MAPK4 Accelerates Progression of Cervical Squamous Cell Carcinoma by Positively Regulating SLC3A2 Expression
-
摘要:目的
探索MAPK4在宫颈鳞状细胞癌(CSCC)中的作用以鉴定候选的预后预测生物标志物和分子治疗靶点。
方法在TCGA队列中进行Kaplan-Meier生存分析并利用临床样本开展免疫组织化学实验探索MAPK4与患者预后的相关性;基于MAPK4 mRNA水平构建列线图。Western Blot、CCK-8、克隆形成和Transwell细胞功能实验明确MAPK4在宫颈鳞状细胞癌中的异常表达和作用;DIA蛋白组测序鉴定MAPK4调节的效应分子;敲降MAPK4并过表达效应分子,联合上述细胞功能实验揭示MAPK4调节效应分子介导肿瘤进程的作用。
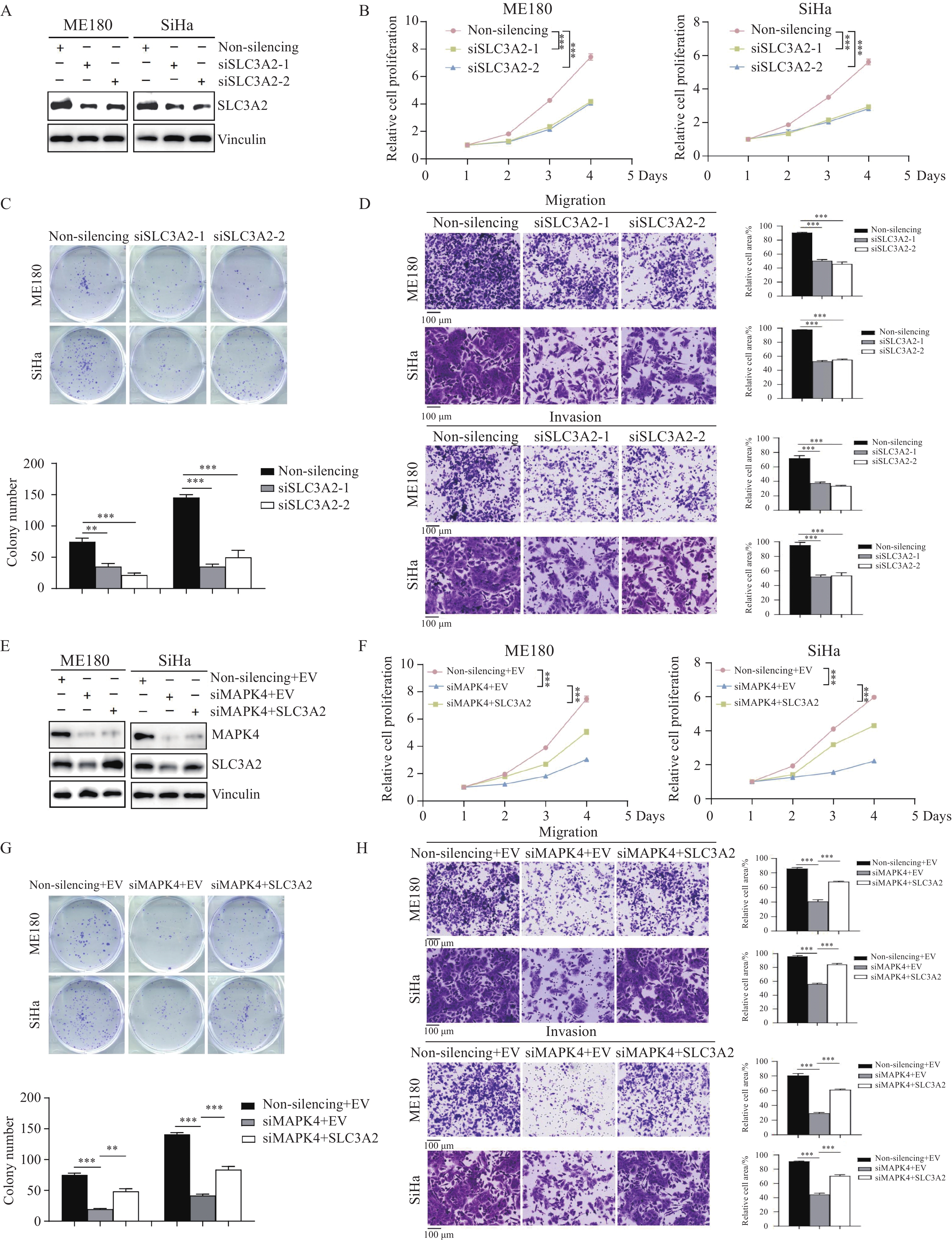
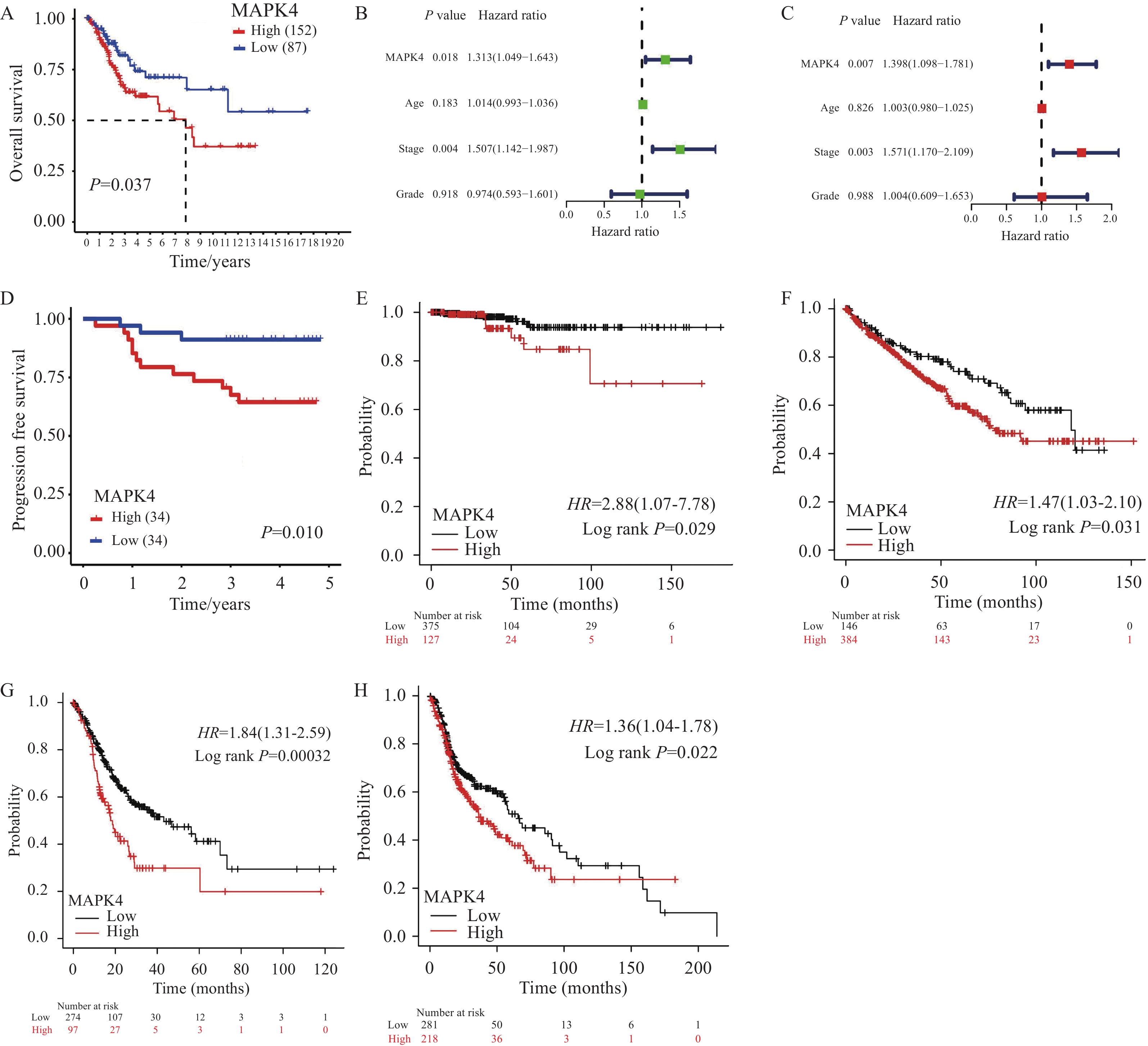
结果MAPK4 mRNA水平升高与蛋白高表达的CSCC患者预后更差;基于MAPK4构建的列线图可准确预测患者1、3、5年生存率。相比于正常宫颈组织,MAPK4蛋白在肿瘤中表达上调,敲降MAPK4可显著抑制宫颈鳞状细胞癌细胞ME180和SiHa增殖、克隆形成和迁移侵袭能力。SLC3A2是MAPK4的下游效应分子,敲降MAPK4后过表达SLC3A2可减弱敲降MAPK4对细胞增殖、克隆形成和迁移侵袭的抑制作用。
结论MAPK4是宫颈鳞状细胞癌患者候选的预后预测生物标志物。MAPK4正向调节SLC3A2蛋白表达加速肿瘤进程,是宫颈鳞状细胞癌患者潜在的分子治疗靶点。
Abstract:ObjectiveTo explore the role of MAPK4 in cervical squamous cell carcinoma (CSCC) for the identification of candidate prognostic prediction biomarkers and molecular therapeutic targets.
MethodsThe TCGA cohort was subjected to Kaplan-Meier survival analysis. Immunohistochemistry experiments were conducted on clinical samples to explore the correlation between MAPK4 and patient prognosis. A nomogram was constructed based on MAPK4 mRNA levels. Western blot, CCK-8, colony formation, and Transwell cell function experiments were performed to clarify the abnormal expression and role of MAPK4 in CSCC. DIA proteome sequencing was used to identify effector molecules regulated by MAPK4. Combined with the above cell function experiments, the knockdown of MAPK4 and the overexpression of effector molecules revealed that MAPK4 regulated effector molecules to mediate tumor progression.
ResultsCSCC patients with elevated MAPK4 mRNA levels and high protein expression have a worse prognosis. The constructed nomogram based on MAPK4 can accurately predict the 1-, 3-, and 5-year survival rates of patients. Compared with that in normal cervical tissues, MAPK4 protein expression was up-regulated in tumors. MAPK4 knockdown substantially inhibited the proliferation, colony formation, migration, and invasion of CSCC ME180 and SiHa cells. SLC3A2 is a downstream effector molecule of MAPK4. Overexpression SLC3A2 can weaken the inhibitory effect of MAPK4 knockdown on cell proliferation, colony formation, migration, and invasion.
ConclusionMAPK4 is a candidate prognostic biomarker for patients with CSCC. MAPK4 positively regulates SLC3A2 protein expression and accelerates tumor progression, making it a potential molecular therapeutic target for patients with CSCC.
-
Key words:
- Cervical squamous cell carcinoma /
- MAPK4 /
- SLC3A2 /
- Tumor progression /
- Biomarkers /
- Therapeutic targets
-
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:余 竟:实验设计、数据分析、实验实施及文章撰写邓 露:文献调研及部分实验实施赵雨亭:数据分析袁振龙:统计学分析、文章校对吴令英:基金支持、思路设计、文章审阅
-
-
[1] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[2] Singh D, Vignat J, Lorenzoni V, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative[J]. Lancet Glob Health, 2023, 11(2): e197-e206. doi: 10.1016/S2214-109X(22)00501-0
[3] Kalliala I, Athanasiou A, Veroniki AA, et al. Incidence and mortality from cervical cancer and other malignancies after treatment of cervical intraepithelial neoplasia: a systematic review and meta-analysis of the literature[J]. Ann Oncol, 2020, 31(2): 213-227. doi: 10.1016/j.annonc.2019.11.004
[4] Guo C, Qu X, Tang X, et al. Spatiotemporally deciphering the mysterious mechanism of persistent HPV-induced malignant transition and immune remodelling from HPV-infected normal cervix, precancer to cervical cancer: Integrating single-cell RNA-sequencing and spatial transcriptome[J]. Clin Transl Med, 2023, 13(3): e1219. doi: 10.1002/ctm2.1219
[5] Sivars L, Jylhä C, Crona Guterstam Y, et al. Cell-free human papillomavirus (HPV) DNA is a sensitive biomarker for prognosis and for early detection of relapse in locally advanced cervical cancer[J]. Clin Cancer Res, 2024, 30(13): 2764-2771. doi: 10.1158/1078-0432.CCR-23-3941
[6] Yang M, Du J, Lu H, et al. Global trends and age-specific incidence and mortality of cervical cancer from 1990 to 2019: an international comparative study based on the Global Burden of Disease[J]. BMJ Open, 2022, 12(7): e055470. doi: 10.1136/bmjopen-2021-055470
[7] Lin S, Sun Y, Cao C, et al. Single-nucleus RNA sequencing reveals heterogenous microenvironments and specific drug response between cervical squamous cell carcinoma and adenocarcinoma[J]. EBioMedicine, 2023, 97: 104846. doi: 10.1016/j.ebiom.2023.104846
[8] Bahar ME, Kim HJ, Kim DR. Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies[J]. Signal Transduct Target Ther, 2023, 8(1): 455. doi: 10.1038/s41392-023-01705-z
[9] Iroegbu JD, Ijomone OK, Femi-Akinlosotu OM, et al. ERK/MAPK signalling in the developing brain: Perturbations and consequences[J]. Neurosci Biobehav Rev, 2021, 131: 792-805. doi: 10.1016/j.neubiorev.2021.10.009
[10] Jin H, Huang X, Pan Q, et al. The EIF3H-HAX1 axis increases RAF-MEK-ERK signaling activity to promote colorectal cancer progression[J]. Nat Commun, 2024, 15(1): 2551. doi: 10.1038/s41467-024-46521-3
[11] Lin X, Ye R, Li Z, et al. KIAA1429 promotes tumorigenesis and gefitinib resistance in lung adenocarcinoma by activating the JNK/ MAPK pathway in an m(6)A-dependent manner[J]. Drug Resist Updat, 2023, 66: 100908. doi: 10.1016/j.drup.2022.100908
[12] Dimri M, Humphries A, Laknaur A, et al. NAD(P)H Quinone Dehydrogenase 1 Ablation Inhibits Activation of the Phosphoinositide 3-Kinase/Akt Serine/Threonine Kinase and Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Pathways and Blocks Metabolic Adaptation in Hepatocellular Carcinoma[J]. Hepatology, 2020, 71(2): 549-568. doi: 10.1002/hep.30818
[13] Park HB, Baek KH. E3 ligases and deubiquitinating enzymes regulating the MAPK signaling pathway in cancers[J]. Biochim Biophys Acta Rev Cancer, 2022, 1877(3): 188736. doi: 10.1016/j.bbcan.2022.188736
[14] Sullivan RJ, Infante JR, Janku F, et al. First-in-Class ERK1/2 Inhibitor Ulixertinib (BVD-523) in Patients with MAPK Mutant Advanced Solid Tumors: Results of a Phase I Dose-Escalation and Expansion Study[J]. Cancer Discov, 2018, 8(2): 184-195. doi: 10.1158/2159-8290.CD-17-1119
[15] Weekes C, Lockhart A, Lorusso P, et al. A Phase Ib Study to Evaluate the MEK Inhibitor Cobimetinib in Combination with the ERK1/2 Inhibitor GDC-0994 in Patients with Advanced Solid Tumors[J]. Oncologist, 2020, 25(10): 833-e1438. doi: 10.1634/theoncologist.2020-0292
[16] Aberg E, Perander M, Johansen B, et al. Regulation of MAPK-activated protein kinase 5 activity and subcellular localization by the atypical MAPK ERK4/MAPK4[J]. J Biol Chem, 2006, 281(46): 35499-35510. doi: 10.1074/jbc.M606225200
[17] Kant S, Schumacher S, Singh MK, et al. Characterization of the atypical MAPK ERK4 and its activation of the MAPK-activated protein kinase MK5[J]. J Biol Chem, 2006, 281(46): 35511-35519. doi: 10.1074/jbc.M606693200
[18] Demichev V, Messner CB, Vernardis SI, et al. DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput[J]. Nat Methods, 2020, 17(1): 41-44. doi: 10.1038/s41592-019-0638-x
[19] Malagon T, Franco EL, Tejada R, et al. Epidemiology of HPV-associated cancers past, present and future: towards prevention and elimination[J]. Nat Rev Clin Oncol, 2024, 21(7): 522-538. doi: 10.1038/s41571-024-00904-z
[20] Brisson M, Kim JJ, Canfell K, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries[J]. Lancet, 2020, 395(10224): 575-590. doi: 10.1016/S0140-6736(20)30068-4
[21] Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis[J]. Lancet Glob Health, 2020, 8(2): e191-e203. doi: 10.1016/S2214-109X(19)30482-6
[22] Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer[J]. N Engl J Med, 1999, 340(15): 1144-1153. doi: 10.1056/NEJM199904153401502
[23] Shen T, Wang W, Zhou W, et al. MAPK4 promotes prostate cancer by concerted activation of androgen receptor and AKT[J]. J Clin Invest, 2021, 131(4): 135465. doi: 10.1172/JCI135465
[24] Wang W, Han D, Cai Q, et al. MAPK4 promotes triple negative breast cancer growth and reduces tumor sensitivity to PI3K blockade[J]. Nat Commun, 2022, 13(1): 245. doi: 10.1038/s41467-021-27921-1
[25] Han D, Wang W, Jeon JH, et al. Cooperative activation of PDK1 and AKT by MAPK4 enhances cancer growth and resistance to therapy[J]. PLoS Biol, 2023, 21(8): e3002227. doi: 10.1371/journal.pbio.3002227
[26] Wang W, Shen T, Dong B, et al. MAPK4 overexpression promotes tumor progression via noncanonical activation of AKT/mTOR signaling[J]. J Clin Invest, 2019, 129(3): 1015-1029. doi: 10.1172/JCI97712
[27] Chen J, Yang J, Liu Y, et al. MAPK4 facilitates angiogenesis by inhibiting the ERK pathway in non-small cell lung cancer[J]. Cancer Innov, 2024, 3(3): e117. doi: 10.1002/cai2.117
[28] Fort J, de la Ballina LR, Burghardt HE, et al. The structure of human 4F2hc ectodomain provides a model for homodimerization and electrostatic interaction with plasma membrane[J]. J Biol Chem, 2007, 282(43): 31444-31452. doi: 10.1074/jbc.M704524200
[29] Verrey F, Closs EI, Wagner CA, et al. CATs and HATs: the SLC7 family of amino acid transporters[J]. Pflugers Arch, 2004, 447(5): 532-542. doi: 10.1007/s00424-003-1086-z
[30] Rodriguez CF, Escudero-Bravo P, Diaz L, et al. Structural basis for substrate specificity of heteromeric transporters of neutral amino acids[J]. Proc Natl Acad Sci U S A, 2021, 118(49): e2113573118. doi: 10.1073/pnas.2113573118
[31] Bridges RJ, Natale NR, Patel SA. System xc(-) cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS[J]. Br J Pharmacol, 2012, 165(1): 20-34. doi: 10.1111/j.1476-5381.2011.01480.x
[32] Digomann D, Kurth I, Tyutyunnykova A, et al. The CD98 Heavy Chain Is a Marker and Regulator of Head and Neck Squamous Cell Carcinoma Radiosensitivity[J]. Clin Cancer Res, 2019, 25(10): 3152-3163. doi: 10.1158/1078-0432.CCR-18-2951
[33] Wu P, Zhao L, Kong G, et al. Study on the Role and Mechanism of SLC3A2 in Tumor-Associated Macrophage Polarization and Bladder Cancer Cells Growth[J]. Technol Cancer Res Treat, 2024, 23: 15330338241246649.
[34] Wu F, Xiong G, Chen Z, et al. SLC3A2 inhibits ferroptosis in laryngeal carcinoma via mTOR pathway[J]. Hereditas, 2022, 159(1): 6. doi: 10.1186/s41065-022-00225-0



 吴令英: 博士,主任医师,博士生导师,北京协和医学院长聘教授和“教学名师”。中国临床肿瘤学会副理事长,中国妇幼保健协会常务理事,中国临床肿瘤学会妇科肿瘤专家委员会主任委员,中国妇幼保健协会妇幼微创专业委员会主任委员,中国抗癌协会妇科肿瘤专业委员会副主任委员,中国临床肿瘤学会临床研究专家委员会副主任委员,中国老年学及老年医学学会妇科分会副会长和中华医学会妇科肿瘤专业委员会常委等。Cancer Biology & Medicine、Oncology and Translational Medicine、《中华妇产科杂志》和《中华放射肿瘤学杂志》等杂志编委。长期从事妇科肿瘤的临床治疗与转化医学研究,作为负责人主持近30余项国际和国内多中心临床研究,主持国家重点研发计划项目和国家自然科学基金等20余项科研课题。作为通讯作者将近5年的研究成果发表于Journal of Clinical Oncology、JAMA Oncology和Clinical Cancer Research等杂志,牵头制定“中国临床肿瘤学会卵巢癌诊疗指南”等多项指南
。
吴令英: 博士,主任医师,博士生导师,北京协和医学院长聘教授和“教学名师”。中国临床肿瘤学会副理事长,中国妇幼保健协会常务理事,中国临床肿瘤学会妇科肿瘤专家委员会主任委员,中国妇幼保健协会妇幼微创专业委员会主任委员,中国抗癌协会妇科肿瘤专业委员会副主任委员,中国临床肿瘤学会临床研究专家委员会副主任委员,中国老年学及老年医学学会妇科分会副会长和中华医学会妇科肿瘤专业委员会常委等。Cancer Biology & Medicine、Oncology and Translational Medicine、《中华妇产科杂志》和《中华放射肿瘤学杂志》等杂志编委。长期从事妇科肿瘤的临床治疗与转化医学研究,作为负责人主持近30余项国际和国内多中心临床研究,主持国家重点研发计划项目和国家自然科学基金等20余项科研课题。作为通讯作者将近5年的研究成果发表于Journal of Clinical Oncology、JAMA Oncology和Clinical Cancer Research等杂志,牵头制定“中国临床肿瘤学会卵巢癌诊疗指南”等多项指南
。
 下载:
下载: