Pan-Cancer Analysis of Disulfidptosis-Related Genes Affecting Prognosis and Tumor Microenvironment
-
摘要:目的
基于生物信息学方法评估双硫死亡相关基因(DRGs)在泛癌中对预后及免疫的潜在作用。
方法使用TCGA及多种开源在线数据库内包含的泛癌RNA-seq数据、突变情况、临床信息、TMB、MSI、干性评分和肿瘤及免疫微环境数据及多组R语言算法综合分析。使用qPCR对细胞层面的DRGs表达情况行实验验证。
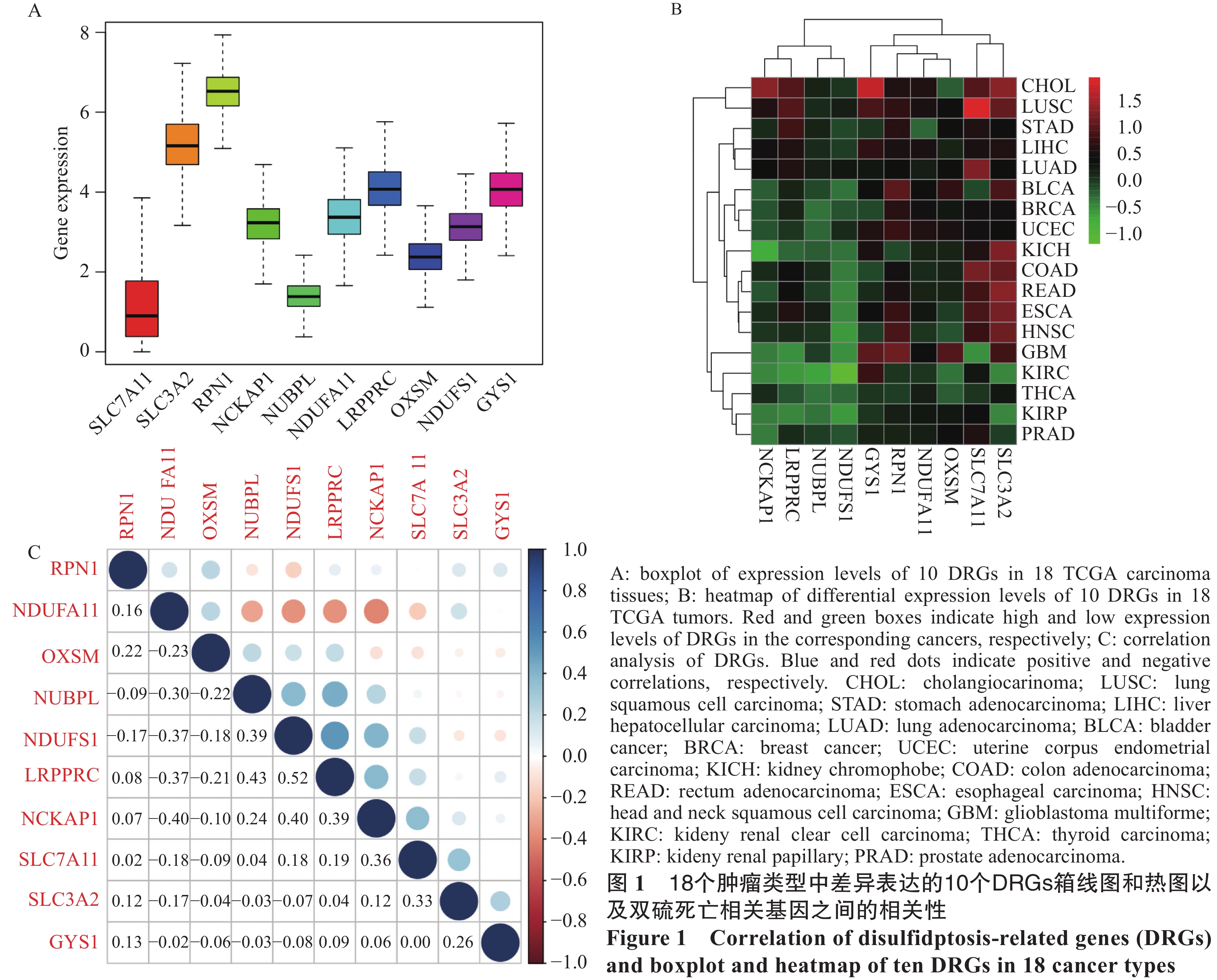
结果LRPPRC、NCKAP1、NDUFS1和NUBPL在肾透明细胞癌中的预后较好(P<0.001),而 SLC7A11、NCKAP1和SLC3A2在肝细胞癌中的预后较差(P<0.01)。在TME分析中,LRPPRC与所有肿瘤类型中的免疫细胞、基质细胞和估计评分均呈负相关。TMB分析结果揭示了DRGs在泛癌中PD-1/PD-L1治疗的潜在研究价值。药物敏感性分析发现SLC7A11(r=0.454)、SLC3A2(r=0.366)和NCKAP1(r=0.455)与Kahalide F明显相关(P<0.01)。实验验证表明了GYS1与NCKAP1在肺腺癌、结肠腺癌、食管鳞癌与肝细胞癌中总体表达高于正常细胞,且存在差异(P<0.05)。
结论通过对DRGs的泛癌分析表明,DRGs可能作为肾透明细胞癌、肺腺癌、肝细胞癌诊断及预后的重要生物标志物。
Abstract:ObjectiveTo assess the potential role of disulfidptosis-related genes (DRGs) in pan-cancer on prognosis and immunity on the basis of bioinformatics approaches.
MethodsPan-cancer RNA-seq data, mutation profiles, clinical information, TMB, MSI, stemness scores, and tumor and immune microenvironment data contained in TCGA and various open-source online databases, and multi-group R-language algorithms were used for comprehensive analysis. The expression levels of DRGs at the cellular level were experimentally validated using qPCR.
ResultsLRPPRC, NCKAP1, NDUFS1, and NUBPL had a better prognosis in renal clear cell carcinoma (P<0.001), whereas SLC7A11, NCKAP1, and SLC3A2 had a worse prognosis in hepatocellular carcinoma (P<0.001). TME analysis showed that LRPPRC was negatively correlated with immune cells, stromal cells, and estimated scores in all tumor types. TMB analysis revealed the potential research value of DRGs for PD-1/PD-L1 therapy in pan-cancer. Drug sensitivity analysis showed that SLC7A11 (r=0.454), SLC3A2 (r=0.366), and NCKAP1 (r=0.455) were significantly associated with Kahalide F (P<0.01). Experimental validation demonstrated the overall higher expression levels of GYS1 and NCKAP1 than normal cells in lung adenocarcinoma, colon adenocarcinoma, esophageal squamous carcinoma, and hepatocellular carcinoma (P<0.05).
ConclusionPan-cancer analysis of DRGs indicates that DRGs may serve as important biomarkers for the diagnosis and prognosis of renal clear-cell carcinoma, lung adenocarcinoma, and hepatocellular carcinoma.
-
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:孙敬阳:文章写作、数据分析蒋荣轩、侯理仁、董欢欢:数据整理及管理林亦瀚、董妞妞:数据可视化分析张广健:研究设计张言鹏:研究设计、基金项目负责人
-
-
[1] Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants[J]. Chin Med J (Engl), 2022, 135(5): 584-590. doi: 10.1097/CM9.0000000000002108
[2] Cortes J, Perez-García JM, Llombart-Cussac A, et al. Enhancing global access to cancer medicines[J]. CA Cancer J Clin, 2020, 70(2): 105-124. doi: 10.3322/caac.21597
[3] Peng F, Liao M, Qin R, et al. Regulated cell death (RCD) in cancer: key pathways and targeted therapies[J]. Signal Transduct Target Ther, 2022, 7(1): 286. doi: 10.1038/s41392-022-01110-y
[4] Xia X, Wang X, Cheng Z, et al. The role of pyroptosis in cancer: pro-cancer or pro-"host"?[J]. Cell Death Dis, 2019, 10(9): 650. doi: 10.1038/s41419-019-1883-8
[5] Yan J, Wan P, Choksi S, et al. Necroptosis and tumor progression[J]. Trends Cancer, 2022, 8(1): 21-27. doi: 10.1016/j.trecan.2021.09.003
[6] Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease[J]. Signal Transduct Target Ther, 2022, 7(1): 378. doi: 10.1038/s41392-022-01229-y
[7] Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease[J]. Nat Rev Mol Cell Biol, 2021, 22(4): 266-282. doi: 10.1038/s41580-020-00324-8
[8] Liu X, Nie L, Zhang Y, et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis[J]. Nat Cell Biol, 2023, 25(3): 404-414. doi: 10.1038/s41556-023-01091-2
[9] Goldman MJ, Craft B, Hastie M, et al. Visualizing and interpreting cancer genomics data via the Xena platform[J]. Nat Biotechnol, 2020, 38(6): 675-678. doi: 10.1038/s41587-020-0546-8
[10] Cunningham F, Allen JE, Allen J, et al. Ensembl 2022[J]. Nucleic Acids Res, 2022, 50(D1): D988-D995. doi: 10.1093/nar/gkab1049
[11] Yang W, Soares J, Greninger P, et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells[J]. Nucleic Acids Res, 2013, 41(Database issue): D955-D961.
[12] Palmeri M, Mehnert J, Silk AW, et al. Real-world application of tumor mutational burden-high (TMB-high) and microsatellite instability (MSI) confirms their utility as immunotherapy biomarkers[J]. ESMO Open, 2022, 7(1): 100336. doi: 10.1016/j.esmoop.2021.100336
[13] Tong X, Tang R, Xiao M, et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research[J]. J Hematol Oncol, 2022, 15(1): 174. doi: 10.1186/s13045-022-01392-3
[14] Bejarano L, Jordāo MJC, Joyce JA. Therapeutic Targeting of the Tumor Microenvironment[J]. Cancer Discov, 2021, 11(4): 933-959. doi: 10.1158/2159-8290.CD-20-1808
[15] Niu X, Chen L, Li Y, et al. Ferroptosis, necroptosis, and pyroptosis in the tumor microenvironment: Perspectives for immunotherapy of SCLC[J]. Semin Cancer Biol, 2022, 86(Pt 3): 273-285.
[16] El Omari N, Bakrim S, Khalid A, et al. Anticancer clinical efficiency and stochastic mechanisms of belinostat[J]. Biomed Pharmacother, 2023, 165: 115212. doi: 10.1016/j.biopha.2023.115212
[17] Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy[J]. Science, 2018, 362(6411): eaar3593. doi: 10.1126/science.aar3593



 下载:
下载:







