Clinical Importance of BAIAP2L1 Expression in Cervical Cancer and Its Effect on Malignant Phenotype of Cervical Cancer Cells
-
摘要:目的
探讨BAIAP2L1在宫颈癌中的表达特征及其对肿瘤细胞转移的调控作用。
方法基于公共数据库分析BAIAP2L1表达与临床预后的相关性,通过R语言进行GO通路富集及临床病理关联分析。采用基因沉默技术观察BAIAP2L1敲低对宫颈癌细胞增殖、侵袭迁移及上皮间质转化(EMT)的影响。
结果BAIAP2L1在宫颈癌组织中表达显著上调(Padj<0.001),是患者死亡独立风险因素(HR=2.808,P=0.03),且与总生存期、T/N分期及复发转移显著相关(均P<0.05)。基因功能富集显示其参与肿瘤转移相关通路。敲低BAIAP2L1可显著抑制癌细胞增殖、侵袭迁移能力及EMT进程(均P<0.05)。
结论BAIAP2L1在宫颈癌中表达上调,与不良预后及转移密切相关,靶向抑制该基因可有效遏制肿瘤进展。
Abstract:ObjectiveTo explore the expression characteristics of BAIAP2L1 in cervical cancer (CC) and its regulatory role in tumor cell metastasis.
MethodsThe correlation between BAIAP2L1 expression and clinical prognosis was analyzed by using a public database. GO pathway enrichment and clinicopathological correlation analyses were conducted by employing R language. The effect of BAIAP2L1 knockdown on CC cell proliferation, invasion, migration, and epithelial-mesenchymal transition (EMT) were further investigated through gene silencing approaches.
ResultsBAIAP2L1 expression was significantly upregulated in CC tissues (Padj <0.001) and it was identified as an independent risk factor for patient mortality (HR=2.808, P=0.03). Elevated BAIAP2L1 levels showed significant correlations with poor overall survival, advanced T/N stage, recurrence, and metastasis (all P<0.05). Functional enrichment analysis revealed its involvement in tumor metastasis-related pathways. The knockdown of BAIAP2L1 significantly attenuated CC cell proliferation, invasion, and migration and suppressed key EMT processes (all P<0.05).
ConclusionBAIAP2L1 is overexpressed in CC tissues and associated with patient prognosis and metastasis. The targeted inhibition of BAIAP2L1 can effectively curb tumor progression.
-
Key words:
- BAIAP2L1 /
- Cervical cancer /
- Metastasis /
- Prognosis
-
图 2 BAIAP2L1在宫颈癌临床样本中的表达验证
Figure 2 Validation of BAIAP2L1 expression in clinical CC tissues
A: qRT-PCR detection of BAIAP2L1 expression in paired tumors and adjacent normal cervical tissues from 47 patients with CC, V=153, P<0.000 1, r=0.68; B, C: Western blot analysis of BAIAP2L1 expression in paired tumor and adjacent normal tissues from eight patients with CC. V=3, P=0.039 1, r=0.73.
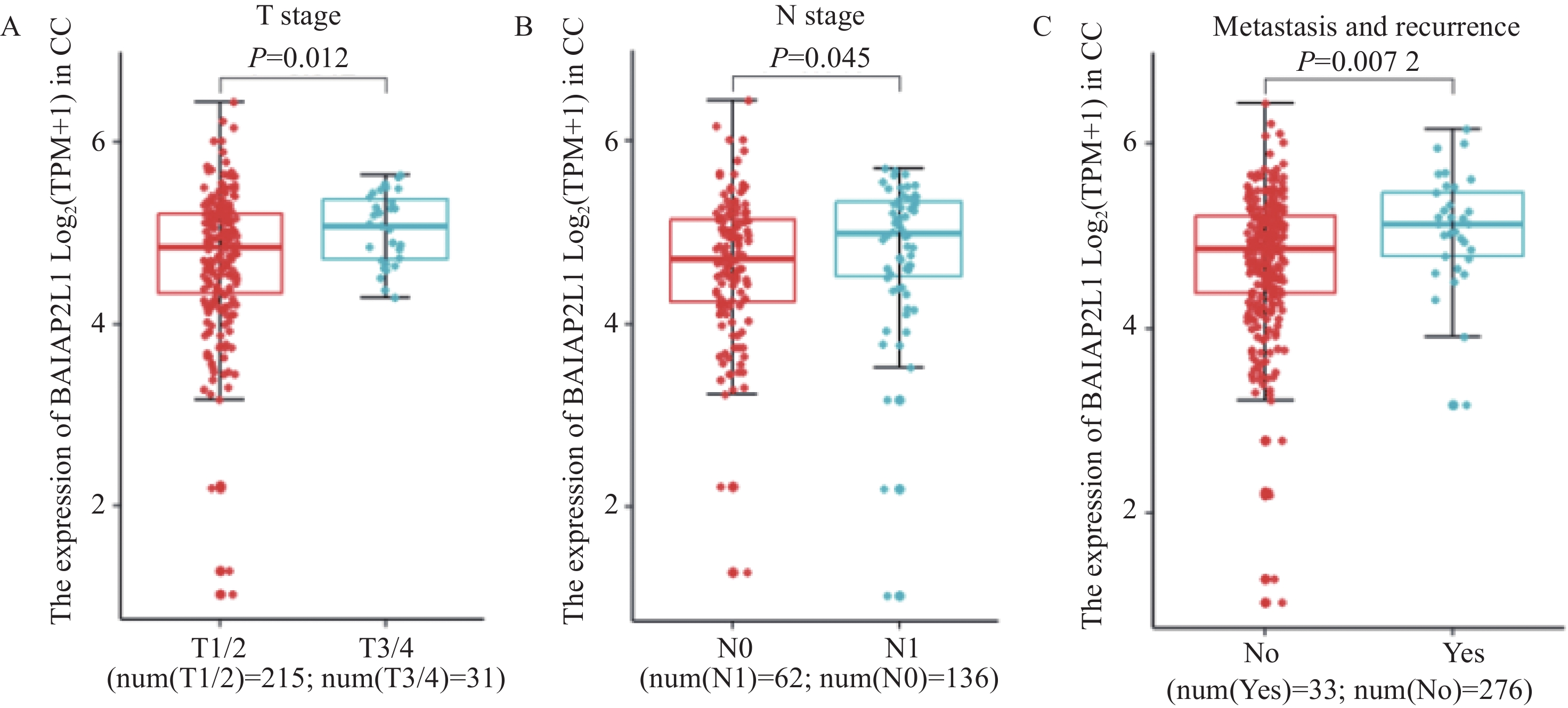
图 3 BAIAP2L1表达与宫颈癌临床病理相关性分析
Figure 3 Correlation between BAIAP2L1 expression and clinicopathological characteristics of CC patients
A-C: expression of BAIAP2L1 mRNA in the T stage (A, W=2398, P=0.012, Cliff's delta=0.28) and N stage (B, W=3465, P=0.045, Cliff's delta=0.18) of CC in the TCGA database and postoperative recurrence and metastasis cases (C, W=3249.5, P=0.007, Cliff's delta=0.29). Box plots: ˉx±s.
图 4 BAIAP2L1表达对细胞侵袭和迁移的影响
Figure 4 Effect of BAIAP2L1 knockdown on CC cell invasion and migration
A, B: Western blot analysis of BAIAP2L1 knockdown in HeLa and SiHa cells (HeLa: NC vs. siRNA-1: t=9.98, df=4, P=0.000 6; NC vs. siRNA-2: t=7.41, df=4, P=0.001 8. SiHa: NC vs. siRNA-1: t=9.37, df=4, P=0.000 7; NC vs. siRNA-2: t=7.34, df=4, P=0.001 8); C-F: Wound healing assay of the migration ability of CC cells upon BAIAP2L1 knockdown (D: NC vs. siRNA-1: t=5.01, df=4, P=0.007; NC vs. siRNA-2: t=6.26, df=4, P=0.003. F: NC vs. siRNA-1: t=5.32, df=4, P=0.006; NC vs. siRNA-2: t=7.92, df=4, P=0.001 4); G-H: Transwell assay of the invasion and migration abilities of CC cells upon BAIAP2L1 knockdown (H: invasion, NC vs. siRNA-1: t=4.96, df=4, P=0.007 7; NC vs. siRNA-2: t=4.56, df=4, P=0.010 3; migration, NC vs. siRNA-1: t=8.21, df=4, P=0.001 2; NC vs. siRNA-2: t=14.6, df=4, P=0.000 1. J: invasion, NC vs. siRNA-1: t=6.00, df=4, P=0.003 9; NC vs. siRNA-2: t=12.69, df=4, P=0.000 2; migration, NC vs. siRNA-1: t=3.29, df=4, P=0.030 3; NC vs. siRNA-2: t=3.87, df=4, P=0.018). Bar graph: mean±s.
图 5 BAIAP2L1表达对宫颈癌细胞增殖(A-B)和克隆(C-E)能力的影响
Figure 5 Effects of BAIAP2L1 expression on the proliferative (A-B) and clonogenic (C-E) capacities of CC cells
A, B: RTCA detection of the proliferation ability of CC cells upon BAIAP2L1 knockdown (A: 64 h NC vs. siRNA-1: t=7.1, df=4, P=0.002 1; NC vs. siRNA-2: t=19.3, df=4, P<0.000 1; B: 64 h NC vs. siRNA-1: t=5.23, df=4, P=0.006 4; NC vs. siRNA-2: t=8.41, df=4, P=0.001 1). C-E: clonogenic assay of the colony formation ability of CC cells upon BAIAP2L1 knockdown (E: HeLa: NC vs. siRNA-1: t=5.72, df=4, P=0.004 6; NC vs. siRNA-2: t=5.98, df=4, P=0.003 9, SiHa: NC vs. siRNA-1: t=11.11, df=4, P=0.000 4; NC vs. siRNA-2: t=19.03, df=4, P<0.000 1). Bar graph: mean±s.
图 6 BAIAP2L1在宫颈癌中的相关通路的富集分析及验证
Figure 6 Enrichment analysis and experimental validation of BAIAP2L1-associated pathways in CC
A: GO enrichment analysis of differentially expressed genes between CC and normal tissues from the TCGA database. B-D: Western blot validation of epithelial-mesenchymal transition markers (N-cadherin, E-cadherin, Vimentin, and ZEB1) upon BAIAP2L1 knockdown in CC cells. E, F: Western blot analysis of AKT signaling pathway components (total AKT and phosphorylated AKT) upon BAIAP2L1 knockdown. Bar graph: mean±s.
表 1 BAIAP2L1差异表达分析部分数据(源自TCGA数据)
Table 1 Partial data of differential expression analysis of BAIAP2L1 (from TCGA data)
Gene symbol log2FC P Padj Change BAIAP2L1 3.057 2.32E−17 2.20E−15 Up RHBDF2 2.179 1.73E−06 2.70E−05 Up ITGB6 5.027 1.88E−09 6.09E−08 Up VDR 2.644 1.92E−08 4.95E−07 Up 表 2 BAIAP2L1表达与临床病理因素的相关性分析 (源自TCGA数据)
Table 2 Correlation of BAIAP2L1 expression with clinical pathological factors (from TCGA database)
Factors BAIAP2L1 expression(n(%)) χ2 r P All High Low Age(years) 2.16 −0.102 0.339 <60 159(76.44) 84(80.77) 75(72.12) ≥60 49(23.56) 20(19.23) 29(27.88) Tumer pathological stage 1.96 0.099 0.376 Ⅰ/Ⅱ 148(73.63) 70(69.31) 78(78.00) Ⅲ/Ⅳ 53(26.37) 31(30.69) 22(22.00) T stage 2.35 0.124 0.309 T1/2 127(83.55) 60(78.95) 67(88.16) T3/4 25(16.45) 16(21.05) 9(11.84) N stage 14.89 0.377 0.001 N0 81(77.14) 28(59.57) 53(91.38) N1 24(22.86) 19(40.43) 5(8.62) M stage 0.01 0.01 0.995 M0 87(88.78) 33(89.19) 54(88.52) M1 11(11.22) 4(10.81) 7(11.48) Tumor differentiation grade 4.27 −0.158 0.118 G1/2 94(54.65) 51(62.96) 43(47.25) G3/4 78(45.35) 30(37.04) 48(52.75) 表 3 宫颈癌患者BAIAP2L1表达及临床病理因素与患者预后的关系分析(源自TCGA 数据)
Table 3 Relationship of BAIAP2L1 expression and clinicopathological factors with prognosis of patients with CC (from TCGA database)
Characteristic N Univariate analysis Multivariate analysis HR(95%CI) P HR(95%CI) P Age (years) 0.105 <60 195 1 ≥60 52 1.636 (0.902, 2.967) Tumor pathological stage 0.001 0.361 (0.096, 1.360) 0.132 Ⅰ/Ⅱ 188 1 Ⅲ/Ⅳ 53 2.747 (1.537, 4.911) Tumor differentiation grade 0.248 G1/2 123 1 G3/4 105 0.686 (0.363, 1.300) T stage <0.001 16.559 (4.310, 63.619) <0.001 T1/2 178 1 T3/4 25 5.218 (2.695, 10.103) N stage 0.002 2.823 (1.225, 6.507) 0.015 N0 111 1 N1 52 3.539 (1.598, 7.837) M stage 0.062 M0 95 1 M1 10 3.325 (0.942, 11.728) BAIAP2L1 expression <0.001 2.808 (1.108, 7.113) 0.03 Low 123 1 High 124 4.772 (2.377, 9.582) -
[1] Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2024, 74(3): 229-263. doi: 10.3322/caac.21834
[2] Tewari KS, Sill MW, Long HR, et al. Improved survival with bevacizumab in advanced cervical cancer[J]. N Engl J Med, 2014, 370(8): 734-743. doi: 10.1056/NEJMoa1309748
[3] Ebadi Zzvieh S, Safari F. The Antitumor Activity of hAMSCs Secretome in HT-29 Colon Cancer Cells Through Downregulation of EGFR/c-Src/IRTKS Expression and p38/ERK1/2 Phosphorylation[J]. Cell Biochem Biophysics, 2022, 80(2): 395-402. doi: 10.1007/s12013-022-01066-4
[4] Chao A, Tsai CL, Jung SM, et al. BAI1-Associated Protein 2-Like 1 (BAIAP2L1) Is a Potential Biomarker in Ovarian Cancer[J]. PLoS One, 2015, 10(7): e0133081. doi: 10.1371/journal.pone.0133081
[5] Williams SV, Hurst CD, Knowles MA. Oncogenic FGFR3 gene fusions in bladder cancer[J]. Hum Mol Genet, 2013, 22(4): 795-803. doi: 10.1093/hmg/dds486
[6] Wang S, Liu Z, Ma YM, et al. Upregulated insulin receptor tyrosine kinase substrate promotes the proliferation of colorectal cancer cells via the bFGF/AKT signaling pathway[J]. Gastroenterol Rep (Oxf), 2021, 9(2): 166-175. doi: 10.1093/gastro/goaa032
[7] Xu Q, Wang J, Sun Y, et al. Efficacy and Safety of Sintilimab Plus Anlotinib for PD-L1-Positive Recurrent or Metastatic Cervical Cancer: A Multicenter, Single-Arm, Prospective Phase Ⅱ Trial[J]. J Clin Oncol, 2022, 40(16): 1795-1805. doi: 10.1200/JCO.21.02091
[8] Song Y, Zhuang G, Li J, et al. BAIAP2L2 facilitates the malignancy of prostate cancer (PCa) via VEGF and apoptosis signaling pathways[J]. Genes Genomics, 2021, 43(4): 421-432. doi: 10.1007/s13258-021-01061-8
[9] Kumar L, Harish P, Malik PS, et al. Chemotherapy and targeted therapy in the management of cervical cancer[J]. Curr Probl Cancer, 2018, 42(2): 120-128. doi: 10.1016/j.currproblcancer.2018.01.016
[10] Deng N, Zhang X, Zhang Y. BAIAP2L1 accelerates breast cancer progression and chemoresistance by activating AKT signaling through binding with ribosomal protein L3[J]. Cancer Science, 2023, 114(3): 764-780. doi: 10.1111/cas.15632
[11] Lu Y, Zhou XY, Zhou CL, et al. Insulin receptor tyrosine kinase substrate (IRTKS) promotes the tumorigenesis of pancreatic cancer via PI3K/AKT signaling[J]. Human Cell, 2022, 35(6): 1885-1899. doi: 10.1007/s13577-022-00770-w
[12] Chen G, Li T, Zhang L, et al. Src-stimulated IRTKS phosphorylation enhances cell migration[J]. FEBS Lett, 2011, 585(19): 2972-2978. doi: 10.1016/j.febslet.2011.08.005
[13] Loh CY, Chai JY, Tang TF, et al. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges[J]. Cells, 2019, 8(10): 1118. doi: 10.3390/cells8101118
[14] Wang YP, Huang LY, Sun WM, et al. Insulin receptor tyrosine kinase substrate activates EGFR/ERK signalling pathway and promotes cell proliferation of hepatocellular carcinoma[J]. Cancer Lett, 2013, 337(1): 96-106. doi: 10.1016/j.canlet.2013.05.019
[15] Nakanishi Y, Akiyama N, Tsukaguchi T, et al. Mechanism of Oncogenic Signal Activation by the Novel Fusion Kinase FGFR3-BAIAP2L1[J]. Mol Cancer Ther, 2015, 14(3): 704-712. doi: 10.1158/1535-7163.MCT-14-0927-T
[16] Chi M, Liu J, Mei C, et al. TEAD4 functions as a prognostic biomarker and triggers EMT via PI3K/AKT pathway in bladder cancer[J]. J Exp Clin Cancer Res, 2022, 41(1): 175. doi: 10.1186/s13046-022-02377-3
[17] Deng R, Lu X, Hong C, et al. Downregulation of TUSC3 promotes EMT and hepatocellular carcinoma progression through LIPC/AKT axis[J]. J Transl Med, 2022, 20(1): 485. doi: 10.1186/s12967-022-03690-3
[18] Cui X, Shang X, Xie J, et al. Cooperation between IRTKS and deubiquitinase OTUD4 enhances the SETDB1-mediated H3K9 trimethylation that promotes tumor metastasis via suppressing E-cadherin expression[J]. Cancer Letters, 2023, 575: 216404. doi: 10.1016/j.canlet.2023.216404




 下载:
下载: