Prospective Study on Tooth Loss and Risk of Esophageal Cancer Among Residents of A Natural Village in Wenfeng District, Anyang City, Henan Province
-
摘要:目的
研究河南省安阳市文峰区某自然村牙齿脱落与食管癌发生的关系。
方法采用前瞻性队列研究方法,自2008年1月至2024年7月对该自然村无症状居民连续随访16年,观察居民牙齿脱落及食管癌发生的情况。资料统计采用卡方检验和二元Logistic回归及限制性立方样条图(RCS)。
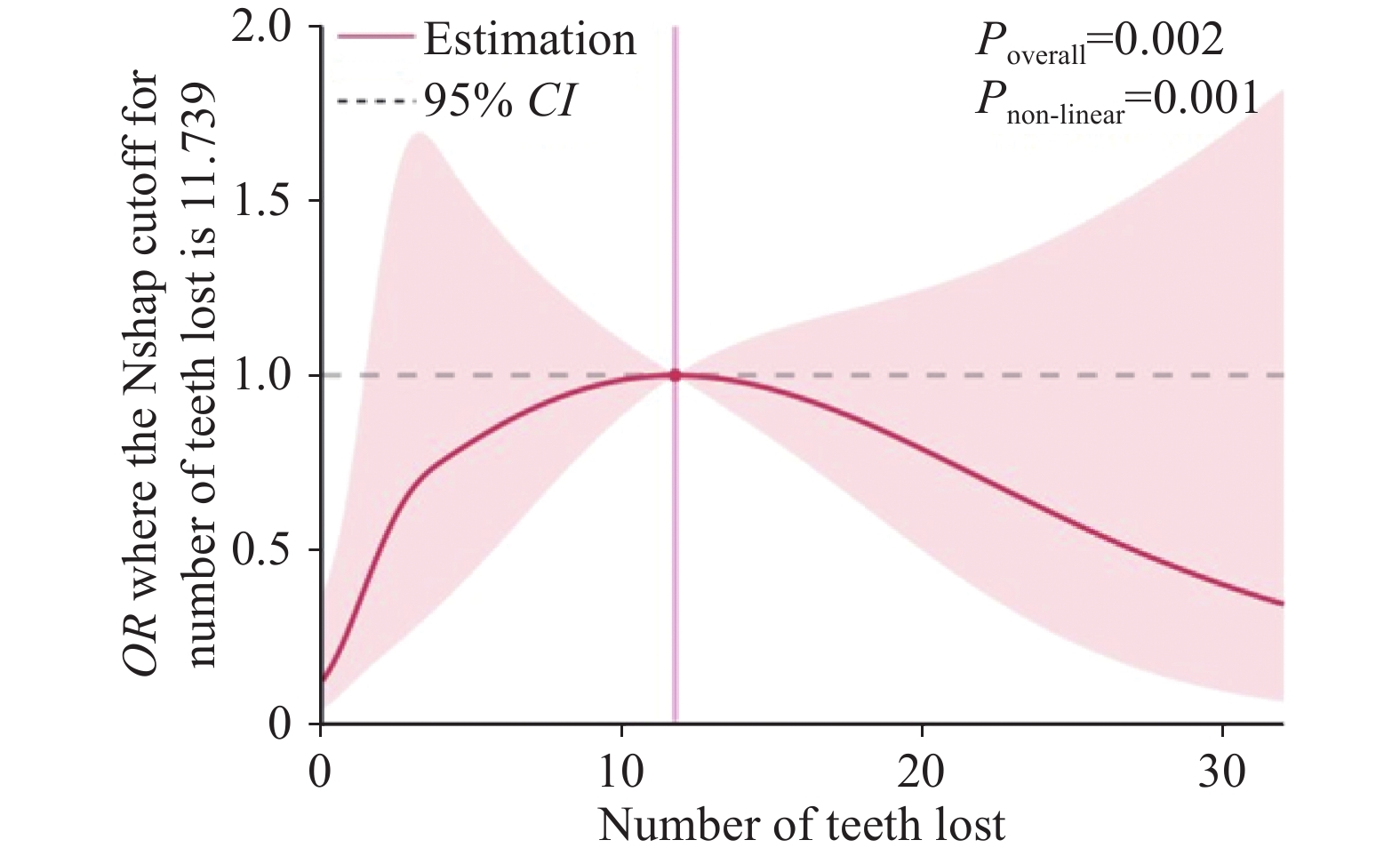
结果总人群711人,失访136人,最终纳入统计575人,其中患食管癌者45人,牙齿有无脱落在食管癌患者中具有明显统计学差异(P<0.05)。Logistic回归分析表明牙齿脱落与食管癌发生有关(OR=3.977,95%CI:1.543~10.255),调整混杂因素后发现,牙齿脱落仍与食管癌发生有显著关系(OR=3.038,95%CI:1.035~8.914)。牙齿脱落颗数与食管癌发生存在非线性剂量-反应关系:当牙齿脱落<12颗时,患食管癌风险会随着牙齿脱落颗数增加而增大;当牙齿脱落≥12颗时,患食管癌风险会随着牙齿脱落颗数增加而降低。
结论该村牙齿脱落是食管癌发生危险因素。当牙齿脱落颗数<12时,随着牙齿脱落颗数增加,患癌风险增大。
Abstract:ObjectiveTo investigate the relationship between tooth loss and the occurrence of esophageal cancer in a natural village in Wenfeng District, Anyang City, Henan Province.
MethodsA prospective cohort study was conducted to observe the occurrence of tooth loss and esophageal cancer among the asymptomatic residents of the natural village for 16 years from January 2008 to July 2024. Data were analyzed by chi-square test, binary logistic regression, and restricted cubic spline.
ResultsAmong the total population of 711 cases, 136 cases were lost to follow-up and 575 cases were included in the final statistics, including 45 cases with esophageal cancer. Significant statistical difference was found between esophageal cancer patients with and without tooth loss (P<0.05). Logistic regression analysis showed that tooth loss was associated with the occurrence of esophageal cancer (OR=3.977, 95%CI: 1.543-10.255). After the adjustment for confounders, tooth loss remained significantly associated with the occurrence of esophageal cancer (OR=3.038, 95%CI: 1.035-8.914). A nonlinear dose-response relationship was observed between the number of teeth lost and the incidence of esophageal cancer. When the number of teeth lost was less than 12, the risk of esophageal cancer increased with the number of teeth lost. When more than 12 teeth were lost, the risk of esophageal cancer decreased as the number of teeth lost increased.
ConclusionTooth loss is a risk factor for the occurrence of esophageal cancer in this natural village. When the number of teeth lost is less than 12, the risk of esophageal cancer increases with the number of teeth lost.
-
Key words:
- Risk factor /
- Tooth loss /
- Esophageal cancer /
- Cohort study
-
0 引言
食管癌是我国常见的上消化道恶性肿瘤,严重危害着国人健康。由于食管癌早期不容易发现,当患者确诊时往往已经进展到中晚期。早期食管癌的5年生存率可达95%,中晚期食管癌5年生存率却不足30%,临床首次确诊的患者中早期食管癌仅占约5%,中晚期食管癌患者高达95%[1-3]。因此,提高早期食管癌的诊断率对提高疗效、改善预后十分重要。食管癌是多种因素长期相互作用所致,其发病的原因和机制尚不明确。已有很多研究报道了牙齿脱落、牙周炎及口腔卫生不良与食管癌均有一定关系。牙周炎与口腔细菌感染有直接关系,也是牙齿脱落的重要因素。有研究团队曾对牙齿脱落与食管癌的关系进行过前瞻性队列研究[4],但诸如在食管癌高发区某自然村连续自然随访16年,探讨牙齿脱落与食管癌关系的前瞻性队列研究鲜有报道。本研究旨在通过前瞻性队列研究,探讨河南省安阳市文峰区某自然村牙齿脱落与食管癌的关系。
1 资料与方法
1.1 研究对象
本研究以河南省安阳市文峰区某自然村为研究现场,该区域地处沿太行山食管癌高发区(平原地区),常驻人口约
4200 人。按照样本量计算公式计算得出,最低样本量为353人。样本量计算公式如下:$ n=\dfrac{{{\textit{z}}}^{2}.p.(1-p)}{{e}^{2}} $,$ n_{\text{调整}}=\dfrac{n}{1+(n-1) / N} $。在满足最低样本量基础上,按照随机抽样原则选取无症状居民711人作为初始研究对象。研究过程中因以下原因失访136人:无法取得联系(8人)、迁移外地(11人)、中途退出(16人)、意外死亡(15人)、因健康状况无法继续参加(13人)、家属拒绝配合(73人),最终纳入有效样本共575人,其中男244人,女331人,年龄38~97岁,平均年龄76.55±8.76岁。纳入标准:(1)年龄≥18岁;(2)自愿参加本研究项目并能持续配合随访;(3)生于本村或长期居住在该村。排除标准:(1)在流调过程中失联或迁居外地的人员;(2)拒绝配合流调的人员;(3)家属拒绝配合流调的非食管癌去世人员。1.2 调查内容与方法
2008年1月对研究对象进行问卷调查和常规体格检查。问卷调查内容包括基本信息、吸烟史、饮酒史、喜食热食、反流性食管病、饮食不规律、食用腌菜、肿瘤家族史及牙齿状况。部分流调内容定义:(1)牙齿脱落:恒牙有无脱落及脱落颗数;(2)吸烟:每天>5根;(3)饮酒:每周>2次,每次>100 ml;(4)喜食热食:饭菜做好后立即食用;(5)饮食不规律:不吃早饭或吃饭时间不定;(6)食用腌菜:每天或每周食用2~3次以上为经常吃,仅在冬季食用为不经常吃。本课题经伦理委员会批准(国家自然基金委项目伦理号:2018-0215),并与受访者签署了知情同意书。所有受检对象均自愿参加长期随访队列并配合流调,每年入户随访1次,以2024年7月作为随访终点,记录随访对象牙齿脱落情况及食管癌发生情况。对于确诊患者,追溯到确诊医院,复印其病理报告。流调表由郑州大学第一附属医院河南省食管癌重点开放实验室提供。流调员均经过严格培训,流调内容整理由双人审查、核对,并由独立质检员随机抽取15%样本进行复核,以确保数据整理的准确性。
1.3 牙齿脱落筛查标准
本次参与流调人员均经过了口腔医生相关培训,牙齿脱落情况统计采用“被流调人员自述+流调人员口腔探查”模式。根据牙齿脱落情况分为正常组和脱落组。根据恒牙脱落颗数分为3级:脱落1~3颗为1级,脱落4~6颗为2级,脱落≥7颗为3级。
1.4 统计学方法
采用SPSS 26.0软件分析数据,牙齿有无脱落与食管癌关系的差异性比较采用卡方检验;牙齿脱落情况与食管癌的关系采用二元Logistic回归分析,先进行单因素分析,再将单因素分析中有统计学意义的指标纳入多因素分析;应用限制性立方样条图(RCS)分析牙齿脱落颗数与食管癌患病风险的非线性剂量-反应关系,限制性立方样条图选择4个节点:第5、35、65和95百分位数来拟合曲线。选择4个节点是基于文献、统计效率和实际应用的综合考虑,4个节点能够在灵活性和稳定性之间取得平衡,适合大多数研究场景[5-6]。检验水准α=0.05。
2 结果
2.1 研究队列资料
本研究对食管癌患者人数统计以4年为一个时间节点进行总结,见表1。男性、吸烟、饮酒、牙齿脱落、饮食不规律、反流性食管病、肿瘤家族史等因素是食管癌的危险因素,见表2。
表 1 食管癌患者历年发病情况(n)Table 1 Incidence of esophageal cancer patients over the years (n)Influencing factor Year 2008–
20122013–
20162017–
20202021–
2024Gender Male 15 8 6 4 Female 5 2 3 2 Age (years) ≤50 0 0 0 0 >50 20 10 9 6 Smoking Yes 12 6 5 4 No 8 4 4 2 Drinking Yes 13 7 6 3 No 7 3 3 3 Tooth loss Yes 19 8 8 5 No 1 2 1 1 Like to eat hot food Yes 3 3 1 3 No 17 7 8 3 Irregular diet Yes 11 5 8 4 No 9 5 1 2 Reflux esophageal disease Yes 10 8 5 5 No 10 2 4 1 Family history of cancer Yes 15 7 8 4 No 5 3 1 2 Consumption of
pickled vegetablesOften 0 0 1 0 Seldom 20 10 8 6 表 2 研究对象基本信息Table 2 Basic information of research objectInfluencing factor Normal
population (n)Esophageal cancer
patients (n)χ2 P OR (95%CI) Gender 30.957 <0.001 Female* 319 12 1.000 (Ref) Male 211 33 4.158 (2.100–8.233) Age (years) 1.315 0.251 -- ≤50 26 0 >50 504 45 Smoking 21.716 <0.01 No* 387 18 1.000 (Ref) Yes 143 27 4.059 (2.170–7.595) Drinking 19.748 <0.001 No 362 16 1.000 (Ref) Yes 168 29 3.906 (2.065–7.386) Tooth loss 9.389 <0.001 No 176 5 1.000 (Ref) Yes 354 40 3.977 (1.543–10.255) Level 1 142 11 2.658 0.103 2.727 (0.92–8.029) Level 2 87 12 8.256 0.004 4.855 (1.658–14.217) Level 3 125 17 9.231 0.002 4.787 (1.721–13.318) Hot food preference 5.211 0.022 No* 321 35 1.000 (Ref) Yes 209 10 0.439 (0.213–0.905) Irregular diet 112.204 <0.001 No* 487 17 1.000 (Ref) Yes 43 28 18.65 (9.464–36.766) Reflux esophageal disease 30.410 <0.001 No* 402 17 1.000 (Ref) Yes 128 28 7.021 (3.471–14.203) Family history of cancer 37.367 <0.001 No* 368 11 1.000 (Ref) Yes 162 34 4.541 (1.999–10.314) Consumption of pickled vegetables 3.094 0.078 Seldom* 466 44 1.000 (Ref) Often 64 1 0.165 (0.022–1.222) Note: *: the reference layer in binary categorical variables. 2.2 牙齿脱落与食管癌发生关系
采用二元Logistic回归分析,单因素分析结果显示牙齿脱落与食管癌发生有显著关系(P=0.004,OR=3.977,95%CI:1.543~10.255);将具有统计学意义的单因素纳入多因素分析。首先对Logistic回归多因素分析模型拟合优度评估:Hosmer-Lemeshow检验结果为χ2=9.387,P=0.311,表明Logistic回归多因素分析模型的预测概率与实际观测值之间没有显著差异,模型能够较好地拟合数据。其次对纳入Logistic回归多因素分析模型的自变量进行共线性分析,VIF值均<3,表明自变量间没有共线性,见表3。多因素分析结果显示牙齿脱落与食管癌发生仍有显著关系(P=0.043,OR=3.038,95%CI:1.035~8.914)。
表 3 纳入Logistic回归多因素分析的自变量共线性分析VIF值Table 3 Variance inflation factor (VIF) values for multicollinearity analysis of independent variables included in Logistic regression multivariate analysisIndependent
variableGender Age Tooth loss Smoking Drinking Hot food
preferenceIrregular
dietFamily history
of cancerReflux
esophageal diseaseVIF 1.14 2.36 1.11 2.65 2.39 1.13 1.28 1.07 1.09 Notes: VIF standard: 0<VIF<3, no collinearity between independent variables; 3≤VIF<10, not serious collinearity; VIF≥10, severe collinearity. 2.3 牙齿脱落颗数与食管癌发生的剂量-反应关系
χ2检验结果发现:牙齿脱落1级与正常组比较无统计学差异,牙齿脱落2级及3级与正常组比较差异有统计学意义,见表2。二元Logistic回归进行单因素分析,结果显示:牙齿脱落2级的OR=4.855,95%CI:1.658~14.217,P=0.004;牙齿脱落3级的OR=4.787,95%CI:1.721~13.318,P=0.002。调整性别、吸烟、饮酒、喜食热食、不规律饮食、肿瘤家族史、反流性食管病等混杂因素后,牙齿脱落2级无统计学意义,OR=3.316,95%CI:0.928~11.845,P=0.065;牙齿脱落3级与食管癌发生有显著关系,得到的OR=4.793,95%CI:1.437~15.993,P=0.011,见表4。应用RCS模型分析得出牙齿脱落颗数与食管癌发生风险存在显著的“倒U形”非线性剂量-反应关系( Pnon-linear=0.001,P<0.05):当牙齿脱落<12颗时,患食管癌风险随着牙齿脱落颗数增加而增大;当牙齿脱落≥12颗时,患食管癌风险会随着牙齿脱落颗数增加而降低,见图1。
表 4 牙齿脱落颗数与食管癌发生关系Table 4 Relationship between the number of teeth lost and occurrence of esophageal cancerTooth loss level Model 1 Model 2 OR 95%CI OR 95%CI Level 1 2.727 0.926–8.029 1.727 0.485–6.156 Level 2 4.855 1.658–14.217 3.316 0.928–11.845 Level 3 4.787 1.721–13.318 4.793 1.437–15.993 Notes: Model 1: before adjustments for covariates; Model 2: after adjustments for gender, smoking, drinking, hot food preference, irregular diet, family history of tumor, reflux esophageal disease, and other factors. 3 讨论
本研究对河南省安阳市文峰区某自然村无症状居民连续随访16年,采用前瞻性队列研究探讨该自然村牙齿脱落与食管癌发生关系。这种研究模式在中国乃至世界对食管癌的研究中都较为少见,比较客观地反映了该自然村牙齿脱落与食管癌发生的关系。
本研究对象牙齿脱落现象较为普遍(394/711),村民对此未引起足够重视。牙齿脱落会改变饮食习惯,对食物咀嚼产生一定影响,导致部分坚硬食物无法充分咀嚼,从而在吞咽过程中损伤食管黏膜。长期反复损伤食管黏膜,容易继发食管炎,导致食管黏膜反复损伤修复和增生,这可能对食管癌发生产生重要影响[7-8]。本研究数据显示牙齿脱落是该地区患食管癌的危险因素。采用二元Logistic回归分析,单因素分析得到的OR值和95%CI为3.977(1.149~9.412),P<0.05;调整性别、吸烟、饮酒、喜食热食、不规律饮食、肿瘤家族史、反流性食管炎等混杂因素后,牙齿脱落与食管癌发生仍有显著关系,OR值和95%CI为3.038(1.035~8.914),P<0.05。应用RCS模型得出牙齿脱落颗数与食管癌发生存在非线性剂量—反应关系,当牙齿脱落<12颗时,患食管癌风险会随着牙齿脱落颗数增加而增大,这与既往研究[9-11]报道相似,即牙齿脱落是食管癌发生的危险因素,随着牙齿脱落颗数增加,患食管癌风险加大,食管癌患者死亡风险也增加,并对食管癌患者术后预后产生影响;当牙齿脱落颗数≥12颗时,患食管癌风险会随着牙齿脱落颗数增加而降低,出现这种现象可能与患者牙齿脱落过多导致咀嚼功能下降,引发饮食结构变化有关:只能食用较为软烂、容易吞咽的食物,这对食管黏膜损伤力度明显下降,可能影响食管癌发生风险。
有研究[12-13]报道口腔健康状况与食管癌的关系,口腔健康以“牙齿脱落” “牙周炎”为重要评价指标,结果显示口腔健康不良是食管癌发生的独立危险因素,且与吸烟、饮酒无关。既往多项研究[14-17]报道牙齿脱落与食管癌发生相关,有增加患食管鳞癌和食管腺癌的风险,且二者具有剂量—反应关系,并可增加癌症患者死亡风险,降低癌症患者生存率。此外,牙齿脱落还会增加食管癌术后肺炎发生的并发症及预后不良风险[18-19]。另有文献[20-21]报道牙周炎是食管癌发生的危险因素,可能与口腔细菌感染有关。两项国内对照研究[22-23]均显示食管癌患者较健康人群口腔菌群中普雷沃氏菌属、卟啉单胞菌、链球菌属、韦荣氏球菌属的相对丰度显著升高,这种口腔微生物菌群的变化与食管癌发生风险有关,并可能对预测食管癌的发展有重要意义。一项国外研究[24]报道在325例切除的食管癌标本中检测到具核梭杆菌的含量明显高于正常人食管组织,74例(23%)检测到具核酸杆菌DNA阳性与肿瘤分期显著相关,并与癌症特异性生存率显著相关,这表明它可作为一种预后生物标志物。综上,口腔健康不良、牙齿脱落、牙周炎均是食管癌发生的危险因素,三者之间又存在一定相关性。牙齿脱落与牙周炎反映了口腔健康状况,二者常存在因果关系,即牙周炎可造成牙槽骨吸收,牙齿松动、脱落。三者在食管癌发生过程中是否存在协同作用或者因果效应,目前尚未有明确报道,需要更多的研究和数据去验证。本研究尚存在不足之处。样本量相对较小,且样本均来自该自然村随机流调人员,结果可能会有偏差,尚需在日后工作中继续研究。坚持早晚刷牙,保持良好口腔卫生,定期检查口腔健康状态。因此,在该自然村食管癌的防治工作中,应加大宣讲保持口腔卫生、预防牙周炎、防止牙齿脱落等方面的医学常识,提高居民自我预防意识。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:王静静:人员流调、整理数据、统计分析、论文撰写与修改徐瑞华、赵学科、魏梦霞:论文初审与修改张艳芳、张强、宋昕、郭军芳、韩雪娜、付亚如、李贝、刘军清、雷玲玲、刘敏:人员流调、数据收集及参考文献整理鲍启德、王立东:提供论文整体构思、统计方法、修改意见、审核文章 -
表 1 食管癌患者历年发病情况(n)
Table 1 Incidence of esophageal cancer patients over the years (n)
Influencing factor Year 2008–
20122013–
20162017–
20202021–
2024Gender Male 15 8 6 4 Female 5 2 3 2 Age (years) ≤50 0 0 0 0 >50 20 10 9 6 Smoking Yes 12 6 5 4 No 8 4 4 2 Drinking Yes 13 7 6 3 No 7 3 3 3 Tooth loss Yes 19 8 8 5 No 1 2 1 1 Like to eat hot food Yes 3 3 1 3 No 17 7 8 3 Irregular diet Yes 11 5 8 4 No 9 5 1 2 Reflux esophageal disease Yes 10 8 5 5 No 10 2 4 1 Family history of cancer Yes 15 7 8 4 No 5 3 1 2 Consumption of
pickled vegetablesOften 0 0 1 0 Seldom 20 10 8 6 表 2 研究对象基本信息
Table 2 Basic information of research object
Influencing factor Normal
population (n)Esophageal cancer
patients (n)χ2 P OR (95%CI) Gender 30.957 <0.001 Female* 319 12 1.000 (Ref) Male 211 33 4.158 (2.100–8.233) Age (years) 1.315 0.251 -- ≤50 26 0 >50 504 45 Smoking 21.716 <0.01 No* 387 18 1.000 (Ref) Yes 143 27 4.059 (2.170–7.595) Drinking 19.748 <0.001 No 362 16 1.000 (Ref) Yes 168 29 3.906 (2.065–7.386) Tooth loss 9.389 <0.001 No 176 5 1.000 (Ref) Yes 354 40 3.977 (1.543–10.255) Level 1 142 11 2.658 0.103 2.727 (0.92–8.029) Level 2 87 12 8.256 0.004 4.855 (1.658–14.217) Level 3 125 17 9.231 0.002 4.787 (1.721–13.318) Hot food preference 5.211 0.022 No* 321 35 1.000 (Ref) Yes 209 10 0.439 (0.213–0.905) Irregular diet 112.204 <0.001 No* 487 17 1.000 (Ref) Yes 43 28 18.65 (9.464–36.766) Reflux esophageal disease 30.410 <0.001 No* 402 17 1.000 (Ref) Yes 128 28 7.021 (3.471–14.203) Family history of cancer 37.367 <0.001 No* 368 11 1.000 (Ref) Yes 162 34 4.541 (1.999–10.314) Consumption of pickled vegetables 3.094 0.078 Seldom* 466 44 1.000 (Ref) Often 64 1 0.165 (0.022–1.222) Note: *: the reference layer in binary categorical variables. 表 3 纳入Logistic回归多因素分析的自变量共线性分析VIF值
Table 3 Variance inflation factor (VIF) values for multicollinearity analysis of independent variables included in Logistic regression multivariate analysis
Independent
variableGender Age Tooth loss Smoking Drinking Hot food
preferenceIrregular
dietFamily history
of cancerReflux
esophageal diseaseVIF 1.14 2.36 1.11 2.65 2.39 1.13 1.28 1.07 1.09 Notes: VIF standard: 0<VIF<3, no collinearity between independent variables; 3≤VIF<10, not serious collinearity; VIF≥10, severe collinearity. 表 4 牙齿脱落颗数与食管癌发生关系
Table 4 Relationship between the number of teeth lost and occurrence of esophageal cancer
Tooth loss level Model 1 Model 2 OR 95%CI OR 95%CI Level 1 2.727 0.926–8.029 1.727 0.485–6.156 Level 2 4.855 1.658–14.217 3.316 0.928–11.845 Level 3 4.787 1.721–13.318 4.793 1.437–15.993 Notes: Model 1: before adjustments for covariates; Model 2: after adjustments for gender, smoking, drinking, hot food preference, irregular diet, family history of tumor, reflux esophageal disease, and other factors. -
[1] 宋昕, 赵学科, 侯志超, 等. 1973年至2019年食管早期浸润鳞癌检出率变化及术后生存影响因素分析[J]. 肿瘤基础与临床, 2020, 33(5): 369-372. [Song X, Zhao XK, Hou ZC, et al. Changes of detection rate of esophageal early invasive squamous cell cancer and influencing factors of postoperative survival from 1973 to 2019[J]. Zhong Liu Ji Chu Yu Lin Chuang, 2020, 33(5): 369-372.] doi: 10.3969/j.issn.1673-5412.2020.05.001 Song X, Zhao XK, Hou ZC, et al. Changes of detection rate of esophageal early invasive squamous cell cancer and influencing factors of postoperative survival from 1973 to 2019[J]. Zhong Liu Ji Chu Yu Lin Chuang, 2020, 33(5): 369-372. doi: 10.3969/j.issn.1673-5412.2020.05.001
[2] Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries[J]. Lancet Glob Health, 2018, 6(5): e555-e567. doi: 10.1016/S2214-109X(18)30127-X
[3] Duggan MA, Anderson WF, Altekruse S, et al. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship[J]. Am J Surg Pathol, 2016, 40(12): e94-e102. doi: 10.1097/PAS.0000000000000749
[4] 范金虎, 孙秀娣, 刘彬, 等. 牙齿缺失与上消化道肿瘤关系前瞻性研究[J]. 中国肿瘤, 2004, 13(9): 561-564. [Fan JH, Sun XD, Liu B, et al. Prospective study on the relationship between tooth loss and upper gastrointestinal cancer[J]. Zhongguo Zhong Liu, 2004, 13(9): 561-564.] doi: 10.3969/j.issn.1004-0242.2004.09.008 Fan JH, Sun XD, Liu B, et al. Prospective study on the relationship between tooth loss and upper gastrointestinal cancer[J]. Zhongguo Zhong Liu, 2004, 13(9): 561-564. doi: 10.3969/j.issn.1004-0242.2004.09.008
[5] 高湘金, 肇晖, 王瑞平. 限制性立方样条在临床研究数据分析中的应用[J]. 上海医药, 2024, 45(13): 29-33. [Gao XJ, Zhao H, Wang RP. Application of restricted cubic splines in clinical study data analysis[J]. Shanghai Yi Yao, 2024, 45(13): 29-33.] doi: 10.3969/j.issn.1006-1533.2024.13.007 Gao XJ, Zhao H, Wang RP. Application of restricted cubic splines in clinical study data analysis[J]. Shanghai Yi Yao, 2024, 45(13): 29-33. doi: 10.3969/j.issn.1006-1533.2024.13.007
[6] 罗剑锋, 金欢, 李宝月, 等. 限制性立方样条在非线性回归中的应用研究[J]. 中国卫生统计, 2010, 27(3): 229-232. [Luo JF, Jin H, Li BY, et al. The application of restricted cubic splines to nonlinear regression[J]. Zhongguo Wei Sheng Tong Ji, 2010, 27(3): 229-232.] doi: 10.3969/j.issn.1002-3674.2010.03.002 Luo JF, Jin H, Li BY, et al. The application of restricted cubic splines to nonlinear regression[J]. Zhongguo Wei Sheng Tong Ji, 2010, 27(3): 229-232. doi: 10.3969/j.issn.1002-3674.2010.03.002
[7] 刘付东, 吴玲玲, 缪伟刚, 等. 江苏省农村地区居民食管癌及癌前病变影响因素分析[J]. 中国肿瘤外科杂志, 2024, 16(2): 116-121. [Liu FD, Wu LL, Miao WG, et al. Analysis of influencing factors of esophageal cancer and precancerous lesions in rural residents of Jiangsu Province[J]. Zhongguo Zhong Liu Wai Ke Za Zhi, 2024, 16(2): 116-121.] doi: 10.3969/j.issn.1674-4136.2024.02.003 Liu FD, Wu LL, Miao WG, et al. Analysis of influencing factors of esophageal cancer and precancerous lesions in rural residents of Jiangsu Province[J]. Zhongguo Zhong Liu Wai Ke Za Zhi, 2024, 16(2): 116-121. doi: 10.3969/j.issn.1674-4136.2024.02.003
[8] 王静静, 付亚如, 刘军清, 等. 中国人群食管癌主要危险因素Meta分析[J]. 临床心身疾病杂志, 2024, 30(3): 115-122. [Wang JJ, Fu YR, Liu JQ, et al. Meta-analysis of major risk factors for esophageal cancer in Chinese population[J]. Lin Chuang Xin Shen Ji Bing Za Zhi, 2024, 30(3): 115-122.] doi: 10.3969/j.issn.1672-187X.2024.03.024 Wang JJ, Fu YR, Liu JQ, et al. Meta-analysis of major risk factors for esophageal cancer in Chinese population[J]. Lin Chuang Xin Shen Ji Bing Za Zhi, 2024, 30(3): 115-122. doi: 10.3969/j.issn.1672-187X.2024.03.024
[9] Chen QL, Zeng XT, Luo ZX, et al. Tooth loss is associated with increased risk of esophageal cancer: evidence from a meta-analysis with dose-response analysis[J]. Sci Rep, 2016, (6): 18900.
[10] Zhang S, Yu P, Wang JB, et al. Association between tooth loss and upper gastrointestinal cancer: A 30-year follow-up of the Linxian Dysplasia Nutrition Interventio Trial Cohort[J]. Thorac Cancer, 2019, 10(4): 966-974. doi: 10.1111/1759-7714.13037
[11] Miura S, Nakamura T, Hasegawa T, et al. Tooth Loss Predicts Long-Term Prognosis of Esophageal Cancer After Esophagectomy[J]. Ann Surg Oncol, 2020, 27(3): 683-690. doi: 10.1245/s10434-019-07903-w
[12] Guha N, Boffetta P, Wunsch Filho V, et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case-control studies[J]. Am J Epidemiol, 2007, 166(10): 1159-1173. doi: 10.1093/aje/kwm193
[13] Abnet CC, Kamangar F, Islami F, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma[J]. Cancer Epidemiol Biomarkers Prev, 2008, 17(11): 3062-3068. doi: 10.1158/1055-9965.EPI-08-0558
[14] Yano Y, Fan JH, Dawsey SM, et al. A long-term follow-up analysis of associations between tooth loss and multiple cancers in the Linxian General Population Cohort[J]. J Natl Cancer Cent, 2021, 1(2): 39-43. doi: 10.1016/j.jncc.2021.01.002
[15] Zhang J, Bellocco R, Sandborgh-Englund G, et al. Poor Oral Health and Esophageal Cancer Risk: A Nationwide Cohort Study[J]. Cancer Epidemiol Biomarkers Prev, 2022, 31(7): 1418-1425. doi: 10.1158/1055-9965.EPI-22-0151
[16] Qian Y, Cai B, Chi F, et al. Alveolar bone loss and tooth loss contribute to increase in cancer mortality among older patients[J]. BMC Oral Health, 2023, 23(1): 1023. doi: 10.1186/s12903-023-03543-5
[17] Wu YJ, Lin TY, Pu XF, et al. Association of tooth loss and periodontal disease with all-cause mortality in cancer survivors: A cohort study based on NHANES[J]. Heliyon, 2024, 10(17): e36813. doi: 10.1016/j.heliyon.2024.e36813
[18] Yamada Y, Yurikusa T, Furukawa K, et al. The Effect of Improving Oral Hygiene through Professional Oral Care to Reduce the Incidence of Pneumonia Post-esophagectomy in Esophageal Cancer[J]. Keio J Med, 2019, 68(1): 17-25.
[19] Watanabe T, Sohda M, Kim M, et al. Preoperative evaluation of oral hygiene may predict the overall survival of patients with esophageal cancer[J]. Esophagus, 2023, 20(1): 99-108. doi: 10.1007/s10388-022-00941-6
[20] 李玉超, 潘亚萍. 牙周炎与肿瘤相关性研究进展[J]. 中国实用口腔科杂志, 2019, 12(5): 307-311. [Li YC, Pan YP. Research progress of the correlation between periodontitis and tumor[J]. Zhongguo Shi Yong Kou Qiang Za Zhi, 2019, 12(5): 307-311.] Li YC, Pan YP. Research progress of the correlation between periodontitis and tumor[J]. Zhongguo Shi Yong Kou Qiang Za Zhi, 2019, 12(5): 307-311.
[21] 顾雯佳, 陆海霞, 张羽, 等. 牙周炎与消化系统癌症相关性研究进展[J]. 口腔疾病防治, 2021, 29(5): 346-350. [Gu WJ, Lu HX, Zhang Y, et al. Research progress on the study of the relationship between periodontitis and cancers of the digestive system[J]. Kou Qiang Ji Bing Fang Zhi, 2021, 29(5): 346-350.] Gu WJ, Lu HX, Zhang Y, et al. Research progress on the study of the relationship between periodontitis and cancers of the digestive system[J]. Kou Qiang Ji Bing Fang Zhi, 2021, 29(5): 346-350.
[22] Chen X, Winckler B, Lu M, et al. Oral Microbiota and Risk for Esophageal Squamous Cell Carcinoma in a High-Risk Area of China[J]. PLoS One, 2015, 10(12): e0143603. doi: 10.1371/journal.pone.0143603
[23] Zhao Q, Yang T, Yan Y, et al. Alterations of Oral Microbiota in Chinese Patients With Esophageal Cancer[J]. Front Cell Infect Microbiol, 2020, 10: 541144. doi: 10.3389/fcimb.2020.541144
[24] Yamamura K, Baba Y, Nakagawa S, et al. Human Microbiome Fusobacterium Nucleatum in Esophageal Cancer Tissue Is Associated with Prognosis[J]. Clin Cancer Res, 2016, 22(22): 5574-5581. doi: 10.1158/1078-0432.CCR-16-1786



 王立东: 郑州大学第一附属医院二级教授,博士生导师,省部共建食管癌防治国家重点实验室主任;国家杰出青年科学基金获得者,国务院有突出贡献专家,中组部联系专家,中原学者。主持13项国家自然科学基金项目、6项“863”重大专项、1项“精准医学”重大专项、1项“973”前期专项、2项美国国立癌症研究所项目和1项香港特区重点项目。发表论文700篇,总他引12 365次,最高影响因子41.307。出版专著14部。获4项省部级科技进步一等奖。获授权发明专利52项,计算机软件著作权55项。培养300余名硕士和博士。从事食管癌防治研究近40年,建立10万例无症状人群食管癌前病变随访队列和70万例食管癌随访队列以及国际标准生物样本库资源共享平台;发现食管癌易感基因PLCE1和致病基因NOTCH1等,揭示食管癌变多阶段演进分子病理特征,丰富了食管癌变“环境—遗传—基因互作”理论,建立食管癌高危人群预警和早期发现液体活检关键技术体系。相关研究已在Nat Genet、Gut、Nat Commun和Cancer Res等期刊上发表
。
王立东: 郑州大学第一附属医院二级教授,博士生导师,省部共建食管癌防治国家重点实验室主任;国家杰出青年科学基金获得者,国务院有突出贡献专家,中组部联系专家,中原学者。主持13项国家自然科学基金项目、6项“863”重大专项、1项“精准医学”重大专项、1项“973”前期专项、2项美国国立癌症研究所项目和1项香港特区重点项目。发表论文700篇,总他引12 365次,最高影响因子41.307。出版专著14部。获4项省部级科技进步一等奖。获授权发明专利52项,计算机软件著作权55项。培养300余名硕士和博士。从事食管癌防治研究近40年,建立10万例无症状人群食管癌前病变随访队列和70万例食管癌随访队列以及国际标准生物样本库资源共享平台;发现食管癌易感基因PLCE1和致病基因NOTCH1等,揭示食管癌变多阶段演进分子病理特征,丰富了食管癌变“环境—遗传—基因互作”理论,建立食管癌高危人群预警和早期发现液体活检关键技术体系。相关研究已在Nat Genet、Gut、Nat Commun和Cancer Res等期刊上发表
。
 下载:
下载: