-
摘要:
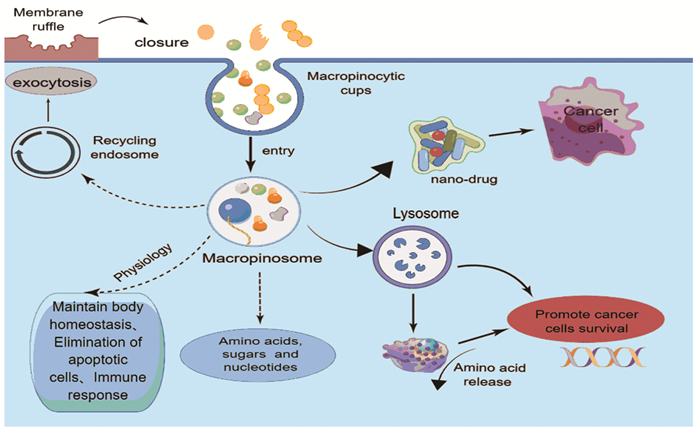
巨胞饮是一种进化上保守的、依赖肌动蛋白的内吞作用形式,巨胞饮涉及多种生理过程,包括营养吸收、抗原呈递以及细胞信号转导和迁移。研究证明致癌基因激活和抑癌失活在消化系统肿瘤中诱导巨胞饮作用,参与肿瘤的发生和进展,而抑制巨胞饮作用可减缓消化系统肿瘤的侵袭表型,提高抗肿瘤药物的疗效。此外,巨胞饮也可作为抗肿瘤药物的递送途径。因此,靶向巨胞饮作用,开发新的治疗消化系统肿瘤的方法,具有较高的研究价值。本文就巨胞饮在机体中发挥的作用、调节巨胞饮的相关信号通路以及巨胞饮在结直肠癌、胰腺导管腺癌、肝癌等消化系统肿瘤中的作用机制进行综述,以期为相关研究提供参考。
Abstract:Macropinocytosis, an evolutionarily conserved, actin-dependent form of endocytosis, is involved in various physiological processes, including nutrient absorption, antigen presentation, and cell signaling transduction and migration. Oncogene activation and tumor suppressor inactivation induce macropinocytosis in tumors in the digestive system, involved in tumorigenesis and progression, whereas the inhibition of macropinocytosis slows the aggressive phenotype of digestive system tumors and improves the efficacy of anti-tumor drugs. Macropinocytosis can also be used as a delivery route for anti-tumor drugs. Therefore, macropinocytosis has been widely studied to develop new methods for the treatment of digestive system tumors.This paper reviews the role of macropinocytosis in the body, the regulation of macropinocytosis-related signaling pathway, as well as the mechanism of macropinocytosis in colorectal cancer, pancreatic ductal adenocarcinoma, liver cancer and other digestive system tumors, to provide reference for related researches.
-
Key words:
- Micropinocytosis /
- Digestive system tumors /
- Signal pathway
-
0 引言
乳腺癌年龄标化发生率为21.6/10万,严重威胁着女性的健康,其发病率持续上升[1]。乳腺癌预后评估方式很多,炎性因子标志物就是其中一类。
研究表明中性粒细胞与淋巴结细胞数目的比值[2]、血小板数目与淋巴细胞数目的比值[3]、淋巴细胞数目与单核细胞数目的比值[4]均与乳腺癌的预后相关。目前关于白蛋白与纤维蛋白原比值和预后的关系研究较少,且白蛋白与纤维蛋白原是临床常见指标,廉价且易于收集,更适用于临床。本试验拟探讨术前白蛋白与纤维蛋白原比值对乳腺癌预后的影响,现报道如下。
1 资料与方法
1.1 一般资料
选取河北医科大学第四医院乳腺中心2009年1月—2012年4月手术切除的230例原发性乳腺浸润性导管癌血液及临床资料作为研究对象。根据《美国癌症联合委员会(AJCC)癌症分期手册》第7版标准进行术后分期:Ⅰ期70例、Ⅱ期126例、Ⅲ期34例。所有患者术前均未进行任何治疗。收集指标包括年龄、总生存期(overall survival, OS)、白细胞(white blood cells, WBC)、红细胞(red blood cells, RBC)、血红蛋白(hemoglobin, HGB)、血小板(platelets, PLT)及病理指标TNM分期、核分级、雌激素(estrogen receptor, ER)、孕激素(progesterone receptor, PR)、人表皮生长因子受体(human epidermal growth factor receptor-2, HER2)、Ki-67、淋巴血管侵袭等。
1.2 随访情况
采用门诊、电话和病历查阅方式对230例患者进行随访,以病理确诊时开始计算生存时间,随访至2016年7月,终止事件为患者死亡。随访时间为7~81月,中位随访时间为67月,均获得完整的随访资料,死亡26例。
1.3 统计学方法
应用易侕统计软件进行统计学分析。计数资料采用χ2检验和Fisher’s精确检验。生存分析采用Kaplan-Meier生存曲线,通过Log rank法进行生存率差别检验,并采用Cox比例回归风险模型进行多因素生存分析。运用易侕软件建立患者生存率模型,检验水准α=0.05。
2 结果
2.1 原发性乳腺浸润性导管癌患者炎性因子标志物水平
230例原发性乳腺浸润性导管癌患者白蛋白与纤维蛋白原比值(albumin to fibrinogen ratio, AFR)在整个人群中的均值为13.23±2.43,因此后续研究中按二分类变量值13.1将患者分为两组。年龄50.57±11.32岁,总生存时间64.48±13.37月,其他炎性因子标志物水平,见表 1。
表 1 230例原发性乳腺浸润性导管癌患者一般资料Table 1 General information of 230 patients with primary breast invasive ductal carcinoma
2.2 纤维蛋白原表达与TNM分期的关系
TNM分期Ⅰ~Ⅲ期患者的纤维蛋白原表达量,见表 2,纤维蛋白原表达与TNM分期负相关,结果见表 2。
表 2 纤维蛋白原与TNM分期的关系Table 2 Relation between fbrinogen and TNM staging
2.3 AFR按二分类后原发性乳腺浸润性导管癌患者人群描述
收集患者术前纤维蛋白原及白蛋白值,计算AFR值,并将AFR进行二分类,取中位数为13.1,经过生存分析可以发现,当AFR≥13.1时,OS为62.3±15.1月;当AFR < 13.1时,OS为66.3±11.0月,两者差异有统计学意义(P=0.015),说明当AFR值较大时,总生存率降低,见表 3。
表 3 AFR二分类后(即AFR < 13.1和AFR≥13.1组)原发性乳腺浸润性导管癌患者临床特征Table 3 Clinicopathological features of breast invasive ductal carcinoma patients after AFR second classification (AFR < 13.1 and AFR≥13.1)
2.4 单因素分析
单因素分析中,AFR的P=0.008478 < 0.05。年龄、WBC、HGB、TNM分期、脉管瘤栓、核分级、ER/PR、p53 < 0.2,我们将单因素分析里P < 0.2的因素纳入多因素分析,单因素分析见表 4。
表 4 乳腺浸润性导管癌患者的单因素分析Table 4 Univariate analysis of breast invasive ductal carcinoma patients
2.5 变量多因素分析
结果显示:AFR、WBC、ER或PR、TNM分期、核分级、p53及脉管瘤栓的P < 0.05,可见AFR、WBC、ER或PR、TNM分期、核分级、p53及脉管瘤栓均是女性浸润性导管癌患者生存的独立预后因素,见表 5。我们以AFR、WBC、ER或PR、TNM、核分级、p53及脉管瘤栓为变量建立乳腺浸润性导管癌预后模型,预测患者3年及5年生存率,算得ROC曲线下面积为0.882,表明有良好的预测价值,见图 1,将以上7个指标对应的量化指标累加起来,得出总数值,总数值越大,对应的生存率越低。从列线图中可以看出AFR越大,总数值越大,患者的3年及5年生存率越小,从整体看AFR占比重最大,在乳腺浸润性导管癌预后的预测中起到重要作用,见图 2。
表 5 影响乳腺浸润性导管癌患者生存的多因素分析Table 5 Multivariate analysis of breast invasive ductal carcinoma patients
2.6 AFR的Kaplan-Meier生存曲线
结果显示当AFR≥13.1时,患者生存率较低(P=0.03)。AFR越大,生存率越低,见图 2。
3 讨论
乳腺癌是女性最常见的恶性肿瘤,且近年来发病率不断上升。尽管自20世纪90年代起,乳腺癌早期筛查技术的发展、辅助化疗、靶向药物的应用使乳腺癌患者的死亡率下降了39%,但是乳腺癌的死亡率仍居高不下,因此选择合适的预后评价指标,对患者的治疗及预后预测具有重要意义。
研究表明白蛋白与纤维蛋白原比(AFR)是很多肿瘤的预后指标[5]。纤维蛋白原是由肝脏产生、相对分子质量为340 kDa的共价二聚体,由α、β、γ三条多肽链组成,是凝血系统的关键物质, 主要参与凝血及止血,与体内伤口愈合、炎性反应及组织损伤的修复关系密切。近年来研究表明纤维蛋白原可以促进肿瘤血管的生成及促生长。其中纤维蛋白原在良性肿瘤患者的表达明显低于恶性肿瘤患者[6]。近年来也有研究证实,纤维蛋白原与癌症的转移相关[7]。关于纤维蛋白原促进肿瘤的转移,目前公认的有以下几种解释,一种可能的解释是,增加凝血水平后通过巨噬细胞的活化和细胞因子及趋化因子,如NF-κB、TNF和巨噬细胞炎性蛋白-1的分泌促进肿瘤生长[8]。Redman等发现纤维蛋白原转染的细胞脂肪生成增加,脂肪是肿瘤细胞代谢的一种重要分子[9]。Palumbo报道,纤维蛋白原缺陷小鼠模型中肺转移的发生率明显减少[10],也发现纤维蛋白原通过阻止自然杀伤细胞对肿瘤细胞的消除而增加肿瘤细胞的转移[11]。纤维蛋白原还可以通过与其他肿瘤生长因子,如VEGF、FGF-2的相互作用促进肿瘤细胞的增殖、转移及黏附[12]。本实验表明,患者的纤维蛋白原水平表达与TNM分期呈负相关(HR=-0.4, P=0.0027),与上述结果相反,因此需进一步查找患者病理标本对患者病理组织中纤维蛋白原的表达进行测定,该实验正在进行中。本实验结果并未发现纤维蛋白原的表达与预后相关。
白蛋白是由肝脏产生,人血清白蛋白主要由α-螺旋组成,其整体结构类似于心脏形状。人血清白蛋白有九个双环,跨越三个同源结构域。白蛋白是人体血浆中最主要的蛋白质,临床上广泛使用白蛋白评估患者的营养状况。研究表明炎性反应发生时,白蛋白水平下降[13]。本实验中也并未发现白蛋白与乳腺癌患者预后相关。推测原因可能为发达地区乳腺癌患病率较高[14],一方面患者的营养状况较好,另一方面乳腺肿瘤为体表肿物,对白蛋白消耗较少。
研究表明AFR要比单一的纤维蛋白与白蛋白能更好的预测预后,如对于心肌梗死及软组织肉瘤的预测[15]。另外研究表明AFR是老年胃癌患者经腹腔镜根治性胃切除术后严重并发症的预测指标[16]。因本实验中我们并未发现独立纤维蛋白与白蛋白与患者的预后相关,发现纤维蛋白原在死亡患者中稍降低,白蛋白在死亡患者中稍增高,二者与预后无统计学意义可能与样本量相关。鉴于二者与总生存期的变化趋势,我们选用AFR作为乳腺导管癌患者的预后评价指标。
本研究表明AFR是乳腺浸润性导管癌的独立预后因素。当患者AFR≥13.1时,患者3年、5年生存率较低。患者来院就诊时可以测定AFR,根据其结果制定相关的治疗方案。Wang等研究认为纤维蛋白原与白蛋白比值指数(fibrinogen-albumin ratio index, FARI)是结肠癌预后的危险因素,该值越大患者的死亡率越高[17]。FARI为纤维蛋白原与白蛋白比值,与我们研究的AFR比值互为倒数,FARI值越大,AFR越小。因此,Wang等与我们的结论相反,其原因可能是肿瘤种类或者研究人群不同,纤维蛋白原与白蛋白的表达水平亦不相同。为查找表达不一致的原因,我们需扩大样本量进一步研究,并查找结果不同的原因。
多因素分析结果显示:AFR、WBC、ER/PR、TNM、核分级、p53及脉管瘤栓在AFR值高低组间差异有统计学意义(均P < 0.05),我们将上述指标建立乳腺浸润性导管癌患者OS预后模型,预测患者生存率,此模型ROC曲线下面积为0.882,模型下面积为0.7至0.8时说明模型具有很好的预测价值[18]。本实验结果说明此模型具有很好的预测能力。
通过多因素分析建立患者女性浸润性导管患者预后模型,预测患者的生存率,结果可靠,在临床上具有很好的预测效能。
综上所述,AFR值越高,患者生存期越短,死亡率越高。可以将它作为女性乳腺浸润性导管癌患者预后的一个指标,而且纤维蛋白原与白蛋白检查为住院患者的基本检查项目,其检查操作简单,收集方便、廉价、可重复,值得临床推广。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:陈 婷:资料收集与分析,论文设计与撰写闫曙光:协助论文撰写李京涛:论文修改魏海梁:文献收集周永学:指导论文撰写 -
表 1 巨胞饮在消化系统肿瘤中的作用及调控因素
Table 1 Role and regulatory factors of macropinocytosis in digestive system tumors

-
[1] Mima K, Kosumi K, Baba Y, et al. The microbiome, genetics, and gastrointestinal neoplasms: the evolving field of molecular pathological epidemiology to analyze the tumor-immune-microbiome interaction[J]. Hum Genet, 2021, 140(5): 725-746. doi: 10.1007/s00439-020-02235-2
[2] Ha KD, Bidlingmaier SM, Liu B. Macropinocytosis Exploitation by Cancers and Cancer Therapeutics[J]. Front Physiol, 2016, 7: 381.
[3] Kerr MC, Teasdale RD. Defining macropinocytosis[J]. Traffic, 2009, 10(4): 364-371. doi: 10.1111/j.1600-0854.2009.00878.x
[4] Palm W. Metabolic functions of macropinocytosis[J]. Philos Trans R Soc Lond B Biol Sci, 2019, 374(1765): 20180285. doi: 10.1098/rstb.2018.0285
[5] Lin XP, Mintern JD, Gleeson PA. Macropinocytosis in Different Cell Types: Similarities and Differences[J]. Membranes (Basel), 2020, 10(8): 177. doi: 10.3390/membranes10080177
[6] 姜萱璟, 张伟晴, 贺丽娜, 等. 巨胞饮作用及其对脑胶质瘤的影响[J]. 现代肿瘤医学, 2021, 29(20): 3668-3672. doi: 10.3969/j.issn.1672-4992.2021.20.034 Jiang XJ, Zhang WQ, He LN, et al. Macropinocytosis and its influence on glioblastoma[J]. Xian Dai Zhong Liu Yi Xue, 2021, 29(20): 3668-3672. doi: 10.3969/j.issn.1672-4992.2021.20.034
[7] Finicle BT, Jayashankar V, Edinger AL. Nutrient scavenging in cancer[J]. Nat Rev Cancer, 2018, 18(10): 619-633. doi: 10.1038/s41568-018-0048-x
[8] Commisso C. The pervasiveness of macropinocytosis in oncological malignancies[J]. Philos Trans R Soc Lond B Biol Sci, 2019, 374(1765): 20180153. doi: 10.1098/rstb.2018.0153
[9] Colin M, Delporte C, Janky R, et al. Dysregulation of Macropinocytosis Processes in Glioblastomas May Be Exploited to Increase Intracellular Anti-Cancer Drug Levels: The Example of Temozolomide[J]. Cancers (Basel), 2019, 11(3): 411. doi: 10.3390/cancers11030411
[10] Jayashankar V, Edinger AL. Macropinocytosis confers resistance to therapies targeting cancer anabolism[J]. Nat Commun, 2020, 11(1): 1121. doi: 10.1038/s41467-020-14928-3
[11] Ramirez C, Hauser AD, Vucic EA, et al. Plasma membrane V-ATPase controls oncogenic RAS-induced macropinocytosis[J]. Nature, 2019, 576(7787): 477-481. doi: 10.1038/s41586-019-1831-x
[12] Unni AM, Lockwood WW, Zejnullahu K, et al. Evidence that synthetic lethality underlies the mutual exclusivity of oncogenic KRAS and EGFR mutations in lung adenocarcinoma[J]. Elife, 2015, 4: e06907. doi: 10.7554/eLife.06907
[13] Commisso C, Davidson SM, Soydaner-Azeloglu RG, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells[J]. Nature, 2013, 497(7451): 633-637. doi: 10.1038/nature12138
[14] Palm W, Araki J, King B, et al. Critical role for PI3-kinase in regulating the use of proteins as an amino acid source[J]. Proc Natl Acad Sci U S A, 2017, 114(41): E8628-E8636.
[15] Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins[J]. Science, 1986, 233(4768): 1061-1068. doi: 10.1126/science.3090687
[16] Zwartkruis FJT, Burgering BMT. Ras and macropinocytosis: trick and treat[J]. Cell Res, 2013, 23(8): 982-983. doi: 10.1038/cr.2013.79
[17] Amyere M, Payrastre B, Krause U, et al. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C[J]. Mol Biol Cell, 2000, 11(10): 3453-3467. doi: 10.1091/mbc.11.10.3453
[18] Lee SW, Zhang Y, Jung M, et al. EGFR-Pak Signaling Selectively Regulates Glutamine Deprivation-Induced Macropinocytosis[J]. Dev Cell, 2019, 50(3): 381-392.e5. doi: 10.1016/j.devcel.2019.05.043
[19] Liu H, Sun M, Liu Z, et al. KRAS-enhanced macropinocytosis and reduced FcRn-mediated recycling sensitize pancreatic cancer to albumin-conjugated drugs[J]. J Control Release, 2019, 296: 40-53. doi: 10.1016/j.jconrel.2019.01.014
[20] Redelman-Sidi G, Iyer G, Solit DB, et al. Oncogenic activation of Pak1-dependent pathway of macropinocytosis determines BCG entry into bladder cancer cells[J]. Cancer Res, 2013, 73(3): 1156-1167. doi: 10.1158/0008-5472.CAN-12-1882
[21] Takenaka T, Nakai S, Katayama M, et al. Effects of gefitinib treatment on cellular uptake of extracellular vesicles in EGFR-mutant non-small cell lung cancer cells[J]. Int J Pharm, 2019, 572: 118762. doi: 10.1016/j.ijpharm.2019.118762
[22] Zdżalik-Bielecka D, Poświata A, Kozik K, et al. The GAS6-AXL signaling pathway triggers actin remodeling that drives membrane ruffling, macropinocytosis, and cancer-cell invasion[J]. Proc Natl Acad Sci U S A, 2021, 118(28): e2024596118. doi: 10.1073/pnas.2024596118
[23] Redelman-Sidi G, Binyamin A, Gaeta I, et al. The Canonical Wnt Pathway Drives Macropinocytosis in Cancer[J]. Cancer Res, 2018, 78(16): 4658-4670. doi: 10.1158/0008-5472.CAN-17-3199
[24] Ghoshal P, Singla B, Lin H, et al. Nox2-Mediated PI3K and Cofilin Activation Confers Alternate Redox Control of Macrophage Pinocytosis[J]. Antioxid Redox Signal, 2017, 26(16): 902-916. doi: 10.1089/ars.2016.6639
[25] Sivanand S, Vander Heiden MG. Transcriptional activation of macropinocytosis by the Hippo pathway following nutrient limitation[J]. Genes Dev, 2020, 34(19-20): 1253-1255. doi: 10.1101/gad.343632.120
[26] Srivastava RK, Li C, Khan J, et al. Combined mTORC1/mTORC2 inhibition blocks growth and induces catastrophic macropinocytosis in cancer cells[J]. Proc Natl Acad Sci U S A, 2019, 116(49): 24583-24592. doi: 10.1073/pnas.1911393116
[27] Dai M, Yan G, Wang N, et al. In vivo genome-wide CRISPR screen reveals breast cancer vulnerabilities and synergistic mTOR/Hippo targeted combination therapy[J]. Nat Commun, 2021, 12(1): 3055. doi: 10.1038/s41467-021-23316-4
[28] Palm W, Park Y, Wright K, et al. The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1[J]. Cell, 2015, 162(2): 259-270. doi: 10.1016/j.cell.2015.06.017
[29] Zhu G, Pei L, Xia H, et al. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer[J]. Mol Cancer, 2021, 20(1): 143. doi: 10.1186/s12943-021-01441-4
[30] Hanada K, Kawada K, Nishikawa G, et al. Dual blockade of macropinocytosis and asparagine bioavailability shows synergistic anti-tumor effects on KRAS-mutant colorectal cancer[J]. Cancer Lett, 2021, 522: 129-141. doi: 10.1016/j.canlet.2021.09.023
[31] Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, et al. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis[J]. J Control Release, 2017, 266: 100-108. doi: 10.1016/j.jconrel.2017.09.019
[32] Takakura H, Nakao T, Narita T, et al. Citrus limonL. -Derived Nanovesicles Show an Inhibitory Effect on Cell Growth in p53-Inactivated Colorectal Cancer Cells via the Macropinocytosis Pathway[J]. Biomedicines, 2022, 10(6): 1352. doi: 10.3390/biomedicines10061352
[33] Cancer Genome Atlas Research Network. Electronic address: andrew_aguirre@dfci.harvard.edu; Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma[J]. Cancer Cell, 2017, 32(2): 185-203.e13. doi: 10.1016/j.ccell.2017.07.007
[34] Encarnación-Rosado J, Kimmelman AC. Harnessing metabolic dependencies in pancreatic cancers[J]. Nat Rev Gastroenterol Hepatol, 2021, 18(7): 482-492. doi: 10.1038/s41575-021-00431-7
[35] Rai V, Agrawal S. Targets (Metabolic Mediators) of Therapeutic Importance in Pancreatic Ductal Adenocarcinoma[J]. Int J Mol Sci, 2020, 21(22): 8502. doi: 10.3390/ijms21228502
[36] Xiong X, Rao G, Roy RV, et al. Ubiquitin-binding associated protein 2 regulates KRAS activation and macropinocytosis in pancreatic cancer[J]. FASEB J, 2020, 34(9): 12024-12039. doi: 10.1096/fj.201902826RR
[37] Thu PM, Zheng ZG, Zhou YP, et al. Phellodendrine chloride suppresses proliferation of KRAS mutated pancreatic cancer cells through inhibition of nutrients uptake via macropinocytosis[J]. Eur J Pharmacol, 2019, 850: 23-34. doi: 10.1016/j.ejphar.2019.01.060
[38] Zhang YF, Li Q, Huang PQ, et al. A low amino acid environment promotes cell macropinocytosis through the YY1-FGD6 axis in Ras-mutant pancreatic ductal adenocarcinoma[J]. Oncogene, 2022, 41(8): 1203-1215. doi: 10.1038/s41388-021-02159-9
[39] Ikeda M, Okusaka T, Mitsunaga S, et al. Safety and Pharmacokinetics of Lenvatinib in Patients with Advanced Hepatocellular Carcinoma[J]. Clin Cancer Res, 2016, 22(6): 1385-1394. doi: 10.1158/1078-0432.CCR-15-1354
[40] Zhang MS, Cui JD, Lee D, et al. Hypoxia-induced macropinocytosis represents a metabolic route for liver cancer[J]. Nat Commun, 2022, 13(1): 954. doi: 10.1038/s41467-022-28618-9
[41] Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma[J]. Gastro-enterology, 2019, 156(2): 477-491.e1. doi: 10.1053/j.gastro.2018.08.065
[42] Byun JK, Lee S, Kang GW, et al. Macropinocytosis is an alternative pathway of cysteine acquisition and mitigates sorafenib-induced ferroptosis in hepatocellular carcinoma[J]. J Exp Clin Cancer Res, 2022, 41(1): 98. doi: 10.1186/s13046-022-02296-3
[43] Demarest CT, Chang AC. The Landmark Series: Multimodal Therapy for Esophageal Cancer[J]. Ann Surg Oncol, 2021, 28(6): 3375-3382. doi: 10.1245/s10434-020-09565-5
[44] He S, Zhao C, Tao H, et al. A recombinant scFv antibody-based fusion protein that targets EGFR associated with IMPDH2 downregulation and its drug conjugate show therapeutic efficacy against esophageal cancer[J]. Drug Deliv, 2022, 29(1): 1243-1256. doi: 10.1080/10717544.2022.2063454
[45] Jiang Y, Guo H, Tong T, et al. lncRNA lnc-POP1-1 upregulated by VN1R5 promotes cisplatin resistance in head and neck squamous cell carcinoma through interaction with MCM5[J]. Mol Ther, 2022, 30(1): 448-467. doi: 10.1016/j.ymthe.2021.06.006
[46] Song S, Xia X, Qi J, et al. Silmitasertib-induced macropinocytosis promoting DDP intracellular uptake to enhance cell apoptosis in oral squamous cell carcinoma[J]. Drug Deliv, 2021, 28(1): 2480-2494. doi: 10.1080/10717544.2021.2000677
[47] Lu Y, Zheng Z, Yuan Y, et al. The Emerging Role of Exosomes in Oral Squamous Cell Carcinoma[J]. Front Cell Dev Biol, 2021, 9: 628103. doi: 10.3389/fcell.2021.628103
[48] Cao J, Zhang M, Xie F, et al. Exosomes in head and neck cancer: Roles, mechanisms and applications[J]. Cancer Lett, 2020, 494: 7-16. doi: 10.1016/j.canlet.2020.07.005
[49] Sasabe E, Tomomura A, Liu H, et al. Epidermal growth factor/epidermal growth factor receptor signaling blockage inhibits tumor cell-derived exosome uptake by oral squamous cell carcinoma through macropinocytosis[J]. Cancer Sci, 2022, 113(2): 609-621. doi: 10.1111/cas.15225
-
期刊类型引用(5)
1. 彭晴,李洪涛,黄高忠. 纤维蛋白原与白蛋白比值在预测恶性肿瘤预后中的研究进展. 中国慢性病预防与控制. 2022(05): 391-393 .  百度学术
百度学术
2. 王羿翔,李明山,董理鸣,薛东炜,刘屹立,王平. 术前白蛋白与纤维蛋白原比值对非肌层浸润性膀胱癌患者预后的价值. 现代肿瘤医学. 2022(18): 3384-3388 .  百度学术
百度学术
3. 张岚,李群锋,冯耀霞. 系统性炎症指标与浸润性乳腺癌淋巴结转移及临床预后的相关性. 中国卫生检验杂志. 2022(12): 1482-1485 .  百度学术
百度学术
4. 崔杰,刘鑫. 纤维蛋白原与白蛋白比值与激素受体阳性/人类表皮生长因子受体2阴性乳腺癌新辅助化疗疗效相关性分析. 陕西医学杂志. 2022(11): 1351-1354+1359 .  百度学术
百度学术
5. 刘晓丽,黄海伟. 纤维蛋白原与白蛋白比值(FAR)对IB-IIA期宫颈癌患者预后的预测作用. 中国继续医学教育. 2021(31): 163-167 .  百度学术
百度学术
其他类型引用(2)



 下载:
下载: