Role of Lactate Dehydrogenase in Tumor Metabolism and Progress of LDH-targeted Drugs
-
摘要:
能量代谢异常是肿瘤的特征性病理变化之一。有研究表明,肿瘤细胞即使在有氧情况下,也选择糖酵解方式并在乳酸脱氢酶A(LDHA)帮助下生成乳酸和少量ATP(即著名的瓦伯格效应)。细胞内过多的乳酸将在细胞膜上单羧酸转运蛋白4(MCT4)作用下转运至细胞外,并导致肿瘤细胞微环境pH值降低,同时肿瘤细胞微环境中的乳酸又可以被MCT1转运至邻近相对富氧区域的肿瘤细胞,并在LDHB的作用下,重新转变成丙酮酸用于氧化磷酸化。LDH在肿瘤能量代谢重编程中的重要作用,使其成为抗肿瘤治疗的新靶点。本文回顾了LDH与肿瘤细胞能量代谢之间的关系,并重点讨论了LDH在肿瘤发生发展中的作用,以及以LDH为靶点抗肿瘤药物研究的进展。
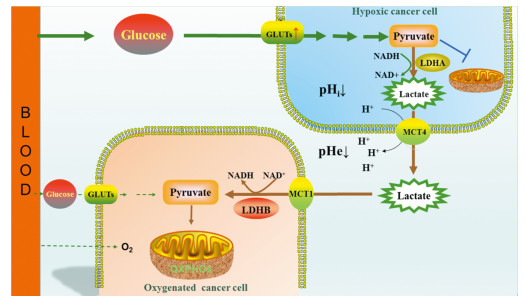
Abstract:Abnormal energy metabolism is one of the main hallmarks of cancer. Studies have shown that tumor cells, even in the presence of oxygen, favored glycolysis to produce lactic acid and a small amount of ATP with the help of LDHA in the cytosol (the famous Warburg effect). The excessive lactate is then exported to the extracellular space in the presence of monocarboxylate transporters (MCTs, primarily MCT4), resulting in the decrease of microenvironment pH value. On the other hand, the lactate in the tumor microenvironment will be re-absorbed in adjacent oxygenated tumor cells through the transporter MCT1 and finally transformed into pyruvate for oxidative phosphorylation under the effect of LDHB. The important effects of LDH in tumor energy metabolism reprogramming make it a new target for tumor therapy. In this review, we discussed briefly on the relation between LDH and energy metabolism of tumor cells, especially its effects on the process of tumor as well as the progress of LDH-targeted anti-tumor drugs.
-
Key words:
- Lactate /
- Lactic dehydrogenase /
- Warburg effect /
- Pyruvate metabolism
-
Competing interests: The authors declare that they have no competing interests.作者贡献丘金梅:论文构思、撰写与修改龚娟、谢青池:参与论文撰写及文献收集与整理谢志忠:论文构思、写作指导及审阅
-
[1] Warburg O. On the origin of cancer cells[J]. Science, 1956, 123(3191): 309-314.
[2] Felmlee MA, Jones RS, Rodriguez-Cruz V, et al. Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease[J]. Pharmacol Rev, 2020, 72(2): 466-485. http://www.researchgate.net/publication/339752470_Monocarboxylate_Transporters_SLC16_Function_Regulation_and_Role_in_Health_and_Disease
[3] Pucino V, Cucchi D, Mauro C. Lactate transporters as therapeutic targets in cancer and inflammatory diseases[J]. Expert Opin Ther Targets, 2018, 22(9): 735-743. http://www.ncbi.nlm.nih.gov/pubmed/30106309
[4] Contreras-Baeza Y, Sandoval PY, Alarcón R. Monocarboxylate transporter 4 (MCT4) is a high affinity transporter capable of exporting lactate in high-lactate microenvironments[J]. J Biol Chem, 2019, 294(52): 20135-20147. http://www.jbc.org/content/294/52/20135.short
[5] Guan X, Bryniarski MA, Morris ME. In Vitro and In Vivo Efficacy of the Monocarboxylate Transporter 1 Inhibitor AR-C155858 in the Murine 4T1 Breast Cancer Tumor Model[J]. AAPS J, 2018, 21(1): 3.
[6] Benjamin D, Robay D, Hindupur SK, et al. Dual Inhibition of the Lactate Transporters MCT1 and MCT4 Is Synthetic Lethal with Metformin due to NAD+ Depletion in Cancer Cells[J]. Cell Rep, 2018, 25(11): 3047-3058. e4. http://www.researchgate.net/publication/329979812_Dual_Inhibition_of_the_Lactate_Transporters_MCT1_and_MCT4_Is_Synthetic_Lethal_with_Metformin_due_to_NAD_Depletion_in_Cancer_Cells
[7] Srinivas SR, Gopal E, Zhuang L, et al. Cloning and functional identification of slc5a12 as a sodium-coupled low-affinity transporter for monocarboxylates (SMCT2)[J]. Biochem J, 2005, 392(Pt 3): 655-664. http://europepmc.org/abstract/MED/16104846
[8] Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer[J]. Cancer Res, 2011, 71(22): 6921-6925. http://carcin.oxfordjournals.org/cgi/ijlink?linkType=ABST&journalCode=canres&resid=71/22/6921
[9] Haas R, Smith J, Rocher-Ros V, et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions[J]. PLoS Biol, 2015, 13(7): e1002202. http://pubmedcentralcanada.ca/pmcc/articles/PMC4504715/
[10] Guan X, Morris ME. Pharmacokinetics of the Monocarboxylate Transporter 1 Inhibitor AZD3965 in Mice: Potential Enterohepatic Circulation and Target-Mediated Disposition[J]. Pharm Res, 2019, 37(1): 5. doi: 10.1007%2Fs11095-019-2735-z
[11] Hou L, Zhao Y, Song GQ, et al. Interfering cellular lactate homeostasis overcomes Taxol resistance of breast cancer cells through the microRNA-124-mediated lactate transporter (MCT1) inhibition[J]. Cancer Cell Int, 2019, 19: 193. http://www.ncbi.nlm.nih.gov/pubmed/31367191
[12] Chen C, Zhu YH, Huang JA. Clinical evaluation of potential usefulness of serum lactate dehydrogenase level in follow-up of small cell lung cancer[J]. J Cancer Res Ther, 2018, 14(Supplement): S336-S340. http://europepmc.org/abstract/MED/29970686
[13] Zhang X, Guo M, Fan J, et al. Prognostic significance of serum LDH in small cell lung cancer: A systematic review with meta-analysis[J]. Cancer Biomark, 2016, 16(3): 415-423. http://europepmc.org/abstract/MED/27062698
[14] Xu XD, Shao SX, Jiang HP, et al. Warburg effect or reverse Warburg effect? A review of cancer metabolism[J]. Oncol Res Treat, 2015, 38(3): 117-122. http://europepmc.org/abstract/med/25792083
[15] Zong WX, Rabinowitz JD, White E. Mitochondria and Cancer[J]. Mol Cell, 2016, 61(5): 667-676.
[16] Epstein T, Gatenby RA, Brown JS. The Warburg effect as an adaptation of cancer cells to rapid fluctuations in energy demand[J]. PLoS One, 2017, 12(9): e0185085. http://www.ncbi.nlm.nih.gov/pubmed/28922380
[17] de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, et al. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches[J]. Front Oncol, 2019, 9: 1143. http://www.researchgate.net/publication/336976433_lactate_in_the_regulation_of_tumor_microenvironment_and_therapeutic_approaches
[18] Brooks GA. The Science and Translation of Lactate Shuttle Theory[J]. Cell Metab, 2018, 27(4): 757-785. http://europepmc.org/abstract/MED/29617642
[19] Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells?[J] Trends Biochem Sci, 2016, 41(3): 211-218. http://www.ncbi.nlm.nih.gov/pubmed/26778478
[20] Cui J, Shi M, Xie D, et al. FOXM1 promotes the warburg effect and pancreatic cancer progression via transactivation of LDHA expression[J]. Clin Cancer Res, 2014, 20(10): 2595-2606. http://pubmedcentralcanada.ca/pmcc/articles/PMC4024335/
[21] Jiang W, Zhou F, Li N, et al. FOXM1-LDHA signaling promoted gastric cancer glycolytic phenotype and progression[J]. Int J Clin Exp Pathol, 2015, 6: 6756-6763. http://www.ncbi.nlm.nih.gov/pubmed/26261559
[22] Su Y, Yu QH, Wang XY, et al. JMJD2A promotes the Warburg effect and nasopharyngeal carcinoma progression by transactivating LDHA expression[J]. BMC Cancer, 2017, 17(1): 477. http://pubmedcentralcanada.ca/pmcc/articles/PMC5504777/
[23] Jiang F, Ma S, Xue Y, et al. LDH-A promotes malignant progression via activation of epithelial-to-mesenchymal transition and conferring stemness in muscle-invasive bladder cancer[J]. Biochem Biophys Res Commun, 2016, 469(4): 985-992. http://www.ncbi.nlm.nih.gov/pubmed/26721441
[24] Jiang P, Du W, Wu M. Regulation of the pentose phosphate pathway in cancer[J]. Protein Cell, 2014, 5(8): 592-602. http://www.cell.com/trends/biochemical-sciences/references/S0968-0004(14)00106-6
[25] Martinez-Outschoorn UE, Lin Z, Trimmer C, et al. Cancer cells metabolically' fertilize' the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: Implications for PET imaging of human tumors[J]. Cell Cycle, 2011, 10(15): 2504-2520. doi: 10.4161/cc.10.15.16585
[26] Wang SJ, Xie J, Li JJ, et al. Cisplatin suppresses the growth and proliferation of breast and cervical cancer cell lines by inhibiting integrin β5-mediated glycolysis[J]. Am J Cancer Res, 2016, 6(5): 1108-1117. http://www.ncbi.nlm.nih.gov/pubmed/27294003
[27] McCleland ML, Adler AS, Deming L, et al. Lactate dehydrogenase B is required for the growth of KRAS-dependent lung adenocarcinomas[J]. Clin Cancer Res, 2013, 19(4): 773-784.
[28] Li C, Chen Y, Bai PP, et al. LDHB may be a significant predictor of poor prognosis in osteosarcoma[J]. Am J Transl Res, 2016, 8(11): 4831-4843. http://europepmc.org/articles/PMC5126326/
[29] Sun W, Zhang X, Ding X, et al. Lactate dehydrogenase B is associated with the response to neoadjuvant chemotherapy in oral squamous cell carcinoma[J]. PLoS One, 2015, 10(5): e0125976. http://pubmedcentralcanada.ca/pmcc/articles/PMC4431727/
[30] Kumar S, Xie H, Scicluna P, et al. MiR-375 Regulation of LDHB Plays Distinct Roles in Polyomavirus-Positive and -Negative Merkel Cell Carcinoma[J]. Cancers (Basel), 2018, 10(11): 443.
[31] Kim JH, Kim EL, Lee YK, et al. Decreased lactate dehydrogenase B expression enhances claudin 1-mediated hepatoma cell invasiveness via mitochondrial defects[J]. Exp Cell Res, 2011, 317(8): 1108-1118. http://europepmc.org/abstract/MED/21356207
[32] Wu GY, Yuan SC, Chen ZP, et al. The KLF14 Transcription Factor Regulates Glycolysis by Downregulating LDHB in Colorectal Cancer[J]. Int J Biol Sci, 2019, 15(3): 628-635. http://www.researchgate.net/publication/330966450_The_KLF14_Transcription_Factor_Regulates_Glycolysis_by_Downregulating_LDHB_in_Colorectal_Cancer
[33] Maekawa M, Taniguchi T, Ishikawa J, et al. Promoter hypermethylation in cancer silences LDHB, eliminating lactate dehydrogenase isoenzymes 1-4[J]. Clin Chem, 2003, 49(9): 1518-1520. http://www.ncbi.nlm.nih.gov/pubmed/12928234
[34] Leiblich A, Cross SS, Catto JW, et al. Lactate dehydrogenase-B is silenced by promoter hypermethylation in human prostate cancer[J]. Oncogene, 2006, 25(20): 2953-2960.
[35] Cui JJ, Quan M, Jiang WH, et al. Suppressed expression of LDHB promotes pancreatic cancer progression via inducing glycolytic phenotype[J]. Med Oncol, 2015, 32(5): 143. doi: 10.1007/s12032-015-0589-8
[36] McCleland ML, Adler AS, Shang Y, et al. An integrated genomic screen identifies LDHB as an essential gene for triple-negative breast cancer[J]. Cancer Res, 2012, 72(22): 5812-5823.
[37] Brown NJ, Higham SE, Perunovic B, et al. Lactate dehydrogenase-B is silenced by promoter methylation in a highfrequency of human breast cancers[J]. PLoS One, 2013, 8(2): e57697. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3578788/
[38] Hussien R, Brooks GA. Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines[J]. Physiol Genomics, 2011, 43(5): 255-264. http://www.europepmc.org/articles/PMC3068517/
[39] Liao AC, Li CF, Shen KH, et al. Loss of lactate dehydrogenase B subunit expression is correlated with tumour progression and independently predicts inferior disease-specific survival in urinary bladder urothelial carcinoma[J]. Pathology, 2011, 43(7): 707-712. http://www.sciencedirect.com/science/article/pii/S0031302516329403
[40] Shi L, Yan H, An S, et al. SIRT5-mediated deacetylation of LDHB promotes autophagy and tumorigenesis in colorectal cancer[J]. Mol Oncol, 2019, 13(2): 358-375. http://www.ncbi.nlm.nih.gov/pubmed/30443978
[41] Takeo N, Fujiwara S, Sakai T, et al. Hereditary Lactate Dehydrogenase M-subunit Deficiency With Late-Developing Pustular Psoriasis-Like Lesions[J]. J Dermatol, 2016, 43(12): 1429-1432. http://www.onacademic.com/detail/journal_1000039519858510_675b.html
[42] Koukourakis M, Tsolou A, Pouliliou S, et al. Blocking LDHA Glycolytic Pathway Sensitizes Glioblastoma Cells to Radiation and Temozolomide[J]. Biochem Biophys Res Commun, 2017, 491(4): 932-938. https://www.sciencedirect.com/science/article/abs/pii/S0006291X17314973
[43] Van Poznak C, Seidman AD, Reidenberg MM, et al. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase Ⅰ/Ⅱ clinical trial[J]. Breast Cancer Res Treat, 2001, 66(3): 239-248.
[44] Zhang SL, He Y, Tam KY. Targeting cancer metabolism to develop human lactate dehydrogenase (hLDH) 5 inhibitors[J]. Drug Discov Today, 2018, 23(7): 1407-1415. http://www.sciencedirect.com/science/article/pii/S1359644617305329
[45] Ždralević M, Brand A, Di Ianni L, et al. Double genetic disruption of lactate dehydrogenases A and B is required to ablate the "Warburg effect" restricting tumor growth to oxidative metabolism[J]. J Biol Chem, 2018, 293(41): 15947-15961.



 下载:
下载: