吻合[3]、Uncut Roux-en-Y吻合[4]、自牵引后离断(self-pulling and latter transected, SPLT)Delta吻合(Delta-SPLT)[5]。全胃切除术后多以Roux-en-Y吻合为基础,衍生出食道-空肠吻合口的Overlap吻合[6]、T-shaped吻合[7]、功能性端端吻合(functional end-to-end anastomotic technique, FEEA)[8]、π式吻合[9]、自牵引后离断(SPLT)[10]吻合等多种方式。本文就完全腹腔镜远端胃及全胃切除术后消化道重建方法、临床应用现状、优势与不足等方面进行阐述,并对近端胃切除术后消化道重建方法进行阐述。
1 远端胃切除术后消化道重建
目前仍以BillrothⅠ式、BillrothⅡ式、Roux-en-Y吻合作为远端胃切除术后重建的常用方法。Jiang等[11]通过Meta分析显示,三种重建术式在术后恢复及并发症方面均无差异,而BillrothⅠ式吻合相较其他两种吻合手术时间更短,操作更简便;Roux-en-Y吻合降低了术后残胃炎的发生率,但Cai等[12]发现Roux-en-Y吻合术后胃排空延迟的发生率更高。So等[13]通过对比Billroth Ⅱ式与Roux-en-Y吻合发现,Roux-en-Y吻合术后残胃炎发生率更低,而在术后远期的营养状况及术后生活质量(quality of life, QOL)方面两者无明显差异。He等[14]通过Meta分析认为,考虑到术后的长期收益,Roux-en-Y吻合是远端胃切除术后更好的选择。目前临床医师在吻合方式选择上仍以自己的习惯与偏好为主,但不可否认三种方式各有利弊。
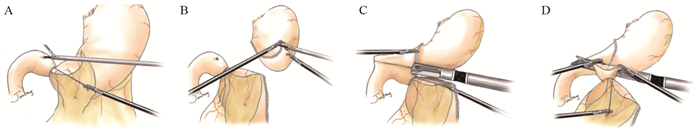
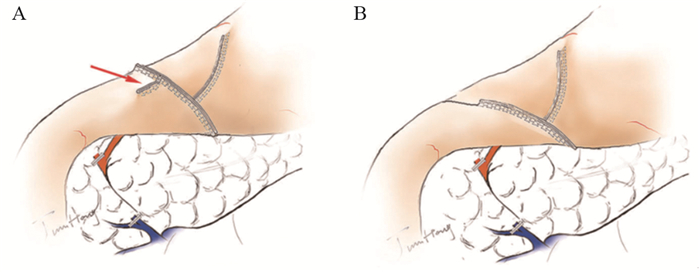
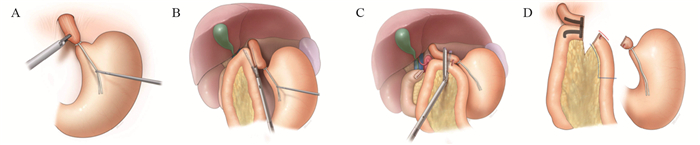
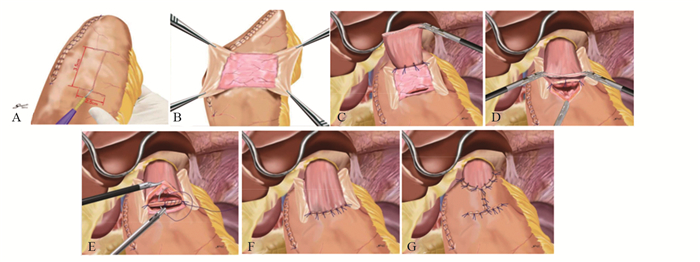
上世纪日韩等国远端胃术后重建方式以BillrothⅠ式为主,这与他们更高的早期胃癌检出率有关[15],更早的分期意味着术中可保留更多的残胃体积,便于行BillrothⅠ式吻合。随着腹腔镜技术及吻合器的发展,2002年Kanaya等[3]首先报道了Delta吻合,利用直线切割闭合器实施完全腹腔镜下残胃与十二指肠后壁的端端功能性BillrothⅠ式吻合,该方法安全易行,术后无显著的倾倒综合征及残胃炎,进食情况好,获得更好的生活质量[16]。Tanimura等[17]于2008年报道的三角吻合(triangulating stapling technique, TST)改进了Delta吻合,其吻合口张力小,适用于残胃容积较小的患者,但操作过程中消化道内容物易溢出而增加腹腔感染的概率[18],因此Omori等[18]于2014年报道了改良的体内三角吻合术(intracorporeal triangulating anastomotic technique, INTACT)并将其应用于单孔腹腔镜远端胃切除术,其优势在于关闭共同开口后,用直线闭合器一并切除胃、十二指肠残端及共同开口的钉线,切除缺血组织而提高了安全性,见图 1[2]。我国蒿汉坤团队于2017年报道了自牵引后离断Delta吻合(Delta SPLT)[5]应用于完全腹腔镜远端胃切除术,通过在幽门处系绳,离断胃体后将远端胃向左下腹牵引,于残胃大弯与十二指肠球之间置入直线闭合器,行残胃后壁与十二指肠长轴侧侧吻合,再关闭共同开口并一并切除远端胃,形成三角吻合,见图 2,其吻合口钉线较Dleta吻合减少,通过小样本研究[19]发现此术式可以节省直线闭合器,操作过程更加简便,相关并发症发生率与Delta吻合相近,见图 3。
![]() 图 2 自牵引后离断Delta吻合[19]Figure 2 Self-pulling and latter transected method (Delta SPLT) anastomosis[19]A: Pylorus tether; B: After severing the gastric body, it was pulled to the left lower abdomen, and a hole in the anterior wall of the duodenal bulb was opened; C: A linear occluder was placed between the greater curvature of the remnant stomach and the duodenal bulb to anastomose the posterior wall of the remnant stomach with the long axis of the duodenum; D: The common opening was closed, and the distal stomach was excised together to form a triangular anastomosis.
图 2 自牵引后离断Delta吻合[19]Figure 2 Self-pulling and latter transected method (Delta SPLT) anastomosis[19]A: Pylorus tether; B: After severing the gastric body, it was pulled to the left lower abdomen, and a hole in the anterior wall of the duodenal bulb was opened; C: A linear occluder was placed between the greater curvature of the remnant stomach and the duodenal bulb to anastomose the posterior wall of the remnant stomach with the long axis of the duodenum; D: The common opening was closed, and the distal stomach was excised together to form a triangular anastomosis.BillrothⅡ式吻合常于BillrothⅠ式吻合张力过大时采用,其操作简单,相比Roux-en-Y吻合保留了肠管的连续性,但其碱性反流性胃炎(alkaline reflux gastritis, ARG)发生率高,加用Braun吻合或转为Roux-en-Y吻合均为降低ARG发生率的可选择术式[20],且Roux-en-Y吻合的效果更加确切[21],但其Roux停滞综合征(roux stasis syndrome, RSS)的发生率达10%~30%[22]。时至今日,BillrothⅡ+Braun与Roux-en-Y哪一种作为更好的吻合方式仍处于探索之中。Uncut Roux-en-Y(URY)吻合于1988年由Van Stiegmann等[23]首次提出,该吻合方式在BillrothⅡ+Braun吻合的基础上以无刀片的直线闭合器闭合了胃-空肠吻合口与Braun吻合口之间的输入袢空肠,防止胆汁及胰液通过胃-空肠吻合口进入残胃,同时保全了空肠神经肌肉的连续性。近年来已有文献[24-25]证实腹腔镜下URY吻合操作简便,能够有效降低RSS和ARG的发生率,且食物残留比例更低,Zhou等[26]通过小样本实验也初步验证了单孔腹腔镜下远端胃切除行URY吻合安全可行,故Uncut Roux-en-Y吻合是远端胃切除术后理想的消化道重建方式。
2 全胃切除术后消化道重建
日本2012年一项问卷调查数据[27]显示,145个医疗机构在全胃切除术后约95%(138个)选择Roux-en-Y重建,1.4%(2个)选择空肠间置法,0.7%(1个)选择双通道吻合重建。淋巴结廓清程度及术后肿瘤复发也是影响重建方式选择的重要条件,对于术后腹膜转移风险高的患者不适宜行间置空肠法,而Roux-en-Y吻合适用于各种条件下的消化道重建[28]。保留十二指肠自然通路的重建方式虽然被提倡,而Yang等[29]通过Meta分析显示,是否保留十二指肠通路在术后并发症、远期生活质量等方面无显著差异,而保留十二指肠通路的重建方式大大延长了手术时间。Roux-en-Y吻合具有操作简便、吻合口少、术后抗反流效果好等特点,仍是全胃切除术后的主流重建方式。
对于吻合器的选择,在传统的开腹及腹腔镜辅助全胃切除术大多选择圆形吻合器行食管-空肠的端侧吻合。腹腔镜辅助手术的吻合方式可采用反穿刺法及OrVil吻合,需借助腹部小切口进行圆形吻合器的置入。在完全腹腔镜手术中,受鞘卡的尺寸限制,普遍选用直线吻合器。Umemura等[30]通过Meta分析发现使用圆形吻合器更易发生吻合口狭窄及吻合口瘘,而直线吻合器在完全腹腔镜操作时难度更高,且对食管残端长度的要求更高,圆形吻合器是否适用于全腹腔镜下的腔内吻合仍需进一步研究[31],有必要开展圆形吻合器与直线吻合器的前瞻性临床研究[28]。
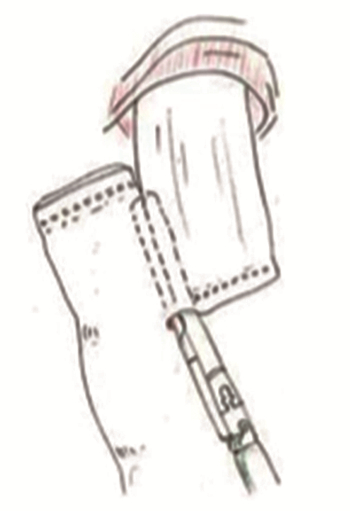
完全腹腔镜下食管-空肠的吻合是完全腹腔镜全胃切除术的关键操作步骤,食管-空肠侧侧吻合是常用的吻合方式,Inaba等[6]于2010年报道了名为Overlap的食管-空肠侧侧吻合方式,见图 4,目前广泛应用于全腹腔镜全胃切除术后的食管-空肠吻合。为了避免可能造成的吻合口狭窄,其吻合口的共同开口使用腔镜下手工缝合关闭[32],这无疑增加了手术操作的难度,以及吻合口易回缩入胸腔造成吻合口瘘引起胸腔感染等严重的并发症,Naiag等[7]于2013年将吻合口改为T形吻合,见图 5[2]。与圆形吻合器所做的食管-空肠端侧吻合类似,T形吻合可以有效防止吻合口缩入胸腔。Ebihara等[8]报道的功能性端端吻合(FEEA)比Overlap操作更加简便且安全可行。Kwon等[9]于2016年报道的π式吻合将胃切除、关闭共同开口及空肠离断三步合为一步,节省时间并能降低成本,见图 6。我国蒿汉坤团队[10]报道的SPLT吻合同样是一种简单而安全的吻合方式,具有一定的发展潜力。这两种术式均可以通过牵拉胃使食管下段良好暴露,便于手术操作,降低了吻合的难度。
![]() 图 6 π式吻合[9]Figure 6 Pi-Shaped anastomosis[9]A: A rope was tied at the cardia and pulled down, and a hole in the lower part of the esophagus was opened; B: Side-to-side anastomosis of the esophagus and jejunum was conducted by a linear occluder; C: The lower segment of the esophagus and jejunum was severed with a linear occluder; D: Π-shaped anastomosis was formed, and jejunum-jejunal anastomosis was continued after removing the total stomach.
图 6 π式吻合[9]Figure 6 Pi-Shaped anastomosis[9]A: A rope was tied at the cardia and pulled down, and a hole in the lower part of the esophagus was opened; B: Side-to-side anastomosis of the esophagus and jejunum was conducted by a linear occluder; C: The lower segment of the esophagus and jejunum was severed with a linear occluder; D: Π-shaped anastomosis was formed, and jejunum-jejunal anastomosis was continued after removing the total stomach.3 近端胃切除术后消化道重建
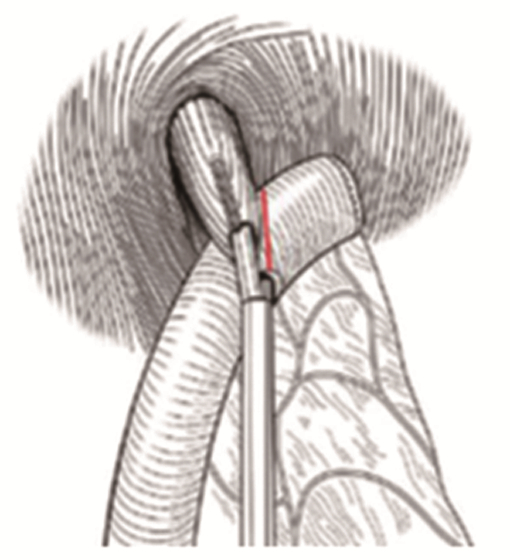
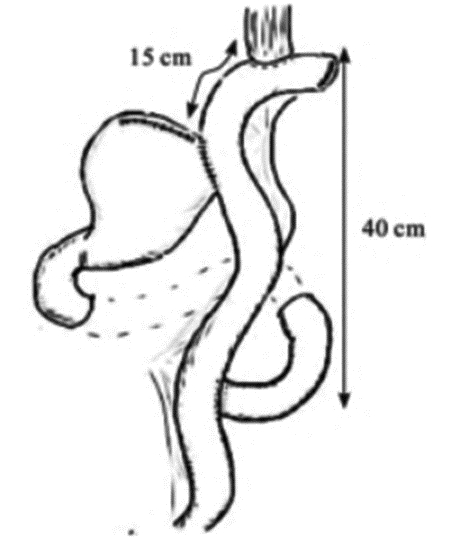
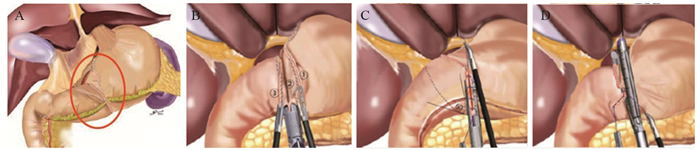
目前广为接受的近端胃切除术的适应证为日本第5版《胃癌治疗指南》[1]中所规定的:切除后能保留1/2远端胃的早期胃癌。近端胃切除保留了部分胃组织,改善术后的营养状况[33],但其吻合口狭窄及反流性食管炎等并发症的发生率高[34]。研究者们致力于降低近端胃切除术后的并发症,传统的吻合方式为食管-残胃吻合,以圆形吻合器将食管下端与残胃后壁进行吻合,术后反流与吻合口狭窄的发生率高[35]。食管-管型胃吻合通过切除胃窦部组织,将残胃改为管状,减少了胃酸的分泌,增加了反流距离,进而降低了反流,有更好的生活质量[36],但切除了更多的胃组织。间置空肠法将一段10~15 cm的小肠间置于食管与残胃之间,可有效预防反流性食管炎,术后营养状况等生活质量也较好,但其操作困难,多用于开腹手术[37-38]。食管胃吻合双肌瓣成形术(Kamikawa法)由Kuroda等[39]于2016年报道,该术式通过将食管残端置入胃壁肌层下隧道内,形成双肌瓣包裹,起到单向阀作用,类似于再造贲门,见图 7,可通过开腹辅助或完全腹腔镜下操作完成,在预防术后反流性食管炎及远期生活质量等方面取得了良好的效果[40-41],但该术式操作相对复杂,国内开展中心较少,其吻合口瘘等手术并发症发生率仍需进一步大样本研究。双通道法由Aikou等[42]报道用于近端胃切除术,见图 8[43],相比全胃切除Roux-en-Y吻合术后安全性无差异,且术后贫血发生率更低[44],并发症发生率不高于传统术式,抗反流功能及术后营养状况更好[45-46],同样适用于完全腹腔镜下操作[47],是近端胃切除术后的理想吻合方式。但其吻合口多、操作相对复杂、学习曲线较长以及各吻合口之间的距离、残胃是否固定、淋巴结清扫等相关问题仍需进一步研究,其抗反流效果尚无高级别循证医学证据支持,需大样本多中心的对照研究数据支持[48]。
![]() 图 7 食管胃吻合双肌瓣成形术[39]Figure 7 Double-Flap Technique as an Antireflux Procedure[39]A: H-shaped mark was made on the anterior wall of remnant stomach, with a size of 2.5cm×3.5cm, 3-4cm from the top; B: The muscle flap was dissected between the mucosal layer and the muscular layer; C: Suture the posterior wall of the lower esophagus and the upper edge of the muscle flap; D: Fix the opening between the lower esophagus and the mucosal layer of the gastric wall; E: Continuous suture anastomosis; F: Suture anterior wall; G: The anastomosis was covered and sutured with double seromuscular flaps.
图 7 食管胃吻合双肌瓣成形术[39]Figure 7 Double-Flap Technique as an Antireflux Procedure[39]A: H-shaped mark was made on the anterior wall of remnant stomach, with a size of 2.5cm×3.5cm, 3-4cm from the top; B: The muscle flap was dissected between the mucosal layer and the muscular layer; C: Suture the posterior wall of the lower esophagus and the upper edge of the muscle flap; D: Fix the opening between the lower esophagus and the mucosal layer of the gastric wall; E: Continuous suture anastomosis; F: Suture anterior wall; G: The anastomosis was covered and sutured with double seromuscular flaps.4 生理学、生物力学重建的探索
胃切除术破坏了消化道的解剖结构及延续性,消化道重建的关注点不仅在于重建解剖结构,更应使其符合生理,对于降低术后反流及增强远期营养状况具有积极意义。空肠储袋的研究是该理论的一次探索。全胃切除术后消化道丧失了储存食物的功能,原有的生理反射功能受损,易出现营养障碍。Ishigami等[49]在全胃切除Roux-en-Y吻合的基础上于空肠远端增加8 cm的空肠储袋,相比不增加空肠储袋的患者组,其术后6个月的BMI与术后1年的食物摄入均明显提高。其他研究者通过研究也发现增加空肠储袋的患者恢复更快、体质更好,并且操作不会提高手术并发症的发生率[50-51]。然而也有学者认为[31],制作空肠储袋的过程切断了小肠的轮状肌,损害了小肠的收缩蠕动,降低了肠壁的张力,以及制作过长的空肠储袋可能影响储袋的排空,空肠储袋的最佳长度与排空能力是该手术方式的制约点[28],消化道重建中是否增加储袋仍需要大样本、更严谨的临床试验[28]。基于生理学、生物力学的研究,日本学者池田正视设计了一种His角,假穹窿成形,逆J型间置空肠储袋的重建方法[52],结果显示其腹痛、进食不适、消化不良、倾倒综合征发生率均低,无储袋扩张和反流食管炎发生。食管和空肠储袋吻合保留腹段食管,制作His角、假穹窿以及逆J型肠襻小肠的顺蠕动具有防止反流效果。同样在近端胃术后重建中,将吻合口位置改为残胃前壁,并将残胃固定于膈肌脚。重建胃底结构及His角在一定程度上减缓了食管-残胃吻合后反流的发生[53],也是生理学、生物力学重建上的一次探索。
5 展望
随着医疗技术与药物的协同发展,胃癌手术后患者的生存期进一步延长,对消化道重建带来的生活质量上的要求也随之提高。腹腔镜微创手术的技术进展对医生提出了新的考验,在注重技术提高的同时,应更多的关注生命的质量,使患者在治疗疾病的过程中最大获益。
Competing interests: The authors declare that they have no competing interests.作者贡献:王子豪:资料收集、文献查阅、文章撰写熊治国:文章修改及审校 -
-
[1] 日本胃癌学会. 胃癌治疗指南[M]. 5版. 东京: 金原出版株式会社, 2018. Japanese Society of Gastric Cancer. Guidelines for treatment of gastric cancer[M]. 5th ed. Tokyo: Kinbara Publishing, 2018.
[2] 李国新, 陈韬. 全腹腔镜胃癌根治术及消化道重建发展现状与前景[J]. 中国实用外科杂志, 2016, 36(9): 929-934. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWK201609002.htm Li GX, Chen T. The current situation and development prospect of digestive tract reconstruction after totally laparoscopic gastrectomy for gastric cancer[J]. Zhongguo Shi Yong Wai Ke Za Zhi, 2016, 36(9): 929-934. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWK201609002.htm
[3] Kanaya S, Gomi T, Momoi H, et al. Delta-shaped anastomosis in totally laparoscopic BillrothⅠ gastrectomy: new technique of intraabdominal gastroduodenostomy[J]. J Am Coll Surg, 2002, 195(2): 284-287. doi: 10.1016/S1072-7515(02)01239-5
[4] Uyama I, Sakurai Y, Komori Y, et al. Laparoscopy-assisted uncut Roux-en-Y operation after distal gastrectomy for gastric cancer[J]. Gastric Cancer, 2005, 8(4): 253-257. doi: 10.1007/s10120-005-0344-5
[5] Hong J, Wang YP, Wang J, et al. A novel method of self-pulling and latter transected delta-shaped Billroth-Ⅰ anastomosis in totally laparoscopic distal gastrectomy[J]. Surg Endosc, 2017, 31(11): 4831. doi: 10.1007/s00464-017-5532-y
[6] Inaba K, Satoh S, Ishida Y, et al. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy[J]. J Am Coll Surg, 2010, 211(6): e25-e29. doi: 10.1016/j.jamcollsurg.2010.09.005
[7] Nagai E, Ohuchida K, Nakata K, et al. Feasibility and safety of intracorporeal esophagojejunostomy after laparoscopic total gastrectomy: inverted T-shaped anastomosis using linear staplers[J]. Surgery, 2013, 153(5): 732-738. doi: 10.1016/j.surg.2012.10.012
[8] Ebihara Y, Okushiba S, Kawarada Y, et al. Outcome of functional end-to-end esophagojejunostomy in totally laparoscopic total gastrectomy[J]. Langenbecks Arch Surg, 2013, 398(3): 475-479. doi: 10.1007/s00423-013-1051-z
[9] Kwon IG, Son YG, Ryu SW. Novel Intracorporeal Esophagojejunostomy Using Linear Staplers During Laparoscopic Total Gastrectomy: pi-Shaped Esophagojejunostomy, 3-in-1 Technique[J]. J Am Coll Surg, 2016, 223(3): e25-e29. doi: 10.1016/j.jamcollsurg.2016.06.011
[10] Hong J, Wang YP, Wang J, et al. A novel method of self-pulling and latter transected reconstruction in totally laparoscopic total gastrectomy: feasibility and short-term safety[J]. Surg Endosc, 2017, 31(7): 2968-2976. doi: 10.1007/s00464-016-5314-y
[11] Jiang H, Li Y, Wang T. Comparison of Billroth I, Billroth Ⅱ, and Roux-en-Y reconstructions following distal gastrectomy: A systematic review and network meta-analysis[J]. Cir Esp (Engl Ed), 2021, 99(6): 412-420. doi: 10.1016/j.ciresp.2020.09.003
[12] Cai Z, Zhou Y, Wang C, et al. Optimal reconstruction methods after distal gastrectomy for gastric cancer: A systematic review and network meta-analysis[J]. Medicine (Baltimore), 2018, 97(20): e10823. doi: 10.1097/MD.0000000000010823
[13] So JB, Rao J, Wong AS, et al. Roux-en-Y or Billroth Ⅱ Reconstruction After Radical Distal Gastrectomy for Gastric Cancer: A Multicenter Randomized Controlled Trial[J]. Ann Surg, 2018, 267(2): 236-242. doi: 10.1097/SLA.0000000000002229
[14] He L, Zhao Y. Is Roux-en-Y or Billroth-Ⅱ reconstruction the preferred choice for gastric cancer patients undergoing distal gastrectomy when Billroth I reconstruction is not applicable? A meta-analysis[J]. Medicine (Baltimore), 2019, 98(48): e17093. doi: 10.1097/MD.0000000000017093
[15] Yoshino K. History of gastric cancer surgery[J]. Nihon Geka Gakkai Zasshi, 2000, 101(12): 855-860.
[16] Kanaya S, Kawamura Y, Kawada H, et al. The delta-shaped anastomosis in laparoscopic distal gastrectomy: analysis of the initial 100 consecutive procedures of intracorporeal gastroduodenostomy[J]. Gastric Cancer, 2011, 14(4): 365-371. doi: 10.1007/s10120-011-0054-0
[17] Tanimura S, Higashino M, Fukunaga Y, et al. Intracorporeal Billroth 1 reconstruction by triangulating stapling technique after laparoscopic distal gastrectomy for gastric cancer[J]. Surg Laparosc Endosc Percutan Tech, 2008, 18(1): 54-58. doi: 10.1097/SLE.0b013e3181568e63
[18] Omori T, Masuzawa T, Akamatsu H, et al. A simple and safe method for Billroth I reconstruction in single-incision laparoscopic gastrectomy using a novel intracorporeal triangular anastomotic technique[J]. J Gastrointest Surg, 2014, 18(3): 613-616. doi: 10.1007/s11605-013-2419-7
[19] Hong J, Wang YP, Wang J, et al. The safety and feasibility of intra-corporeal gastroduodenostomy using a self-pulling and latter transected method (Delta SPLT) in totally laparoscopic distal gastrectomy[J]. J Surg Oncol, 2021, 123 Suppl 1: S25-S29.
[20] 李树春, 臧露. 远端胃切除术BillrothⅡ+Braun吻合的研究进展[J]. 中华胃肠外科杂志, 2018, 21(8): 956-960. doi: 10.3760/cma.j.issn.1671-0274.2018.08.023 Li SC, Zang L. Research advance in Billroth Ⅱ with Braun anastomosis after distal gastrectomy[J]. Zhonghua Wei Chang Wai Ke Za Zhi, 2018, 21(8): 956-960. doi: 10.3760/cma.j.issn.1671-0274.2018.08.023
[21] Nimeri A, Al ST, Maasher A. Conversion of one anastomosis gastric bypass/mini gastric bypass to Roux-en-Y gastric bypass for bile reflux gastritis after failed Braun jejunojejunostomy[J]. Surg Obes Relat Dis, 2017, 13(2): 361-363. doi: 10.1016/j.soard.2016.10.022
[22] Masui T, Kubora T, Nakanishi Y, et al. The flow angle beneath the gastrojejunostomy predicts delayed gastric emptying in Roux-en-Y reconstruction after distal gastrectomy[J]. Gastric Cancer, 2012, 15(3): 281-286. doi: 10.1007/s10120-011-0107-4
[23] Van Stiegmann G, Goff JS. An alternative to Roux-en-Y for treatment of bile reflux gastritis[J]. Surg Gynecol Obstet, 1988, 166(1): 69-70.
[24] Wang Q, Ni Q, Yang K, et al. Laparoscopic uncut Roux-en-Y for radical distal gastrectomy: the study protocol for a multirandomized controlled trial[J]. Cancer Manag Res, 2019, 11: 1697-1704. doi: 10.2147/CMAR.S170355
[25] Sah BK, Li J, Yan C, et al. Anastomosis for distal gastrectomy in Chinese patients: uncut roux-Y or roux-Y?[J]. BMC Surg, 2020, 20(1): 7. doi: 10.1186/s12893-019-0672-8
[26] Zhou W, Dong CZ, Zang YF, et al. Initial experience of single-incision plus one port left-side approach totally laparoscopic distal gastrectomy with uncut Roux-en-Y reconstruction[J]. World J Gastroenterol, 2020, 26(31): 4669-4679. doi: 10.3748/wjg.v26.i31.4669
[27] Kumagai K, Shimizu K, Yokoyama N, et al. Questionnaire survey regarding the current status and controversial issues concerning reconstruction after gastrectomy in Japan[J]. Surg Today, 2012, 42(5): 411-418. doi: 10.1007/s00595-012-0159-z
[28] 胡祥. 全腹腔镜全胃切除术消化道重建的热点问题和理性思考[J]. 中华消化外科杂志, 2020, 19(9): 925-930. doi: 10.3760/cma.j.cn115610-20200826-00585 Hu X. Hot issues and rational reflection on digestive tract reconstruction after totally laparoscopic total gastrectomy[J]. Zhonghua Xiao Hua Wai Ke Za Zhi, 2020, 19(9): 925-930. doi: 10.3760/cma.j.cn115610-20200826-00585
[29] Yang YS, Chen LQ, Yan XX, et al. Preservation versus non-preservation of the duodenal passage following total gastrectomy: a systematic review[J]. J Gastrointest Surg, 2013, 17(5): 877-886. doi: 10.1007/s11605-013-2174-9
[30] Umemura A, Koeda K, Sasaki A, et al. Totally laparoscopic total gastrectomy for gastric cancer: literature review and comparison of the procedure of esophagojejunostomy[J]. Asian J Surg, 2015, 38(2): 102-112. doi: 10.1016/j.asjsur.2014.09.006
[31] 蔡清萍. 合理选择全胃切除术后的消化道重建方式[J]. 上海医药, 2021, 42(11): 6-11. doi: 10.3969/j.issn.1006-1533.2021.11.003 Cai QP. Reasonable selection of digestive tract reconstruction methods after total gastrectomy[J]. Shanghai Yi Yao, 2021, 42(11): 6-11. doi: 10.3969/j.issn.1006-1533.2021.11.003
[32] 中华医学会外科学分会. 腹腔镜胃外科手术缝合技术与缝合材料选择中国专家共识(2021版)[J]. 中国实用外科杂志, 2021, 41(5): 495-503. doi: 10.19538/j.cjps.issn1005-2208.2021.05.03 Chinese Society of Surgery, Chinese Medical Association. Chinese expert consensus on suture technique and material selection in laparoscopic gastric surgery(2021 edition)[J]. Zhongguo Shi Yong Wai Ke Za Zhi, 2021, 41(5): 495-503. doi: 10.19538/j.cjps.issn1005-2208.2021.05.03
[33] Ohashi M, Morita S, Fukagawa T, et al. Functional Advantages of Proximal Gastrectomy with Jejunal Interposition Over Total Gastrectomy with Roux-en-Y Esophagojejunostomy for Early Gastric Cancer[J]. World J Surg, 2015, 39(11): 2726-2733. doi: 10.1007/s00268-015-3180-8
[34] An JY, Youn HG, Choi MG, et al. The difficult choice between total and proximal gastrectomy in proximal early gastric cancer[J]. Am J Surg, 2008, 196(4): 587-591. doi: 10.1016/j.amjsurg.2007.09.040
[35] Hosoda K, Yamashita K, Katada N, et al. Potential benefits of laparoscopy-assisted proximal gastrectomy with esophagogastrostomy for cT1 upper-third gastric cancer[J]. Surg Endosc, 2016, 30(8): 3426-3436. doi: 10.1007/s00464-015-4625-8
[36] Ueda Y, Shiraishi N, Toujigamori M, et al. Laparoscopic Proximal Gastrectomy With Gastric Tube Reconstruction[J]. JSLS, 2016, 20(3): e2016. 00046. doi: 10.4293/JSLS.2016.00046
[37] Yabusaki H, Nashimoto A, Matsuki A, et al. Evaluation of jejunal pouch interposition after proximal gastrectomy for early gastric cancer in the upper third of the stomach[J]. Hepatogastroenterology, 2012, 59(119): 2032-2036.
[38] Zhao P, Xiao SM, Tang LC, et al. Proximal gastrectomy with jejunal interposition and TGRY anastomosis for proximal gastric cancer[J]. World J Gastroenterol, 2014, 20(25): 8268-8273. doi: 10.3748/wjg.v20.i25.8268
[39] Kuroda S, Nishizaki M, Kikuchi S, et al. Double-Flap Technique as an Antireflux Procedure in Esophagogastrostomy after Proximal Gastrectomy[J]. J Am Coll Surg, 2016, 223(2): e7-e13. doi: 10.1016/j.jamcollsurg.2016.04.041
[40] Tsumura T, Kuroda S, Nishizaki M, et al. Short-term and long-term comparisons of laparoscopy-assisted proximal gastrectomy with esophagogastrostomy by the double-flap technique and laparoscopy-assisted total gastrectomy for proximal gastric cancer[J]. PLoS One, 2020, 15(11): e242223.
[41] Kuroda S, Choda Y, Otsuka S, et al. Multicenter retrospective study to evaluate the efficacy and safety of the double-flap technique as anti-reflux esophagogastrostomy after proximal gastrectomy (rD-FLAP Study)[J]. Ann Gastroenterol Surg, 2019, 3(1): 96-103. doi: 10.1002/ags3.12216
[42] Aikou T, Natsugoe S, Shimazu H, et al. Antrum preserving double tract method for reconstruction following proximal gastrectomy[J]. Jpn J Surg, 1988, 18(1): 114-115. doi: 10.1007/BF02470857
[43] Yamashita K, Iwatsuki M, Koga Y, et al. Preservation of physiological passage through the remnant stomach prevents postoperative malnutrition after proximal gastrectomy with double tract reconstruction[J]. Surg Today, 2019, 49(9): 748-754. doi: 10.1007/s00595-019-01799-5
[44] 樊俊彦, 钱锋, 刘佳佳, 等. 胃上部癌行根治性近端胃切除双通道消化道重建与全胃切除Roux-en-Y消化道重建的临床疗效比较[J]. 中华胃肠外科杂志, 2019, 22(8): 767-773. Fan JY, Qian F, Liu JJ, et al. Comparison of clinical efficacy between proximal gastrectomy with double tract reconstruction and total gastrectomy with Roux-en-Y reconstruction for proximal gastric cancer[J]. Zhonghua Wei Chuang Wai Ke Za Zhi, 2019, 22(8): 767-773.
[45] Hong J, Wang SY, Hao HK. A Comparative Study of Double-Tract Reconstruction and Roux-en-Y After Gastrectomy for Gastric Cancer[J]. Surg Laparosc Endosc Percutan Tech, 2019, 29(2): 82-89. doi: 10.1097/SLE.0000000000000639
[46] 林宏明, 周俊峰, 王家兴, 等. 腹腔镜辅助近端胃切除双通道吻合在治疗早期近端胃癌中的应用[J]. 中华普通外科杂志, 2019, 34(10): 891-892. https://cdmd.cnki.com.cn/Article/CDMD-10392-1020710054.htm Lin HM, Zhou JF, Wang JX, et al. Application of laparoscopic assisted proximal gastrectomy and double channel anastomosis in the treatment of early proximal gastric cancer[J]. Zhonghua Pu Tong Wai Ke Za Zhi, 2019, 34(10): 891-892. https://cdmd.cnki.com.cn/Article/CDMD-10392-1020710054.htm
[47] 黄昌明, 郑朝辉, 陆俊. 完全腹腔镜胃癌手术消化道重建专家共识及手术操作指南(2018版)[J]. 中国实用外科杂志, 2018, 38(8): 833-839. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWK201808001.htm Huang CM, Zheng CH, Lu J. Expert consensus and operation guidelines for digestive tract reconstruction in total laparoscopic gastric cancer surgery(2018 Edition)[J]. Zhonghua Shi Yong Wai Ke Za Zhi, 2018, 38(8): 833-839. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWK201808001.htm
[48] 李啸文, 王海江, 王盼兴, 等. 近端胃切除术后双通道吻合的研究进展[J]. 现代肿瘤医学, 2021, 29(17): 3109-3113. doi: 10.3969/j.issn.1672-4992.2021.17.034 Li XW, Wang HJ, Wang PX, et al. Research progression and prospect of double-tract reconstruction after proximal gastrectomy[J]. Xian Dai Zhong Liu Yi Xue, 2021, 29(17): 3109-3113. doi: 10.3969/j.issn.1672-4992.2021.17.034
[49] Ishigami S, Aridome K, Arigami T, et al. Roux-en-Y Plus Distal Jejunal Pouch After Total Gastrectomy: A Prospective Study[J]. Anticancer Res, 2018, 38(10): 5837-5841. doi: 10.21873/anticanres.12925
[50] Fein M, Fuchs KH, Thalheimer A, et al. Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy: a randomized trial[J]. Ann Surg, 2008, 247(5): 759-765. doi: 10.1097/SLA.0b013e318167748c
[51] Oida T, Mimatsu K, Kano H, et al. Advantages of jejunal pouch in Roux-en-Y reconstruction[J]. Hepatogastroenterology, 2012, 59(117): 1647-1650.
[52] 胡祥. 日本胃癌外科临床研究和治疗新动向[J]. 中国实用外科杂志, 2020, 40(8): 905-908. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWK202008003.htm Hu X. New trends in clinical research and treatment of gastric cancer surgery in Japan[J]. Zhongguo Shi Yong Wai Ke Za Zhi, 2020, 40(8): 905-908. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWK202008003.htm
[53] 李乐平, 崔怀平, 商亮. 全胃切除与近端胃切除术后消化道重建方式的选择与思考[J]. 中华消化外科杂志, 2021, 20(6): 643-647. Li LP. Cui HP, Shang L. Choice and consideration of digestive tract reconstruction after total gastrectomy and proximal gastrectomy[J]. Zhonghua Xiao Hua Wai Ke Za Zhi, 2021, 20(6): 643-647.
-
期刊类型引用(3)
1. 李强. 左侧后入路和右侧前入路腹腔镜胃癌根治术的临床疗效比较. 中外医学研究. 2024(25): 31-35 .  百度学术
百度学术
2. 于海侠,封安强,谢志远,李德春,邵国庆. X线联合内镜技术在胃癌根治术后吻合口梗阻患者诊疗中的应用研究. 现代消化及介入诊疗. 2024(07): 869-872 .  百度学术
百度学术
3. 曾应文. 胃癌患者腹腔镜术后发生消化道瘘的风险预测模型构建. 现代诊断与治疗. 2023(07): 953-956 .  百度学术
百度学术
其他类型引用(1)



 下载:
下载: