Short-Term Efficacy and Safety of Pazopanib Combined with Chemotherapy in Treatment of Advanced Synovial Sarcoma
-
摘要:目的
探讨培唑帕尼联合化疗治疗晚期滑膜肉瘤(SS)的近期疗效及安全性。
方法回顾性分析接受培唑帕尼联合不同化疗方案治疗的晚期滑膜肉瘤患者64例,其中,采取一线化疗方案26例、二线化疗方案19例、三线化疗方案19例,对不同方案的治疗效果和安全性进行评价。
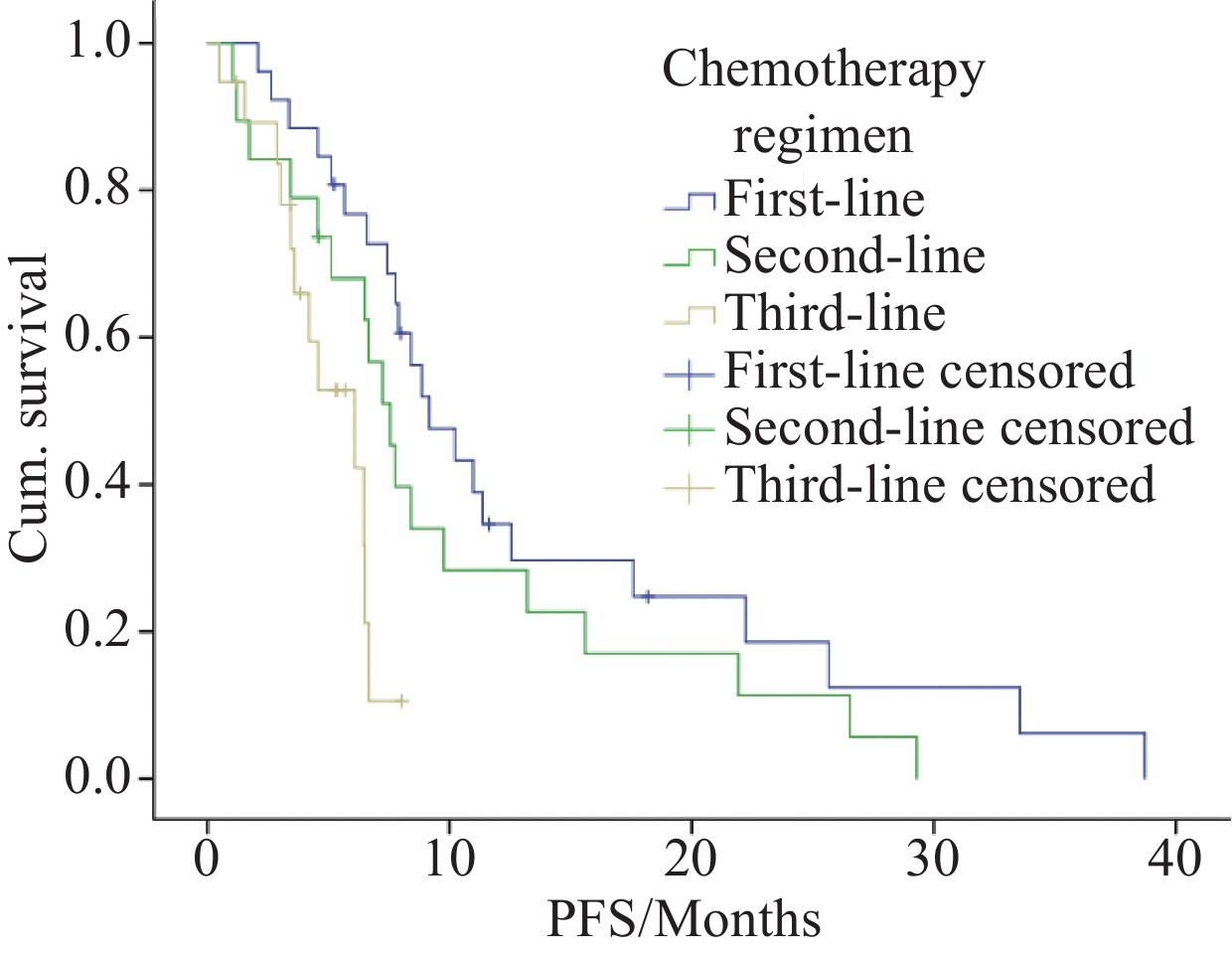
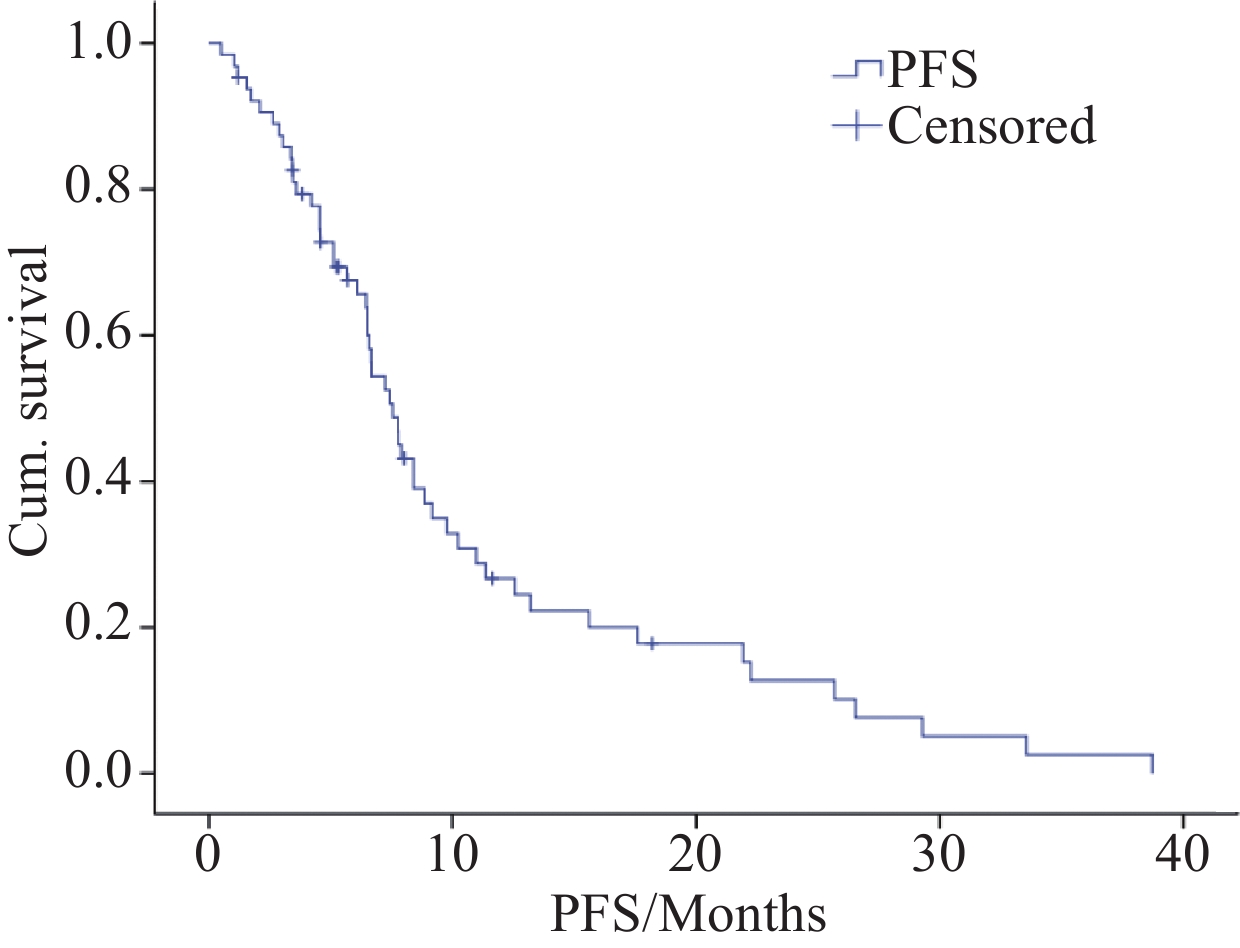
结果64例患者中,33例患者部分缓解,12例患者疾病稳定,19例患者疾病进展,其客观缓解率(ORR)和疾病控制率(DCR)分别为51.6%(33/64)和70.3%(45/64),中位PFS为7.55个月(95%CI:6.320~8.780)。采用一线化疗方案的26例患者ORR为65.4%(17/26),DCR为73.1%(19/26),中位PFS为9.167个月(95%CI:6.362~11.971);采用二线化疗方案的19例患者ORR为47.4%(9/19),DCR为73.7%(14/19),中位PFS为7.55月(95%CI:6.054~9.046);三线化疗方案的19例患者ORR为36.8%(7/19),DCR为63.2%(12/19),中位PFS为6.09月(95%CI:3.158~9.022)。不良反应以Ⅰ/Ⅱ级为主,Ⅲ/Ⅳ级不良事件很少出现,治疗过程中没有死亡案例。
结论培唑帕尼联合化疗治疗晚期滑膜肉瘤的疗效较好,不良反应可以接受。
Abstract:ObjectiveTo investigate the short-term efficacy and safety of pazopanib combined with chemotherapy in the treatment of advanced synovial sarcoma (SS).
MethodsA total of 64 patients with advanced SS treated with pazopanib combined with different chemotherapy regimens were selected. Among them, 26 patients received first-line chemotherapy regimen, 19 patients received second-line chemotherapy regimen, and 19 patients received third-line chemotherapy regimen. The therapeutic efficacy and safety of different treatment regimens were evaluated.
ResultsOut of the 64 patients, 33 achieved partial response (PR), 12 had stable disease (SD), and 19 experienced progression disease (PD). The objective response rate (ORR) and disease control rate (DCR) were 51.6% (33/64) and 70.3% (45/64), respectively, with a median progression-free survival (PFS) of 7.55 months (95%CI: 6.320–8.780 months). Among the 26 patients treated with the first-line chemotherapy regimen, the ORR was 65.4% (17/26), the DCR was 73.1% (19/26), and the median PFS was 9.167 months (95%CI: 6.362–11.971 months). For the 19 patients receiving second-line chemotherapy regimen, the ORR was 47.4% (9/19), the DCR was 73.7% (14/19), and the median PFS was 7.55 months (95%CI: 6.054–9.046 months). Among the 19 patients treated with the third-line chemotherapy regimen, the ORR was 36.8% (7/19), the DCR was 63.2% (12/19), and the median PFS was 6.09 months (95%CI: 3.158–9.022 months). The majority of adverse events were grade Ⅰ/Ⅱ, whereas grade Ⅲ/Ⅳ adverse events rarely occurred. No deaths occurred during the course of treatment.
ConclusionPazopanib combined with chemotherapy shows good efficacy in the treatment of advanced SS, with acceptable adverse events.
-
Key words:
- Synovial sarcoma /
- Pazopanib /
- Molecular targeted therapy /
- Chemotherapy
-
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:王 晶:数据整理,统计分析,文献调研,论文撰写林 峰:研究指导,论文修改
-
表 1 晚期滑膜肉瘤患者的一般特征(N=64)
Table 1 Characteristics of patients with advanced synovial sarcoma (N=64)
Characteristics n (%) Gender Male 40 (62.5) Female 24 (37.5) Age (years) ≥40 27 (42.2) <40 37 (57.8) ECOG 0 42 (65.6) 1 22 (34.4) Primary tumor sites Head-neck 14 (21.9) Limbs 24 (37.5) Trunk 26 (40.6) Metastasis sites Lung 34 (53.1) Lymph node 18 (28.1) Bone 5 (7.8) Adrenal gland 3 (4.7) Spermatic cord 2 (3.1) Intestinal tract 2 (3.1) Chemotherapy regimen First line 26 (40.6) Second line 19 (29.7) Third line 19 (29.7) Treatment Surgery 64 (100.0) Radiotherapy 50 (78.1) Chemotherapy 64 (100.0) 表 2 滑膜肉瘤患者生存预后相关因素分析结果
Table 2 Analysis results of prognostic factors related to survival of synovial sarcoma patients
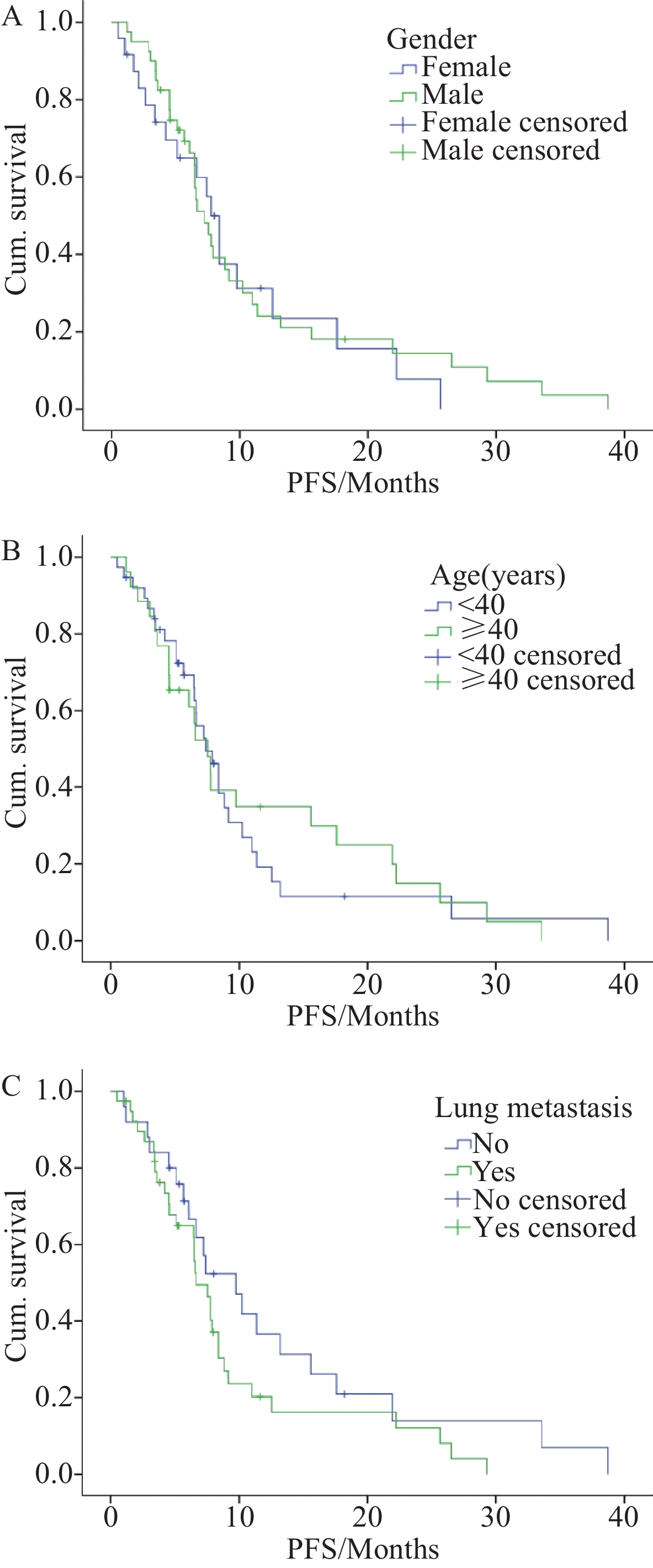
Variable Survival Univariate analysis Multivariate analysis PFS (months) 95%CI χ2 P HR 95%CI P Chemotherapy regimen First line 9.167 6.362–11.971 10.395 0.006 Ref Second line 7.550 6.054–9.046 1.604 0.815–3.160 0.172 Third line 6.090 3.158–9.022 4.020 1.721–9.396 0.001 Gender Female 7.770 6.127–9.413 0.125 0.723 0.836 Ref Male 7.230 5.841–8.619 0.445–1.569 0.576 Age (years) <40 7.430 5.735–9.125 0.039 0.844 0.981 Ref ≥40 7.550 5.627–9.473 0.547–1.761 0.949 Lung metastasis No 9.767 5.616–13.918 1.969 0.161 1.575 Ref Yes 6.660 4.951–8.369 0.868–2.858 0.135 表 3 所有接受治疗的滑膜肉瘤患者不良反应汇总(n(%))
Table 3 Summary of adverse events in all treated patients with synovial sarcoma (n(%))
Adverse events Grade Ⅰ/Ⅱ Grade Ⅲ/Ⅳ Total Fatigue 20 (31.25) 2 (3.13) 22 (34.38) Hand-foot syndrome 11 (17.19) 2 (3.13) 13 (20.31) Nausea/vomiting 14 (21.88) 0 (0.00) 14 (21.88) Diarrhea 23 (35.93) 5 (7.81) 28 (43.75) Stomatitis 8 (12.50) 0 (0.00) 8 (12.50) Hypertension 14 (21.88) 0 (0.00) 14 (21.88) Edema 5 (7.81) 0 (0.00) 5 (7.81) Lightening of skin color 8 (12.50) 0 (0.00) 8 (12.50) Change in hair color 34 (53.13) 0 (0.00) 34 (53.13) Leucopenia 23 (35.94) 2 (3.13) 25 (39.06) Thrombocytopenia 26 (40.63) 5 (7.81) 31 (48.44) Anemia 29 (45.31) 0 (0.00) 29 (45.31) Liver function impairment 14 (21.88) 0 (0.00) 14 (21.88) Kidney function impairment 8 (12.50) 0 (0.00) 8 (12.50) Hypothyroidism 11 (17.19) 0 (0.00) 11 (17.19) -
[1] Aytekin MN, Öztürk R, Amer K, et al. Epidemiology, incidence, and survival of synovial sarcoma subtypes: SEER database analysis[J]. J Orthop Surg (Hong Kong), 2020, 28(2): 2309499020936009.
[2] Gazendam AM, Popovic S, Munir S, et al. Synovial sarcoma: a clinical review[J]. Curr Oncol, 2021, 28(3): 1909-1920. doi: 10.3390/curroncol28030177
[3] Eliason L, Grant L, Francis A, et al. Qualitative study to characterize patient experience and relevance of patient-reported outcome measures for patients with metastatic synovial sarcoma[J]. J Patient Rep Outcomes, 2022, 6(1): 43. doi: 10.1186/s41687-022-00450-1
[4] von Mehren M, Kane JM, Agulnik M, et al. Soft tissue sarcoma, version 2.2022, NCCN Clinical Practice Guidelines in Oncology[J]. J Natl Compr Canc Netw, 2022, 20(7): 815-833. doi: 10.6004/jnccn.2022.0035
[5] 刘书中, 周熹, 宋桉, 等. 滑膜肉瘤全身多发转移1例报道并文献复习[J]. 中国实验诊断学, 2017, 21(5): 908-909. [Liu SZ, Zhou X, Song A, et al. Report and Literature Review of a Case with Multiple Systemic Metastases of Synovial Sarcoma[J]. Zhongguo Shi Yan Zhen Duan Xue, 2017, 21(5): 908-909.] doi: 10.3969/j.issn.1007-4287.2017.05.048 Liu SZ, Zhou X, Song A, et al. Report and Literature Review of a Case with Multiple Systemic Metastases of Synovial Sarcoma[J]. Zhongguo Shi Yan Zhen Duan Xue, 2017, 21(5): 908-909. doi: 10.3969/j.issn.1007-4287.2017.05.048
[6] Palmerini E, Staals EL, Alberghini M, et al. Synovial sarcoma: retrospective analysis of 250 patients treated at a single institution[J]. Cancer, 2009, 115(13): 2988-2998. doi: 10.1002/cncr.24370
[7] 潘伟汉, 张世权. 滑膜肉瘤的治疗进展[J]. 中国骨与关节杂志, 2019, 8(6): 443-448. [Pan WH, Zhang SQ. Progress of synovial sarcoma treatment[J]. Zhongguo Gu Yu Guan Jie Za Zhi, 2019, 8(6): 443-448.] doi: 10.3969/j.issn.2095-252X.2019.06.008 Pan WH, Zhang SQ. Progress of synovial sarcoma treatment[J]. Zhongguo Gu Yu Guan Jie Za Zhi, 2019, 8(6): 443-448. doi: 10.3969/j.issn.2095-252X.2019.06.008
[8] Blay JY, von Mehren M, Jones RL, et al. Synovial sarcoma: characteristics, challenges, and evolving therapeutic strategies[J]. ESMO Open, 2023, 8(5): 101618. doi: 10.1016/j.esmoop.2023.101618
[9] Yang J, Xu Y, Chen Y, et al. Therapeutic perspectives for adult soft tissue sarcoma-updates from the 2022 ASCO annual meeting[J]. Cancer Biol Med, 2022, 19(10): 1496-1501. doi: 10.20892/j.issn.2095-3941.2022.0394
[10] Gronchi A, Palmerini E, Quagliuolo V, et al. Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: final results of a randomized trial from Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) Sarcoma Groups[J]. J Clin Oncol, 2020, 38(19): 2178-2186. doi: 10.1200/JCO.19.03289
[11] Moreau-Bachelard C, Campion L, Toulmonde M, et al. Patterns of care and outcomes of 417 patients with METAstatic SYNovial sarcoma (METASYN): real-life data from the French Sarcoma Group (FSG)[J]. ESMO Open, 2022, 7(2): 100402. doi: 10.1016/j.esmoop.2022.100402
[12] Sleijfer S, Ouali M, van Glabbeke M, et al. Prognostic and predictive factors for outcome to first-line ifosfamide containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG)[J]. Eur J Cancer, 2010, 46(1): 72-83. doi: 10.1016/j.ejca.2009.09.022
[13] von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version L, BENJAMIN 2.2018, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2018, 16(5): 536-563. doi: 10.6004/jnccn.2018.0025
[14] Italiano A, Penel N, Robin YM, et al. Neo/adjuvant chemotherapy does not improve outcome in resected primary synovial sarcoma: a study of the French Sarcoma Group[J]. Ann Oncol, 2009, 20(3): 425-430. doi: 10.1093/annonc/mdn678
[15] Noujaim J, Constantinidou A, Messiou C, et al. Successful ifosfamide rechallenge in soft-tissue sarcoma[J]. Am J Clin Oncol, 2018, 41(2): 147-151. doi: 10.1097/COC.0000000000000243
[16] 李天宇. 阿帕替尼治疗进展期软组织肉瘤的疗效与安全性评估[D]. 武汉: 华中科技大学, 2020. [Li TY. Efficacy and safety of Apatinib in the treatment of advanced soft tissue sarcoma[D]. Wuhan: Huazhong University of Science and Technology, 2020.] Li TY. Efficacy and safety of Apatinib in the treatment of advanced soft tissue sarcoma[D]. Wuhan: Huazhong University of Science and Technology, 2020.
[17] Gutierrez-Sainz L, Martinez-Fdez S, Pedregosa-Barbas J, et al. Efficacy of second and third lines of treatment in advanced soft tissue sarcomas: a real-world study[J]. Clin Transl Oncol, 2023, 25(12): 3519-3526. doi: 10.1007/s12094-023-03221-6
[18] Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial[J]. Lancet Oncol, 2014, 15(4): 415-423. doi: 10.1016/S1470-2045(14)70063-4
[19] Ryan CW, Merimsky O, Agulnik M, et al. PICASSO Ⅲ: A phase Ⅲ, Placebo-Controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma[J]. J Clin Oncol, 2016, 34(32): 3898-3905. doi: 10.1200/JCO.2016.67.6684
[20] Tap WD, Jones RL, van Tine BA, et al. Olaratumaband doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial[J]. Lancet, 2016, 388(10043): 488-497. doi: 10.1016/S0140-6736(16)30587-6
[21] 庄荣源, 王志明, 郭曦, 等. 安罗替尼联合化疗治疗晚期软组织肉瘤的近期疗效及安全性分析[J]. 中国临床医学, 2019, 26(3): 378-381. [Zhang RY, Wang ZM, Guo X, et al. The short-term efficacy and safety of anlotinib combined with chemotherapy in treatment of advanced soft tissue sarcoma[J]. Zhongguo Lin Chuang Yi Xue, 2019, 26(3): 378-381.] doi: 10.12025/j.issn.1008-6358.2019.20190054 Zhang RY, Wang ZM, Guo X, et al. The short-term efficacy and safety of anlotinib combined with chemotherapy in treatment of advanced soft tissue sarcoma[J]. Zhongguo Lin Chuang Yi Xue, 2019, 26(3): 378-381. doi: 10.12025/j.issn.1008-6358.2019.20190054
[22] Tang L, Wang Y, Zhang J, et al. Efficacy and safety of anlotinib in advanced soft tissue sarcoma: results from one of multicenters in a phase ⅡB trial (ALTER0203)[J]. J Clin Oncol, 2019, 37(15_suppl): e22518. doi: 10.1200/JCO.2019.37.15_suppl.e22518
[23] Van Tine BA, Chawla SP, Trent JC, et al. A phase Ⅲ study (APROMISS) of AL3818 (Catequentinib, Anlotinib) hydrochloride monotherapy in subjects with metastatic or advanced synovial sarcoma[J]. J Clin Oncol, 2021, 39 (15 suppl): 11505.
[24] Nakamura T, Matsumine A, Kawai A, et al. The clinical outcome of pazopanib treatment in Japanese patients with relapsed soft tissue sarcoma: A Japanese Musculoskeletal Oncology Group (JMOG) study[J]. Cancer, 2016, 122(9): 1408-1416. doi: 10.1002/cncr.29961
[25] Chi Y, Fang Z, Hong X, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma[J]. Clin Cancer Res, 2018, 24(21): 5233-5238. doi: 10.1158/1078-0432.CCR-17-3766
[26] Hamberg P, Boers-Sonderen MJ, van der Graaf WTA, et al. Pazopanib exposure decreases as a result of an ifosfamide-dependent drug-drug interaction: results of a phase Ⅰ study[J]. Br J Cancer, 2014, 110(4): 888-893. doi: 10.1038/bjc.2013.798
[27] Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase Ⅱ study from the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC Study 62043)[J]. J Clin Oncol, 2009, 27(19): 3126-3132. doi: 10.1200/JCO.2008.21.3223
[28] Gelderblom H, Judson IR, Benson C, et al. Treatment patterns and clinical outcomes with pazopanib in patients with advanced soft tissue sarcomas in a compassionate use setting: results of the SPIRE study[J]. Acta Oncol, 2017, 56(12): 1769-1775. doi: 10.1080/0284186X.2017.1332779
[29] Samuels BL, Chawla SP, Somaiah N, et al. Results of a prospective phase 2 study of pazopanib in patients with advanced intermediate-grade or high-grade liposarcoma[J]. Cancer, 2017, 123(23): 4640-4647. doi: 10.1002/cncr.30926
[30] Bilici A, Koca S, Karaagac M, et al. Real-world outcomes of pazopanib in metastatic soft tissue sarcoma: a retrospective Turkish oncology group (TOG) study[J]. J Cancer Res Clin Oncol, 2023, 149(11): 8243-8253. doi: 10.1007/s00432-023-04766-3
[31] Oh CR, Hong JY, Kim JH, et al. Real-world outcomes of pazopanib treatment in korean patients with advanced soft tissue sarcoma: a multicenter retrospective cohort study[J]. Target Oncol, 2020, 15(4): 485-493. doi: 10.1007/s11523-020-00731-z
[32] van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomized, double-blind, placebo-controlled phase 3 trial[J]. Lancet, 2012, 379(9829): 1879-1886. doi: 10.1016/S0140-6736(12)60651-5
[33] Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma[J]. N Engl J Med, 2013, 369(8): 722-731. doi: 10.1056/NEJMoa1303989
[34] Koca S, Beşiroğlu M, Özçelik M, et al. Pazopanib for metastatic soft-tissue sarcoma: A multicenter retrospective study[J]. J Oncol Pharm Pract, 2021, 27(3): 541-546. doi: 10.1177/1078155220924075



 下载:
下载: