-
摘要:目的
探讨阿帕替尼治疗晚期难治性乳腺癌的临床疗效及安全性。
方法回顾性分析经多线治疗失败的晚期难治性乳腺癌患者29例,给予患者阿帕替尼500 mg/d,餐后半小时口服。观察临床疗效及不良反应发生情况。当出现Ⅲ级或以上不良反应时给予对症治疗及护理,仍不缓解时暂停用药,待不良反应恢复到≤Ⅰ级,再次应用阿帕替尼,并降低剂量为250 mg/d。
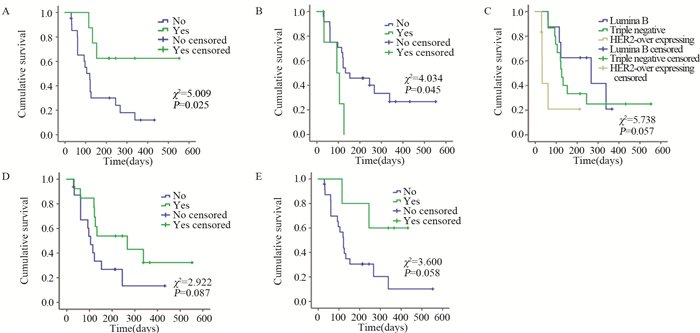
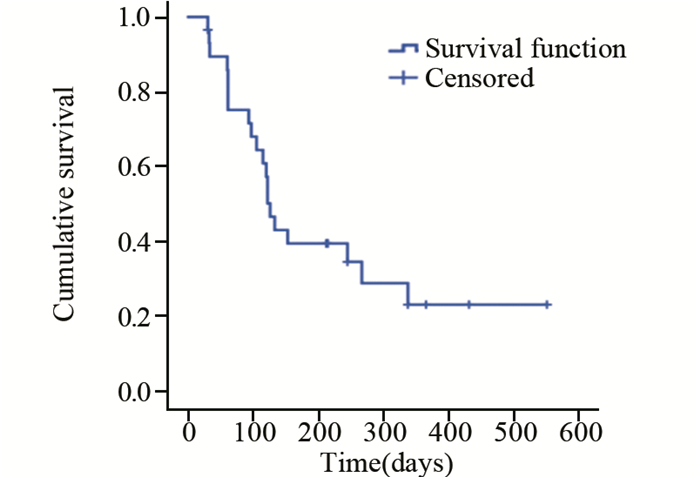
结果CR 0例,PR 37.9%(11/29),SD 44.8%(13/29),PD 17.2%(5/29),疾病控制率(PR+SD)82.8%(24/29)。中位无进展生存期126天。主要不良反应为继发性高血压(27.59%)、手足综合征(20.69%)、继发性蛋白尿(17.24%)、恶心乏力(13.79%)、继发性口腔黏膜炎(17.24%)及腹泻(10.34%),并且Ⅰ~Ⅱ级为主。Log rank单因素分析显示:在晚期难治性乳腺癌中Luminal B型(HER2阴性)及三阴性较HER2阳性型更能从阿帕替尼获益,mPFS分别为:267、126、33 d(P=0.057)。继发性高血压及继发性蛋白尿的患者mPFS更长。Cox回归分析显示:分子分型、继发高血压和继发性蛋白尿是阿帕替尼治疗晚期难治性乳腺癌mPFS的独立影响因素。
结论阿帕替尼治疗晚期难治性Luminal B(HER2阴性)型及三阴性乳腺癌仍有较好的疾病控制率及无进展生存期,不良反应可控。
Abstract:ObjectiveTo evaluate the clinical efficacy and safety of apatinib in the treatment of advanced breast cancer(ABC) patients refractory to multiline treatments.
MethodsWe retrospectively analyzed the clinical data of 29 ABC patients after the failure of multi-line treatments. Patients were treated with apatinib 500 mg/d orally. The clinical efficacy and adverse effects were evaluated. Symptomatic treatment and nursing care were given when the adverse effects were ≥grade Ⅲ and apatinib was suspended if not get relieved. When the adverse effects were ≤gradeⅠ, apatinib was administrated with reduced dosage of 250mg/d.
ResultsAmong 29 patients, there was no case with complete remission, 11(37.9%) patients with partial remission (PR), 13(44.8%) cases with stable disease (SD) and 5(17.2%) patients with progressive disease (PD). The disease control rate (PR+SD) was 82.8% (24/29). The median progression-free survival time (mPFS) was 126 days. The most common adverse effects were secondary hypertension(27.59%), hand-foot syndrome(20.69%), Secondary proteinuria(17.24%), nausea and fatigue(13.79%), Secondary oral mucositis(17.24%) and diarrhea(10.24%). Most of the side effects were degreeⅠ-Ⅱ. Log-rank univariate analysis showed a mPFS of 267 days for Luminal B(HER2-) ABC patients and 126 days for triple-negative group, compared with 33 days for HER2+ ABC patients (P=0.057). Patients with secondary hypertension or transient proteinuria had significantly longer mPFS than those without these adverse effects (P=0.025, P=0.058). Cox regression analysis identified molecular typing, secondary hypertension and proteinuria as the independent influence factors for mPFS of ABC patients treated with apatinib.
ConclusionApatinib has relatively high DCR and PFS in the treatment of Luminal B (HER2-) and triple-negative MBC patients, and adverse reactions could be controlled.
-
Key words:
- Breast cancer /
- Molecular typing /
- Apatinib /
- Clinical efficacy /
- Adverse effect
-
Competing interests: The authors declare that they have no competing interests.作者贡献王静:病例资料收集,数据整理及分析、论文撰写及修改贾敬好:课题构思指导,论文指导及修改刘晶晶:临床资料整理及随访崔志超:临床病例资料收集熊伟、王晓红:课题构思指导
-
表 1 29例晚期难治性乳腺癌患者一般资料
Table 1 Clinical characteristics of 29 advanced breast cancer patients

表 2 阿帕替尼治疗ABC患者预后的单因素分析
Table 2 Univariate analysis of prognosis of ABC patients treated with apatinib

表 3 阿帕替尼治疗ABC患者预后的多因素分析结果
Table 3 Multivariable analysis of prognosis of ABC patients treated with apatinib

表 4 阿帕替尼主要相关不良反应发生情况(n(%))
Table 4 Incidence of main adverse effects related to apatinib (n(%))

-
[1] Cardoso F, Harbeck N, Fallowfield L, et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2012, 23 suppl 7: vii 11-19.
[2] Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis[J]. Nature, 2011, 473(7347): 298-307. doi: 10.1038/nature10144
[3] Huang L, Wei Y, Shen S, et al. Therapeutic effect of apatinib on overall survival is mediated by prolonged progression-free survival in advanced gastric cancer patients[J]. Oncotarget, 2017, 8(17): 29346-20354. doi: 10.18632/oncotarget.12897
[4] Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia[J]. Cancer Cell, 2014, 26(5): 605-622. doi: 10.1016/j.ccell.2014.10.006
[5] Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies[J]. BMC Cancer, 2010, 10: 529. doi: 10.1186/1471-2407-10-529
[6] Anderson BO, Shyyan R, Eniu A, et al. Breast cancer in limited-resource countries: an overview of the breast health global initiative 2005 guidelines[J]. Breast J, 2006, 12 Suppl 1: S3-S15. http://europepmc.org/abstract/MED/16430397
[7] Verheul HMW, Voest EE, Schlingemann RO. Are tumours angiogenesis-dependent?[J]. J Pathol, 2004, 202(1): 5-13. doi: 10.1002/path.1473
[8] Rossari JR, Metzger-Filho O, Paesmans M, et al. Bevacizumab and Breast Cancer: A Meta-Analysis of First-Line Phase Ⅲ Studies and a Critical Reappraisal of Available Evidence[J]. J Oncol, 2012, 2012: 417673. http://www.ncbi.nlm.nih.gov/pubmed/23008712
[9] Vrdoljak E, Marschner N, Zielinski C, et al. Final results of the TANIA randomised phase Ⅲ trial of bevacizumab after progression on first-line bevacizumab therapy for HER2-negative locally recurrent/metastatic breast cancer[J]. Ann Oncol, 2016, 27(11): 2046-2052. doi: 10.1093/annonc/mdw316
[10] Drooger JC, van Tinteren H, de Groot SM, et al. A randomized phase 2 study exploring the role of bevacizumab and a chemotherapy-free approach in HER2-positive metastatic breast cancer: The HAT study (BOOG 2008-2003), a Dutch Breast Cancer Research Group trial[J]. Cancer, 2016, 122(19): 2961-2970. doi: 10.1002/cncr.30141
[11] Martín M, Loibl S, Hyslop T, et al. Evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for hormone receptor-positive metastatic breast cancer: a pooled analysis from the LEA (GEICAM/2006-11_GBG51) and CALGB 40503 (Alliance) trials[J]. Eur J Cancer, 2019, 117: 91-98. doi: 10.1016/j.ejca.2019.06.002
[12] 张化芝, 李晓双, 郭晓烨, 等.阿帕替尼通过抑制糖酵解途径对乳腺癌MDA-MB-231细胞增殖抑制及凋亡的作用[J].肿瘤防治研究, 2019, 46(5): 401-405. doi: 10.3971/j.issn.1000-8578.2019.18.1422 Zhang HZ, Li XS, Guo XY, et al. Apatinib Suppresses Proliferation and Induced Apoptosis of Human Breast Cancer Cell Line MDA-MB-231 Through Glycolytic Inhibition[J]. Zhong Liu Fang Zhi Yan Jiu, 2019, 46(5): 401-405. doi: 10.3971/j.issn.1000-8578.2019.18.1422
[13] Gao Z, Shi M, Wang Y, et al. Apatinib enhanced anti-tumor activity of cisplatin on triple-negative breast cancer through inhibition of VEGFR-2[J]. Pathol Res Pract, 2019, 215(7): 152422. doi: 10.1016/j.prp.2019.04.014
[14] Hu X, Zhang J, Xu B, et al. Multicenter phase Ⅱ study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer[J]. Int J Cancer, 2014, 135(8): 1961-1969. doi: 10.1002/ijc.28829
[15] Hu X, Cao J, Hu W, et al. Multicenter phase Ⅱ study of apatinib in non-triple-negative metastatic breast cancer[J]. BMC Cancer, 2014, 14: 820. doi: 10.1186/1471-2407-14-820
[16] Lin Y, Wu Z, Zhang J, et al. Apatinib for metastatic breast cancer in non-clinical trial setting: Satisfying efficacy regardless of previous anti-angiogenic treatment[J]. Tumour Biol, 2017, 39(6): 1010428317711033. http://europepmc.org/abstract/MED/28639910
[17] Zhu A, Yuan P, Wang J, et al. Apatinib combined with chemotherapy in patients with previously treated advanced breast cancer: An observational study[J]. Oncol Lett, 2019, 17(6): 4768-4778. http://www.researchgate.net/publication/332165686_Apatinib_combined_with_chemotherapy_in_patients_with_previously_treated_advanced_breast_cancer_An_observational_study
[18] Li Y, Zhou Y, Wang Y, et al. Comparison of apatinib and capecitabine (Xeloda) with capecitabine (Xeloda) in advanced triple negative breast cancer as third-line therapy A retrospective study[J]. Medicine (Baltimore), 2018, 97(36): e12222. doi: 10.1097/MD.0000000000012222
[19] 秦叔逵, 李进.阿帕替尼治疗胃癌的临床应用专家共识[J].临床肿瘤学杂志, 2015, 20(9): 841-847. http://www.cnki.com.cn/Article/CJFDTotal-LCZL201509014.htm Qin SQ, Li J. Expert consensus on the clinical application of apatinib for gastric cancer[J]. Lin Chuang Zhong Liu Xue Za Zhi, 2015, 20(9): 841-847. http://www.cnki.com.cn/Article/CJFDTotal-LCZL201509014.htm
[20] Sun D, Hou H, Zhang C, et al. The efficacy and safety of apatinib for refractory malignancies: a review and meta-analysis[J]. Onco Targets Ther, 2018, 11: 6539-6554. doi: 10.2147/OTT.S176429
[21] Fan M, Zhang J, Wang Z, et al. Phosphorylated VEGFR2 and hypertension: potential biomarkers to indicate VEGFdependency of advanced breast cancer in anti-angiogenic therapy[J]. Breast Cancer Res Treat, 2014, 143(1): 141-151. doi: 10.1007/s10549-013-2793-6



 下载:
下载: