Expression of FOXM1 and PLK1 in Colorectal Cancer and Their Relationship with Clinicopathological Features and Prognosis
-
摘要:目的
检测FOXM1和PLK1在结直肠癌组织中的表达及其与患者临床病理特征、预后的关系。
方法回顾性收集接受结直肠癌手术切除治疗的60例患者的结直肠癌组织及癌旁组织(距癌组织边缘>5 cm)。免疫组织化学、蛋白印迹及qRT-PCR法检测FOXM1、PLK1在结直肠癌组织中的表达水平。FOXM1抑制剂FDI-6处理HCT-116人结肠癌细胞,蛋白印迹及qRT-PCR法考察下调FOXM1对PLK1表达水平的影响。
结果FOXM1和PLK1在结直肠癌细胞的细胞质中均高表达,且阳性表达率显著均高于癌旁组织(P<0.05),FOXM1的表达水平与患者的组织分化程度、TNM分期、有无淋巴结转移、浸润深度密切相关(均P<0.05);PLK1的表达水平与患者的TNM分期、有无淋巴结转移、浸润深度密切相关(均P<0.05)。FOXM1、PLK1在结直肠癌组织中的表达水平呈正相关(rs=0.373,P=0.003)。蛋白印迹及qRT-PCR法结果表明,抑制FOXM1表达后PLK1表达水平显著下降。FOXM1、PLK1共表达较FOXM1、PLK1单独表达或同时不表达患者的生存时间更短、预后更差。
结论FOXM1、PLK1均在结直肠癌组织中高表达,FOXM1可能通过PLK1促进结直肠癌发生,其高表达预示患者预后不良,可能是结直肠癌的潜在靶点。
Abstract:ObjectiveTo determine the expression of FOXM1 and PLK1 in colorectal cancer tissues and their relationship with clinicopathological characteristics and prognosis of patients.
MethodsSixty patients who underwent surgical resection of colorectal cancer were retrospectively selected. Colorectal cancer tissues and adjacent tissues (>5 cm from the margins of colorectal cancer tissues) were collected. Immunohistochemistry, Western blot, and qRT-PCR analyses were used to detect the expression levels of FOXM1 and PLK1 in colorectal cancer tissues. Human colon cancer HCT-116 cells were treated with FOXM1 inhibitor FDI-6, and the effect of downregulating FOXM1 on PLK1 expression levels was investigated by Western blot and qRT-PCR.
ResultsFOXM1 and PLK1 were highly expressed in the cytoplasm of colorectal cancer cells, and the positive expression rate was significantly higher than those in adjacent tissues (P<0.05). FOXM1 expression was closely related to the degree of differentiation, TNM stage, lymph node metastasis, and invasion depth (all P<0.05). PLK1 expression was closely related to TNM stage, lymph node metastasis, and invasion depth (all P<0.05). The expression levels of FOXM1 and PLK1 in colorectal cancer tissues were positively correlated (rs=0.373, P=0.003). Western blot and qRT-PCR results showed that the expression level of PLK1 decreased significantly after inhibition of FOXM1 expression. Patients with either FOXM1 or PLK1 expression alone, or with neither expressed, had significantly longer survival time and more favorable prognosis than those with FOXM1 and PLK1 co-expression.
ConclusionFOXM1 and PLK1 are highly expressed in colorectal cancer tissues. FOXM1 may promote colorectal cancer through PLK1, and its high expression suggests poor prognosis of patients and may be a potential target for colorectal cancer.
-
Key words:
- FOXM1 /
- PLK1 /
- Colorectal cancer /
- Correlation /
- Clinicopathological features /
- Prognosis
-
0 引言
结直肠癌作为全球最常见的恶性肿瘤之一,其发病率和死亡率均较高,是全球第三大最常见的癌症和第二大癌症相关死亡病例,严重威胁着人类健康[1]。随着生活方式和饮食结构的改变,结直肠癌的发病率在我国呈逐年上升趋势[2-3]。尽管在诊断技术和治疗方法上取得了显著进展,但结直肠癌患者的预后仍然不尽如人意[4-6]。
在结直肠癌的发生发展过程中,细胞周期调控异常是一个关键因素。叉头框转录因子M1(FOXM1)、polo样激酶1(PLK1)作为细胞周期调控的关键因子,在肿瘤细胞的增殖、侵袭和转移中扮演着重要角色。FOXM1属于转录因子家族,通过对细胞周期中G1/S和G2/M期转换的调控参与细胞周期进展、细胞增殖和分化等多种生物学过程,与肿瘤的恶性程度和预后密切相关[7-8];PLK1作为高度保守的丝氨酸/苏氨酸激酶,参与调控细胞周期中G2/M期转换、中心体成熟、纺锤体形成、染色体分离、胞质分裂等关键进程,其过表达与多种癌症的恶性进展有关[9-12]。本研究旨在探讨FOXM1和PLK1在结直肠癌组织中的表达情况,初步探究二者表达水平之间的调控关系,并分析这两个因子与临床病理特征之间的关系,评估对结直肠癌患者预后的影响。
1 资料与方法
1.1 资料收集
回顾性抽取2021年3月至9月期间在徐州医科大学附属淮安医院接受结直肠癌切除治疗的60例患者,收集患者的年龄、性别、TNM分期等临床信息,本研究方案经医院医学伦理委员会审核批准(批件号:HEYLL201821)。
样本来源:蜡块标本:分别收集60例研究对象的结直肠癌组织、癌旁组织(距离结直肠癌组织边缘>5 cm,经病理科医生诊断为正常的结直肠组织),经过脱水、包埋、切片等过程制成蜡块标本;组织标本:从上述60例研究对象中随机抽取5例患者的手术样本,分别为结直肠癌组织、癌旁组织(距离癌组织>5 cm处取材),放于标本袋中,置于−80℃冰箱保存;细胞标本:HCT-116人结肠癌细胞。
纳入标准:(1)符合《中国结直肠癌诊疗规范2020年版》所规定的结直肠癌诊断准则的患者;(2)经我院手术切除治疗,由病理学检查确诊为结直肠癌的患者;(3)未接受放疗、化疗等手术前辅助治疗的患者;(4)拥有完整临床资料的结直肠癌患者。排除标准:(1)同时患有其他类型的恶性肿瘤;(2)伴随着严重的肝肾功能不全、慢性阻塞性肺病等重大基础性疾病;(3)处于妊娠期或哺乳期的妇女。
1.2 免疫组织化学法
经过脱蜡、水合、清除内源性酶干扰、抗原修复、封闭、抗体解育等步骤后,加入兔抗人FOXM1多克隆抗体(FOXM1,抗体货号:13147-1-AP,稀释比:1∶200,武汉三鹰公司)、兔抗人PLK1多克隆抗体(PLK1,抗体货号:YT5054,稀释比为1∶50,美国Immunoway公司),最后将切片在60℃下加热至干燥,然后加入适量中性香脂密封组织,显微镜下观察染色,在合适的视野下拍照。采用半定量的方法进行评分,根据染色强度,分为未上色、浅黄色、浅棕色、棕褐色,分别记为0分、1分、2分、3分。根据阳性细胞表达面积,分为<5%、5%~25%、≥25%~50%、≥50%四个等级,分别记0分、1分、2分、3分,两项相乘,结果≥1记分为阳性,<1分记为阴性。所有样本均由本院两名病理科医生独立阅片,意见不一致时,协商确定最终实验结果。
1.3 蛋白质印迹法
提取5例患者的结直肠癌及癌旁组织中蛋白,用蛋白酶缓冲液分离FOXM1、PLK1。蛋白在10% SDS/PAGE凝胶中电泳、转膜,以适宜比例稀释一抗、二抗,用FOXM1 GAPDH、PLK1以及GAPDH(抗体货号:10494-1-AP,武汉三鹰公司)蛋白一抗、二抗免疫反应,显影,Image J 1.8分析条带。在HCT-116人结肠癌细胞中加入FOXM1抑制剂FDI-6(抗体货号:HY-112721,美国MedChemExpress公司)后,经过上述步骤后,再次检测PLK1的表达量。
1.4 qRT-PCR法
通过RNA提取试剂盒对5例患者的结直肠癌及癌旁组织进行RNA提取,反转录得到cDNA,以此为模板,采用定量PCR仪进行扩增。反应条件为94℃ 4 min,95℃ 30 s,60℃ 30 s,74℃ 2 min,共40个循环。以β-actin为内参基因,以2−ΔΔCt法计算目的基因相对表达水平,FOXM1的上游引物序列为5'-CGTCGGCCACTGATTCTCAAA-3',下游引物序列为5'-GGCAGGGGATCTCTTAGGTTC-3',PLK1的上游引物序列为5'-CACCAGCACGTCGTAGGATTC-3',下游引物序列为5'-CCGTAGGTAGTATCGGGCCTC-3',β-actin的上游引物序列为5'-ACCGTGAGAAGATGAC CCAGA-3',下游引物序列为5'-AGAGGCGTACAG GGACAGCA-3'。在HCT-116人结肠癌细胞中加入FDI-6,经过上述步骤后,再次检测PLK1的表达量。
1.5 统计学方法
SPSS 27.0软件进行统计学分析,计数资料以例数或百分率来表示,组间采用t检验、卡方检验或Fisher′s精确检验分析。Spearman相关检验分析结肠癌组织中FOXM1和PLK1表达水平的相关性,rs值越大,表示相关性越强。Kaplan-Meier法绘制生存曲线分析评价结直肠癌预后的因素。P<0.05为差异有统计学意义。
2 结果
2.1 FOXM1、PLK1在结直肠癌组织中高表达
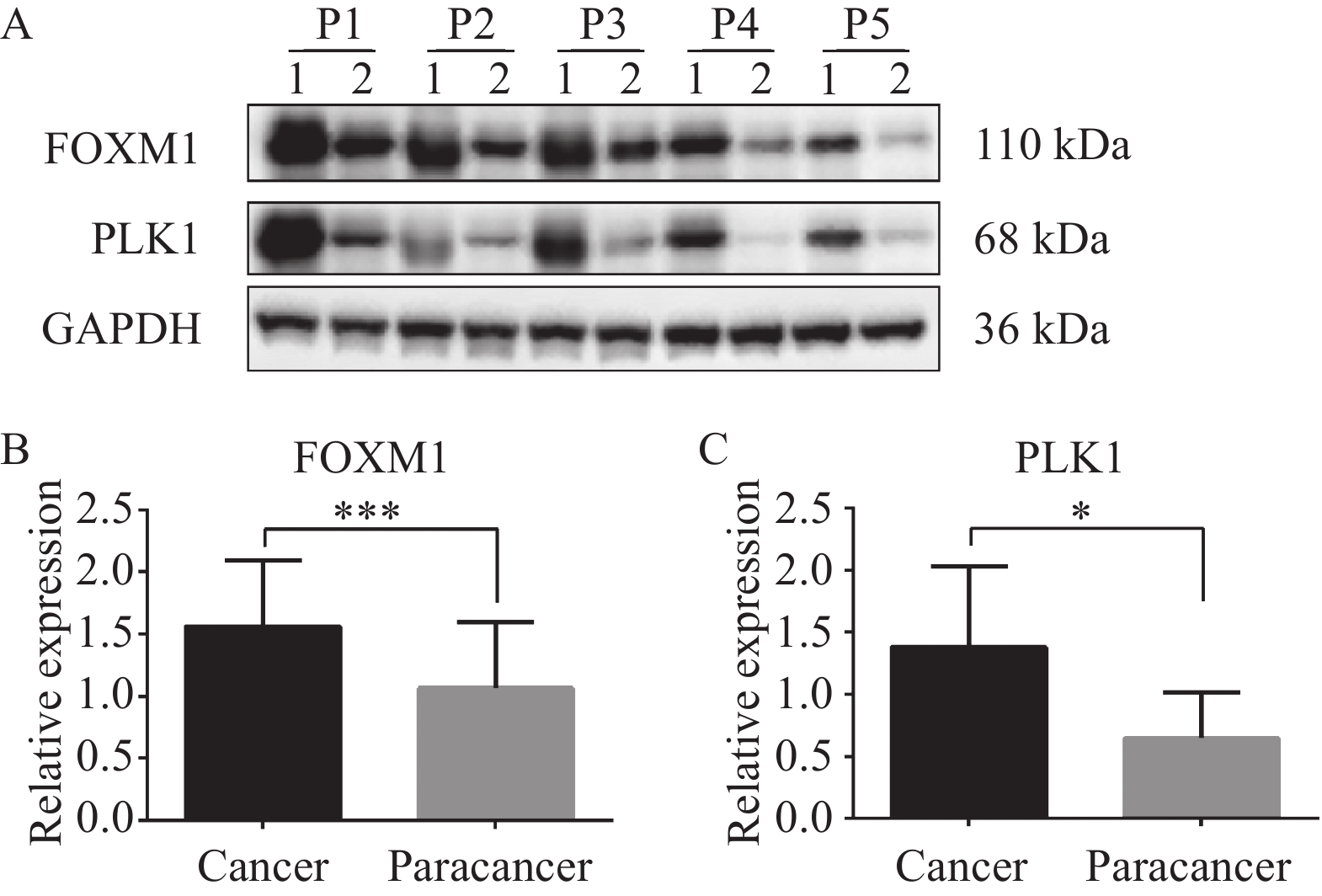
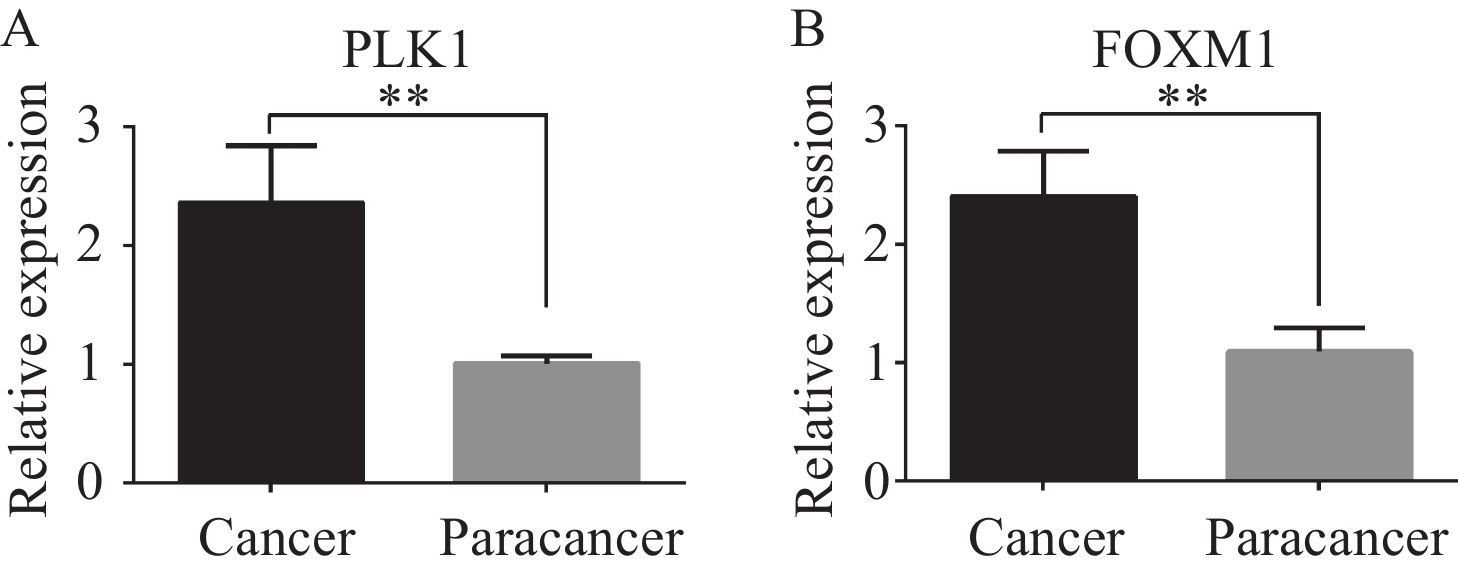
由图1可以观察到在结直肠癌组织中,FOXM1、PLK1均主要在细胞质中高表达,在癌旁组织中不表达,少数在细胞质中呈低表达,在细胞核中不表达。结直肠癌组织中FOXM1阳性表达率和PLK1阳性表达率均显著高于癌旁组织,差异有统计学意义,见表1。蛋白质印迹及qRT-PCR法检测5例结直肠癌组织和癌旁组织中FOXM1和PLK1的表达水平,结果显示:结直肠癌组织中FOXM1和PLK1表达均显著高于癌旁组织,见图2~3。
![]() 图 1 结直肠癌及癌旁组织中FOXM1和PLK1的表达 (免疫组织化学法×200)Figure 1 FOXM1 and PLK1 expression in colorectal cancer and adjacent tissues (IHC ×200)A: the high expression of FOXM1 in colorectal cancer tissues; B: FOXM1 was not expressed or underexpressed in adjacent tissues; C: the high expression of PLK1 in colorectal cancer tissues; D: PLK1 was not expressed or underexpressed in adjacent tissues.表 1 FOXM1、PLK1在结直肠癌中的表达情况Table 1 Expression of FOXM1 and PLK1 in colorectal cancer
图 1 结直肠癌及癌旁组织中FOXM1和PLK1的表达 (免疫组织化学法×200)Figure 1 FOXM1 and PLK1 expression in colorectal cancer and adjacent tissues (IHC ×200)A: the high expression of FOXM1 in colorectal cancer tissues; B: FOXM1 was not expressed or underexpressed in adjacent tissues; C: the high expression of PLK1 in colorectal cancer tissues; D: PLK1 was not expressed or underexpressed in adjacent tissues.表 1 FOXM1、PLK1在结直肠癌中的表达情况Table 1 Expression of FOXM1 and PLK1 in colorectal cancerTissues N Positive expression
rate of FOXM1 (%)Positive expression
rate of PLK1 (%)Colorectal cancer 60 40(66.67) 44(73.33) Paracancer 60 22(36.67) 20(33.33) χ 2 10.812 19.286 P 0.001 <0.001 ![]() 图 2 Western blot法检测结直肠癌及癌旁组织中FOXM1和PLK1表达Figure 2 Expression of FOXM1 and PLK1 in colorectal cancer and adjacent tissues detected by Western blot analysis*: P<0.05, ***: P<0.001. 1: cancer; 2: paracancer. P1-P5: patient 1-5. A: FOXM1, PLK1, and GAPDH protein bands; B: the expression of FOXM1 in colorectal cancer tissue was higher than that in the adjacent tissue; C: the expression of PLK1 protein in colorectal cancer tissue was higher than that in the adjacent tissue.
图 2 Western blot法检测结直肠癌及癌旁组织中FOXM1和PLK1表达Figure 2 Expression of FOXM1 and PLK1 in colorectal cancer and adjacent tissues detected by Western blot analysis*: P<0.05, ***: P<0.001. 1: cancer; 2: paracancer. P1-P5: patient 1-5. A: FOXM1, PLK1, and GAPDH protein bands; B: the expression of FOXM1 in colorectal cancer tissue was higher than that in the adjacent tissue; C: the expression of PLK1 protein in colorectal cancer tissue was higher than that in the adjacent tissue.![]() 图 3 qRT-PCR法检测结直肠癌组织及癌旁组织中PLK1和FOXM1表达Figure 3 Expression of PLK1 and FOXM1 in colorectal cancer tissues and adjacent tissues detected by qRT-PCR**: P<0.01. A: the expression of PLK1 in colorectal cancer tissues was higher than that in adjacent tissues; B: the expression of FOXM1 protein in colorectal cancer tissues was higher than that in adjacent tissues.
图 3 qRT-PCR法检测结直肠癌组织及癌旁组织中PLK1和FOXM1表达Figure 3 Expression of PLK1 and FOXM1 in colorectal cancer tissues and adjacent tissues detected by qRT-PCR**: P<0.01. A: the expression of PLK1 in colorectal cancer tissues was higher than that in adjacent tissues; B: the expression of FOXM1 protein in colorectal cancer tissues was higher than that in adjacent tissues.2.2 FOXM1、PLK1与患者临床病理特征之间的关系
FOXM1阳性表达情况与患者的分化程度、TNM分期、有无淋巴结转移、浸润深度有关(均P<0.05);PLK1阳性表达情况与患者TNM分期、有无淋巴结转移、浸润深度有关(均P<0.05),差异均有统计学意义,见表2。
表 2 FOXM1、PLK1与临床病理特征之间的关系Table 2 Relationship among FOXM1, PLK1, and clinicopathological featuresClinicopathologic features N Positive expression
rate of FOXM1(%)χ2 P Positive expression
rate of PLK1 (%)χ2 P Gender 0.039 0.844 0.002 0.967 Male 41 27(65.85) 30(73.17) Female 19 13(68.42) 14(73.68) Age (years) 0.134 0.714 0.075 0.785 ≤60 32 22(68.75) 23(71.88) >60 28 18(64.29) 21(75.00) TNM stage 15.313 <0.001 10.355 0.001 Ⅰ-Ⅱ 36 17(47.22) 21(58.33) Ⅲ-Ⅳ 24 23(95.83) 23(95.83) Degree of differentiation 9.808 0.002 0.302 0.582 Low 26 23(88.46) 20(76.92) Medium-high 34 17(50.00) 24(70.59) Tumor size (cm) 0.035 0.851 0.006 0.936 <5 23 15(65.22) 17(73.91) ≥5 37 25(65.57) 27(72.97) Lymph node metastases 4.658 0.031 5.084 0.024 Positive 14 6(42.86) 7(50.00) Negative 46 34(73.91) 37(80.43) Depth of infiltration 4.800 0.028 5.455 0.020 T1-T2 30 16(53.33) 18(60.00) T3-T4 30 21(70.00) 26(86.67) Neural invasion 0.400 0.527 0.455 0.507 Positive 15 9(60.00) 10(66.67) Negative 45 31(68.89) 34(75.56) Vascular invasion 0.400 0.527 0.455 0.500 Positive 15 11(73.33) 10(66.67) Negative 45 29(64.44) 34(75.56) 2.3 在结直肠癌组织中FOXM1可以促进PLK1的表达
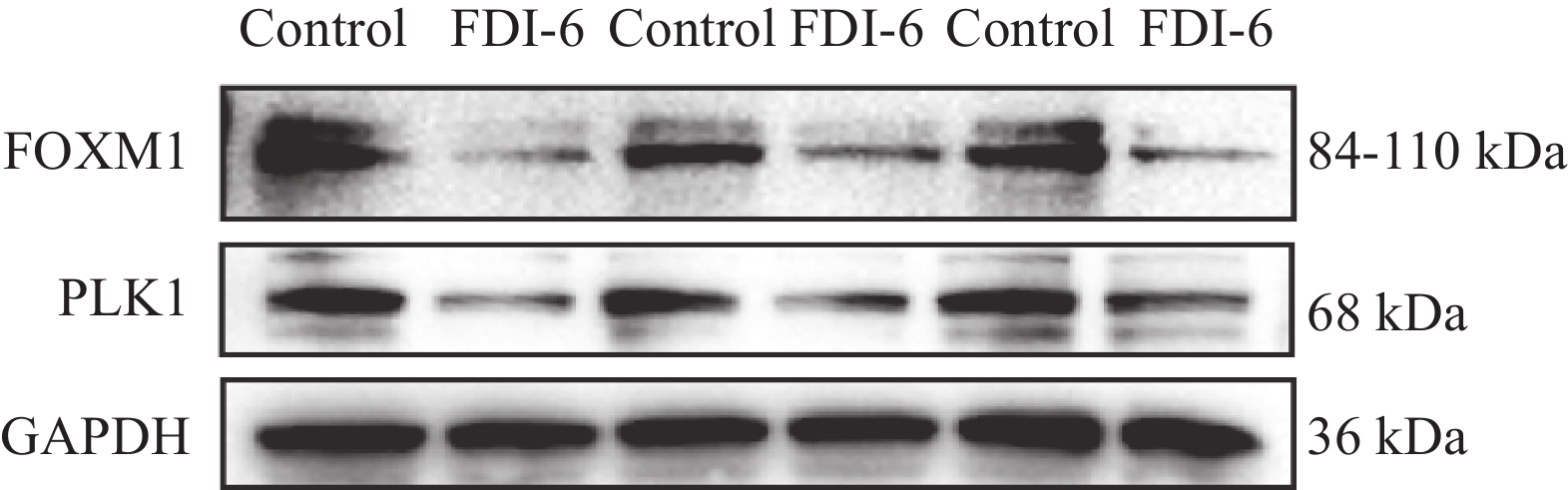
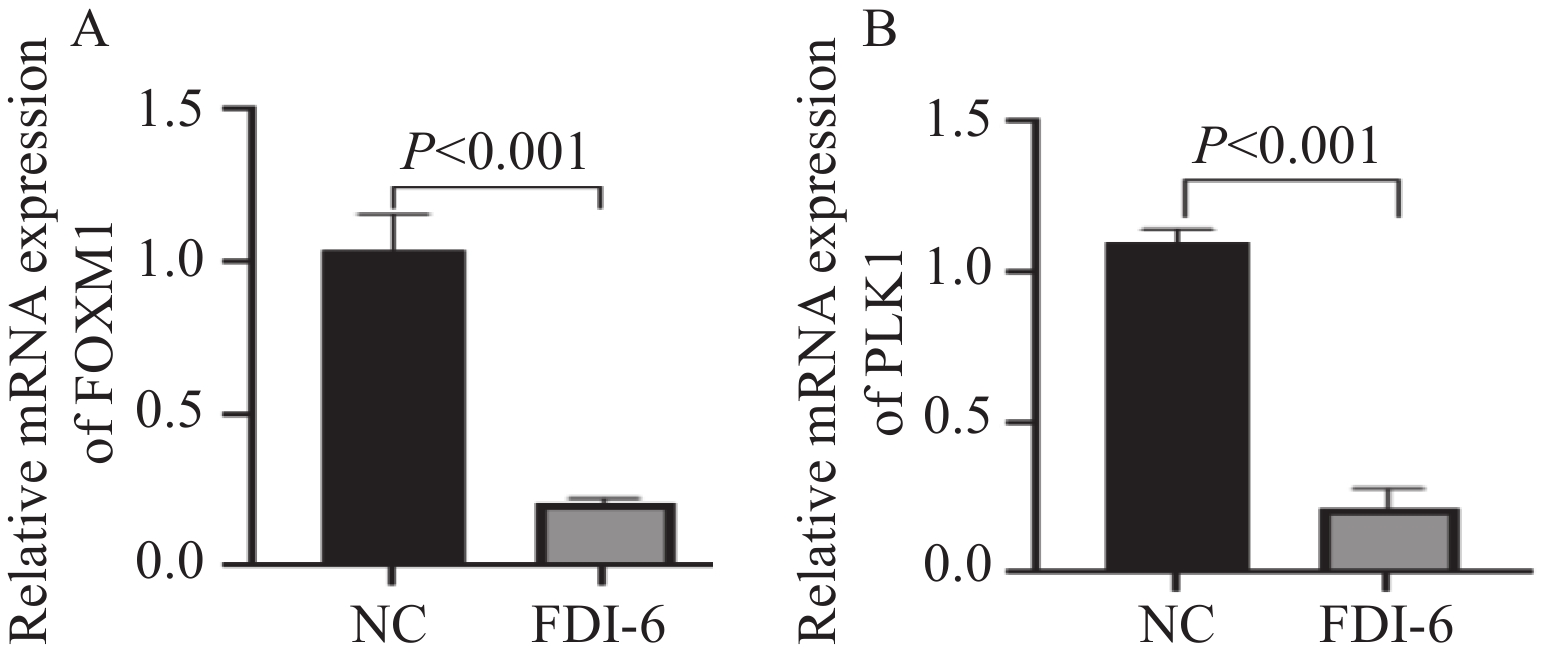
根据统计学结果得出,结直肠癌组织中FOXM1和PLK1的表达呈正相关(rs=0.373,P=0.003),见表3。在HCT-116人结肠癌细胞中加入FOXM1抑制剂FDI-6后,蛋白质印迹法、qRT-PCR法观察到PLK1较未加入FOXM1抑制剂之前表达量明显下降,因此我们推测在结直肠癌细胞中,FOXM1可以促进PLK1的表达,见图4~5。
表 3 结直肠组织中FOXM1与PLK1表达的相关性Table 3 Correlation between FOXM1 and PLK1 expression in colorectal tissuesFOXM1 expression PLK1 expression rs P Positive Negative Positive 34 10 0.373 0.003 Negative 10 10 2.4 FOXM1、PLK1对结直肠癌患者预后的影响
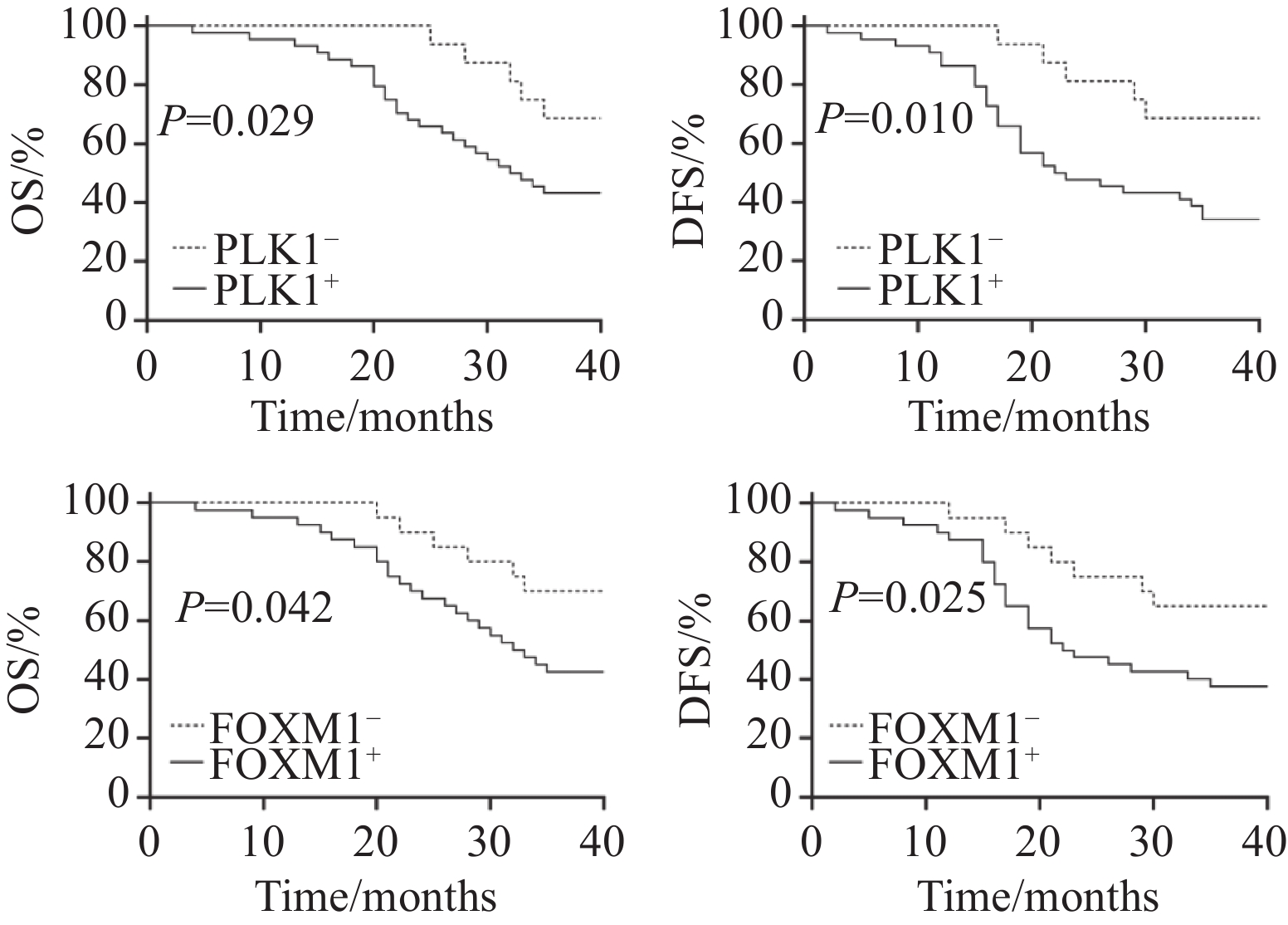
截至2024年9月,60例结直肠癌患者共随访1~36个月,其中1例失访。总生存期为从研究开始到因任何原因导致患者死亡的时间。无病生存期为从研究开始至第一次肿瘤复发或任何原因导致患者死亡的时间。失访患者截至末次随访日,研究结束时仍然存活的患者截至随访结束日。通过生存分析显示,PLK1阳性者的总生存期中位时间是23.6个月,PLK1阴性者的总生存期中位时间是28.6个月,两组总生存期中位时间比较差异有统计学意义(P=0.029);PLK1阳性者的无病生存期中位时间是24.1个月,PLK1阴性者的无病生存期中位时间是29.2个月,两组无病生存期中位时间比较差异有统计学意义(P=0.01),见图6;FOXM1阳性者的总生存期中位时间是22.7个月,FOXM1阴性者的总生存期中位时间是29.3个月,两组总生存期中位时间比较差异有统计学意义(P=0.042);FOXM1阳性者的无病生存期中位时间是22.8个月,FOXM1阴性者的无病生存期中位时间是30.0个月,两组无病生存期中位时间比较差异有统计学意义(P=0.025),见图6。
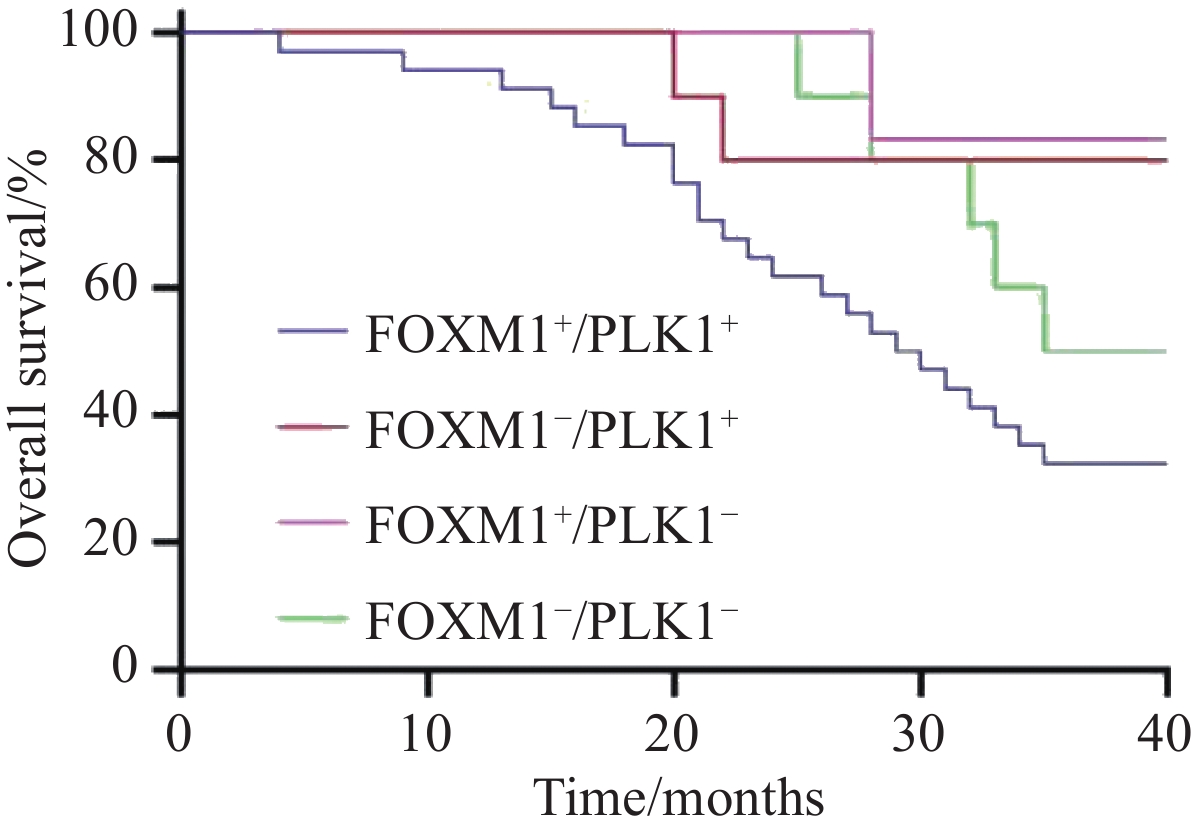
将患者按FOXM1、PLK1的表达情况分为FOXM1+/PLK+、FOXM1+/PLK−、FOXM1−/PLK+、FOXM1−/PLK1−四组,对四组患者总生存期和无病生存期进行生存分析,结果发现FOXM1+/PLK+患者的生存时间显著低于FOXM1+/PLK−、FOXM1−/PLK+、FOXM1−/PLK1−组的患者,差异有统计学意义(均P<0.05),见图7。
3 讨论
结直肠癌是我国常见的恶性肿瘤之一,随着社会人口老龄化进程的加速、饮食结构西方化等危险因素的增加,流行病学预测,未来20年内中国可能成为新发结直肠癌病例数最高的国家之一[13]。结直肠癌的发生、发展及转移过程涉及多种基因的异常表达,FOXM1和PLK1这两个关键的细胞周期调控因子,已在胃癌[14]、食管癌[15]等多种消化道恶性肿瘤中被证实参与肿瘤进展的分子调控网络。
本研究基于免疫组织化学结果,发现在结直肠癌组织中FOXM1和PLK1表达水平显著高于癌旁组织(P<0.05),并利用蛋白质印迹、qRT-PCR实验从蛋白、mRNA水平验证上述实验结果。进一步病理分析显示,FOXM1表达水平与患者的分化程度、TNM分期、有无淋巴结转移、浸润深度相关(P<0.05),PLK1表达水平则与患者的TNM分期、有无淋巴结转移、浸润深度相关(P<0.05)。生存分析表明,FOXM1和PLK1共表达较FOXM1和PLK1单独表达或者同时不表达患者的OS、DFS显著缩短,提示二者与患者不良预后密切相关。这些发现与以往的研究结果一致[16-22]。值得注意的是,本研究在观察同一患者免疫组织化学结果时,发现FOXM1和PLK1的免疫结果评分呈正相关,结合既往研究报道,在食管腺癌中,FOXM1可以通过调控PLK1影响细胞增殖、迁移、侵袭能力[23]。因此,我们推测在结直肠癌中FOXM1可以促进PLK1的表达。为验证这一假说,利用FOXM1抑制剂FDI-6干预HCT-116人结肠癌细胞,通过蛋白质印迹、qRT-PCR实验发现无论是从蛋白水平还是mRNA水平,抑制FOXM1表达后PLK1表达水平可显著下降。
本研究中首次将FOXM1、PLK1在结直肠癌中共同研究,揭示了FOXM1正向调控PLK1的分子机制,并通过免疫组织化学等实验进一步验证了两者与患者临床病理参数及预后的显著相关性,为后续在结直肠癌中研究FOXM1-PLK1信号通路提供初步的实验依据。然而本研究仅通过药理学抑制剂干预FOXM1表达,尚未通过基因编辑技术(如shRNA病毒稳定转染)进行更严谨的验证。另外,未进行PLK1过表达回补实验,未深入探索FOXM1-PLK1轴对结直肠癌细胞增殖、迁移等恶性生物学行为的影响,因此目前还无法直接证明FOXM1-PLK1信号通路在结直肠癌的发展过程中发挥一定的作用。此外本研究样本量较少,未进行相关动物实验,今后可以通过进一步扩大样本量,完善细胞和动物实验,深入开展前瞻性研究,进一步研究FOXM1-PLK1信号通路在结直肠癌中的作用机制,为结直肠癌患者的靶向治疗提供更充分的实验依据。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:韩 雪:课题设计、实验实施、文章撰写与修改刘树青:数据统计分析指导、文章修改指导殷将领:数据收集王昌成:课题指导、文章修改指导 -
表 1 FOXM1、PLK1在结直肠癌中的表达情况
Table 1 Expression of FOXM1 and PLK1 in colorectal cancer
Tissues N Positive expression
rate of FOXM1 (%)Positive expression
rate of PLK1 (%)Colorectal cancer 60 40(66.67) 44(73.33) Paracancer 60 22(36.67) 20(33.33) χ 2 10.812 19.286 P 0.001 <0.001 表 2 FOXM1、PLK1与临床病理特征之间的关系
Table 2 Relationship among FOXM1, PLK1, and clinicopathological features
Clinicopathologic features N Positive expression
rate of FOXM1(%)χ2 P Positive expression
rate of PLK1 (%)χ2 P Gender 0.039 0.844 0.002 0.967 Male 41 27(65.85) 30(73.17) Female 19 13(68.42) 14(73.68) Age (years) 0.134 0.714 0.075 0.785 ≤60 32 22(68.75) 23(71.88) >60 28 18(64.29) 21(75.00) TNM stage 15.313 <0.001 10.355 0.001 Ⅰ-Ⅱ 36 17(47.22) 21(58.33) Ⅲ-Ⅳ 24 23(95.83) 23(95.83) Degree of differentiation 9.808 0.002 0.302 0.582 Low 26 23(88.46) 20(76.92) Medium-high 34 17(50.00) 24(70.59) Tumor size (cm) 0.035 0.851 0.006 0.936 <5 23 15(65.22) 17(73.91) ≥5 37 25(65.57) 27(72.97) Lymph node metastases 4.658 0.031 5.084 0.024 Positive 14 6(42.86) 7(50.00) Negative 46 34(73.91) 37(80.43) Depth of infiltration 4.800 0.028 5.455 0.020 T1-T2 30 16(53.33) 18(60.00) T3-T4 30 21(70.00) 26(86.67) Neural invasion 0.400 0.527 0.455 0.507 Positive 15 9(60.00) 10(66.67) Negative 45 31(68.89) 34(75.56) Vascular invasion 0.400 0.527 0.455 0.500 Positive 15 11(73.33) 10(66.67) Negative 45 29(64.44) 34(75.56) 表 3 结直肠组织中FOXM1与PLK1表达的相关性
Table 3 Correlation between FOXM1 and PLK1 expression in colorectal tissues
FOXM1 expression PLK1 expression rs P Positive Negative Positive 34 10 0.373 0.003 Negative 10 10 -
[1] Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2024, 74(3): 229-263. doi: 10.3322/caac.21834
[2] Stoffel EM, Murphy CC. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults[J]. Gastroenterology, 2020, 158(2): 341-353. doi: 10.1053/j.gastro.2019.07.055
[3] Klimeck L, Heisser T, Hoffmeister M, et al. Colorectal cancer: A health and economic problem[J]. Best Pract Res Clin Gastroenterol, 2023, 66: 101839. doi: 10.1016/j.bpg.2023.101839
[4] Abedizadeh R, Majidi F, Khorasani HR, et al. Colorectal cancer: a comprehensive review of carcinogenesis, diagnosis, and novel strategies for classified treatments[J]. Cancer Metastasis Rev, 2024, 43(2): 729-753. doi: 10.1007/s10555-023-10158-3
[5] Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review[J]. JAMA, 2021, 325(7): 669-685. doi: 10.1001/jama.2021.0106
[6] Fritz CDL, Otegbeye EE, Zong X, et al. Red-flag signs and symptoms for earlier diagnosis of early-onset colorectal cancer[J]. J Natl Cancer Inst, 2023, 115(8): 909-916. doi: 10.1093/jnci/djad068
[7] Bella L, Zona S, Nestal de Moraes G, et al. FOXM1: A key oncofoetal transcription factor in health and disease[J]. Semin Cancer Biol, 2014, 29: 32-39. doi: 10.1016/j.semcancer.2014.07.008
[8] Wonsey DR, Follettie MT. Loss of the forkhead transcription factor FoxM1 causes centrosome amplification and mitotic catastrophe[J]. Cancer Res, 2005, 65(12): 5181-5189. doi: 10.1158/0008-5472.CAN-04-4059
[9] Gheghiani L, Loew D, Lombard B, et al. PLK1 Activation in Late G2 Sets Up Commitment to Mitosis[J]. Cell Rep, 2017, 19(10): 2060-2073. doi: 10.1016/j.celrep.2017.05.031
[10] Severance AL, Latham KE. PLK1 regulates spindle association of phosphorylated eukaryotic translation initiation factor 4E-binding protein and spindle function in mouse oocytes[J]. Am J Physiol Cell Physiol, 2017, 313(5): C501-C515. doi: 10.1152/ajpcell.00075.2017
[11] Maia AR, Garcia Z, Kabeche L, et al. Cdk1 and Plk1 mediate a CLASP2 phospho-switch that stabilizes kinetochore-microtubule attachments[J]. J Cell Biol, 2012, 199(2): 285-301. doi: 10.1083/jcb.201203091
[12] Kalous J, Aleshkina D. Multiple Roles of PLK1 in Mitosis and Meiosis[J]. Cells, 2023, 12(1): 187. doi: 10.3390/cells12010187
[13] Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes[J]. Gut, 2015, 64(10): 1637-1649. doi: 10.1136/gutjnl-2014-309086
[14] Zhao Z, Cai Z, Zhang S, et al. Activation of the FOXM1/ASF1B/PRDX3 axis confers hyperproliferative and antioxidative stress reactivity to gastric cancer[J]. Cancer Lett, 2024, 589: 216796. doi: 10.1016/j.canlet.2024.216796
[15] Zhao M, Lu T, Bi G, et al. PLK1 regulating chemoradiotherapy sensitivity of esophageal squamous cell carcinoma through pentose phosphate pathway/ferroptosis[J]. Biomed Pharmacother, 2023, 168: 115711. doi: 10.1016/j.biopha.2023.115711
[16] Yoshida Y, Wang IC, Yoder HM, et al. The forkhead box M1 transcription factor contributes to the development and growth of mouse colorectal cancer[J]. Gastroenterology, 2007, 132(4): 1420-1431. doi: 10.1053/j.gastro.2007.01.036
[17] Rather TB, Parveiz I, Bhat GA, et al. Evaluation of Forkhead BOX M1 (FOXM1) gene expression in colorectal cancer[J]. Clin Exp Med, 2023, 23(6): 2385-2405.
[18] Xu M, Shao X, Li H, et al. Clinical value and potential association of Rab1A and FoxM1 aberrant expression in colorectal cancer[J]. Sci Rep, 2020, 10(1): 20160. doi: 10.1038/s41598-020-77182-z
[19] Blanchard TG, Czinn SJ, Banerjee V, et al. Identification of Cross Talk between FoxM1 and RASSF1A as a Therapeutic Target of Colon Cancer[J]. Cancers (Basel), 2019, 11(2): 199. doi: 10.3390/cancers11020199
[20] Rödel F, Keppner S, Capalbo G, et al. Polo-like kinase 1 as predictive marker and therapeutic target for radiotherapy in rectal cancer[J]. Am J Pathol, 2010, 177(2): 918-929. doi: 10.2353/ajpath.2010.100040
[21] Jajac Brucic L, Bisof V, Soce M, et al. Polo-like Kinase 1 Expression as a Biomarker in Colorectal Cancer: A Retrospective Two-Center Study[J]. Biomedicines, 2024, 13(1): 54. doi: 10.3390/biomedicines13010054
[22] Wang Y, Wang G, Xiang W, et al. Proteasome activation is critical for cell death induced by inhibitors of polo-like kinase 1 (PLK1) in multiple cancers[J]. Eur J Pharmacol, 2024, 972: 176558. doi: 10.1016/j.ejphar.2024.176558
[23] Dibb M, Han N, Choudhury J, et al. The FOXM1-PLK1 axis is commonly upregulated in oesophageal adenocarcinoma[J]. Br J Cancer, 2012, 107(10): 1766-1775. doi: 10.1038/bjc.2012.424




 下载:
下载: