Effects of UBE2T on Proliferation, Apoptosis, and Epithelial-Mesenchymal Transition of Breast Cancer Cells
-
摘要:目的
探究泛素结合酶E2T(UBE2T)在乳腺癌中的表达和对预后的影响及其可能的作用机制。
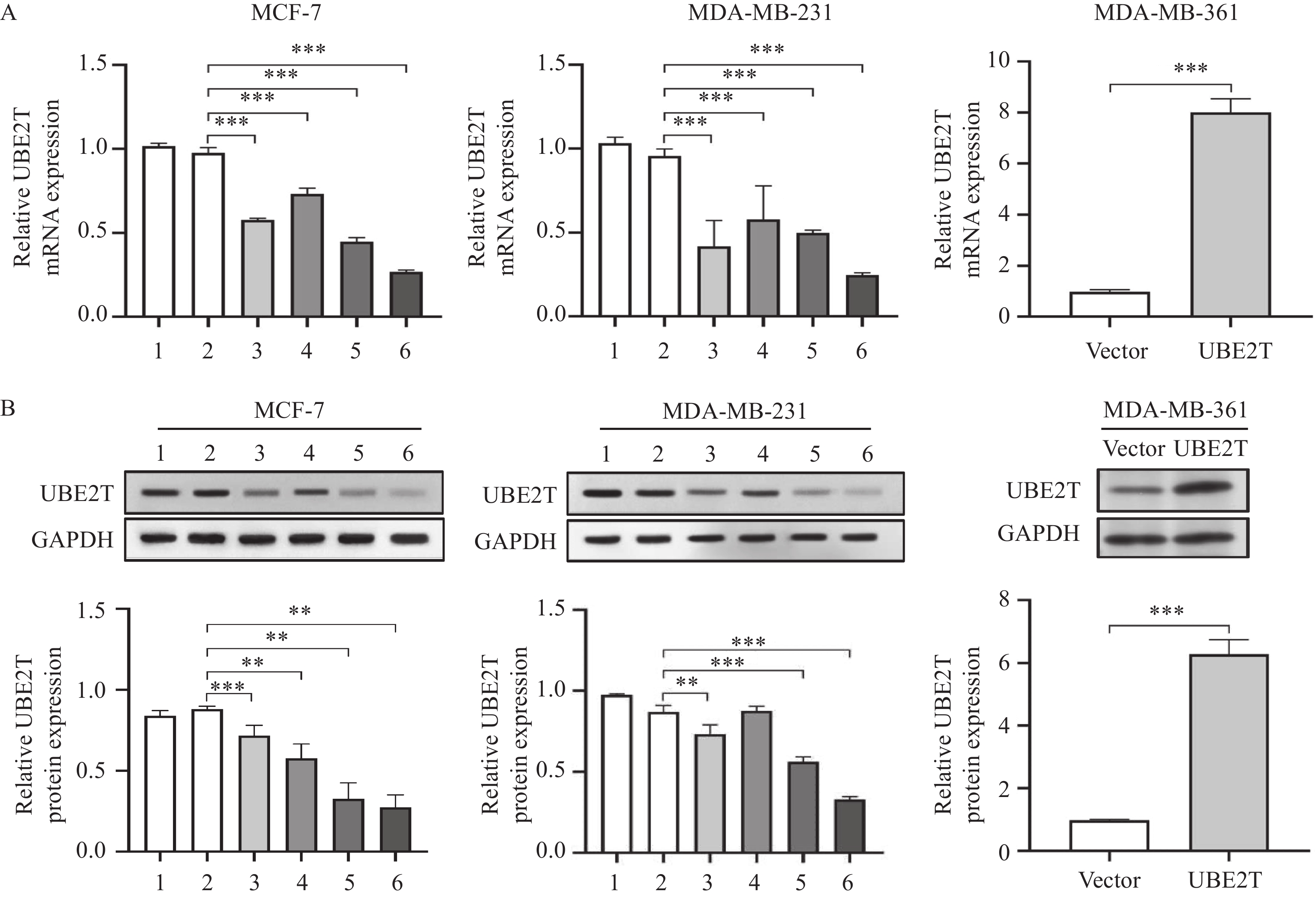
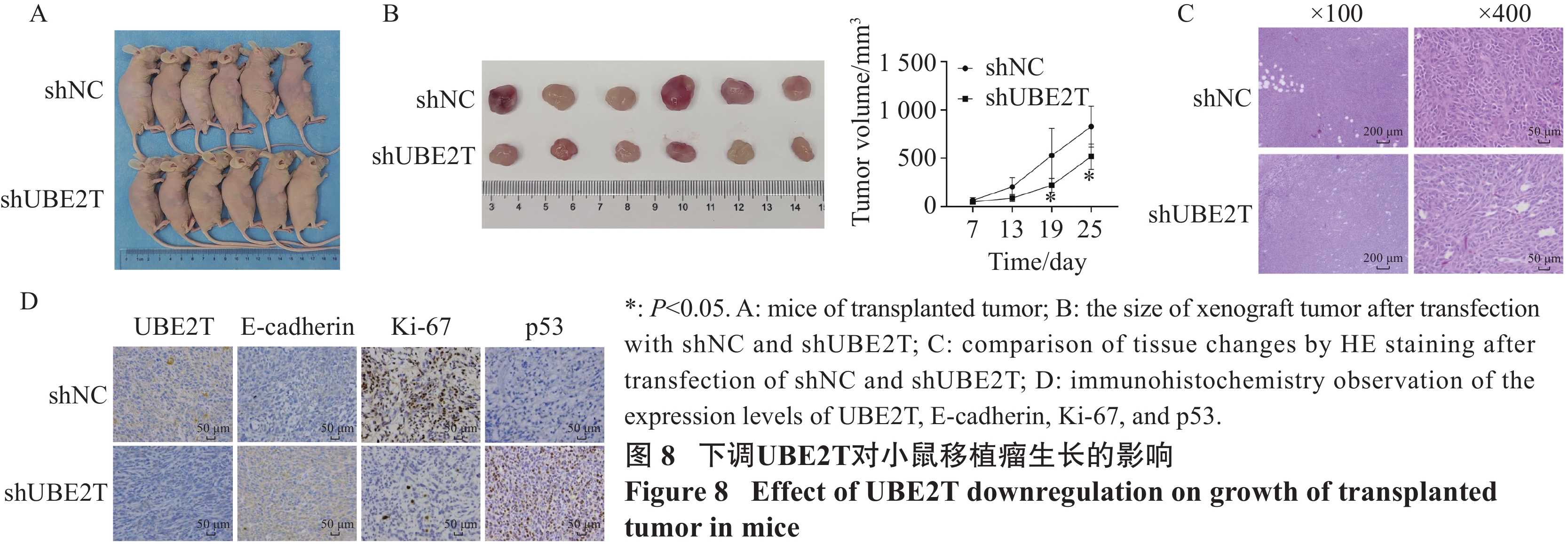
方法采用肿瘤基因组图谱(TCGA)数据库分析UBE2T在乳腺癌组织中的表达情况,Kaplan-Meier生存曲线分析UBE2T高低表达对无病生存期(DFS)和总生存期(OS)的影响。实时定量PCR和Western blot验证UBE2T敲降和过表达效率,分析其对肿瘤细胞生物学行为的影响,Western blot研究UBE2T对细胞上皮-间质转化(EMT)的作用。构建异种移植瘤模型,验证敲降UBE2T对乳腺癌细胞在体内生长的影响。
结果UBE2T在乳腺癌组织和癌旁组织的表达量差异具有统计学意义(P<0.001),在远处转移或分期较晚的乳腺癌患者中表达量较高(均P<0.05),且UBE2T高表达组的DFS和OS降低(均P<0.05)。UBE2T在MCF-7、MDA-MB-231细胞中高表达,在MDA-MB-361细胞中低表达(均P<0.01)。敲降UBE2T后肿瘤细胞增殖能力显著下降,而上调UBE2T表达后细胞增殖能力增强(均P<0.05);沉默组MCF-7、MDA-MB-231细胞凋亡率均较shNC组显著升高,过表达组MAD-MB-361细胞凋亡率下降(均P<0.001);敲降组细胞迁移率较shNC组降低,而过表达组细胞迁移率显著增加(均P<0.01)。此外,敲降组细胞迁移数和侵袭数均低于shNC组,过表达组细胞迁移数和侵袭数均高于对照组(均P<0.01)。下调UBE2T可以降低N-cadherin、Snail和Vimentin的表达(均P<0.05),升高E-cadherin的表达,而上调UBE2T表达则相反(均P<0.01);但TIMER数据库分析结果提示,UBE2T表达与E-cadherin(P<0.001)、N-cadherin(P=0.013)、Snail(P<0.001)正相关,与Vimentin负相关(P<0.001)。体内实验表明下调UBE2T可以减缓移植瘤的生长速度。
结论UBE2T在乳腺癌组织中高表达并影响患者预后,UBE2T可以促进乳腺癌细胞的增殖,并抑制凋亡,同时通过改变EMT相关蛋白的表达,增强肿瘤细胞迁移和侵袭的能力。
Abstract:ObjectiveTo investigate the expression of ubiquitin binding enzyme E2T (UBE2T) in breast cancer (BRCA) and its role and mechanism in the prognosis of BRCA patients.
MethodsThe Tumor Genome Atlas (TCGA) database was used to analyze UBE2T expression in BRCA tissues, and the effects of UBE2T expression on disease-free survival (DFS) and overall survival (OS) were analyzed by Kaplan-Meier (KM) survival curve. In vitro, real-time quantitative PCR and Western blot were used to confirm the knock-down and overexpression efficiency, to analyze its effect on tumor cell biological behavior. The effect of UBE2T on cell epithelial–mesenchymal transition (EMT) was studied by Western blot. A xenograft tumor model was established to verify the effect of UBE2T knockdown on the growth of BRCA cells in vivo.
ResultsThe UBE2T expression levels in BRCA and adjacent tissues were statistically different (P<0.001), and the expression was increased in tissues with distant metastasis or late stage (all P<0.05). The DFS and OS were decreased in the UBE2T high-level group (both P<0.05). UBE2T was highly expressed in MCF-7 and MDA-MB-231 cells and lowly expressed in MDA-MB-361 cells (all P<0.01). After UBE2T was silenced by shRNA, the proliferation ability of tumor cells significantly decreased, whereas it increased after UBE2T up-expression (all P<0.05). The apoptotic rates of MCF-7 and MDA-MB-231 cells in the silent groups were significantly higher than those in the shNC groups, while the apoptotic rates of MAD-MB-361 cells in the overexpression group decreased (all P<0.001). The mobility in the knockdown groups were lower than in the shNC groups, while the mobility in the overexpression group significantly increased (both P<0.01). The migration and invasion cells in the shUBE2T groups were lower than those in the shNC groups, and the migration and invasion cells in the UBE2T group were higher than those in the vector group (all P<0.01). Downregulation of UBE2T decreased the expression levels of N-cadherin, Snail, and Vimentin (all P<0.05) and increased that of E-cadherin; however, the result of UBE2T upregulation was opposite (all P<0.01). TIMER results showed that UBE2T was positively correlated with E-cadherin (P<0.001), N-cadherin (P=0.013), and Snail (P<0.001) and negatively correlated with Vimentin (P<0.001). In vivo experiments showed that downregulation of UBE2T slowed down the growth of transplanted tumors.
ConclusionUBE2T is highly expressed in BRCA tissues and may affect the prognosis. UBE2T can promote the proliferation of BRCA cells, inhibit apoptosis, and increase the migration and invasion abilities by changing the expression levels of EMT-related proteins.
-
Key words:
- Breast cancer /
- UBE2T /
- Prognosis /
- Proliferation /
- Migration /
- Invasion /
- EMT
-
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:刘思琪:研究设计及实施、数据收集与处理、论文撰写孙 欣:数据处理、图表绘制、论文审阅及修改刘 娜:数据处理、论文修改林方才:研究设计、论文审阅及修改
-
表 1 shRNA 序列
Table 1 shRNA sequence
shRNA Forward (5′→3′) Reverse (5′→3′) shNC CCGGGGTTCTCCGAACGTGTCACGTCTCGAG
ACGTGACACGTTCGGAGAACCTTTTTGCAAAAAGGTTCTCCGAACGTGTCACGTCTC
GAGACGTGACACGTTCGGAGAACCCCGGshUBE2T#1 CCGGGCCAACACACCTTATGAGAAACTCGA
GTTTCTCATAAGGTGTGTTGGCTTTTTTAAAAAAGCCAACACACCTTATGAGAAACT
CGAGTTTCTCATAAGGTGTGTTGGCCCGGshUBE2T#2 CCGGGAACCTCCTCAGATCCGATTTCTCGAG
AAATCGGATCTGAGGAGGTTCTTTTTTAAAAAAGAACCTCCTCAGATCCGATTTCTC
GAGAAATCGGATCTGAGGAGGTTCCCGGshUBE2T#3 CCGGTGAGGAAGAGATGCTTGATAACTCGA
GTTATCAAGCATCTCTTCCTCATTTTTTAAAAAATGAGGAAGAGATGCTTGATAACT
CGAGTTATCAAGCATCTCTTCCTCACCGGshUBE2T#4 CCGGGTCCTGGTTCATCTTAGTTAACTCGAG
TTAACTAAGATGAACCAGGACTTTTTTAAAAAAGTCCTGGTTCATCTTAGTTAACTC
GAGTTAACTAAGATGAACCAGGACCCGG -
[1] Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2024, 74(3): 229-263. doi: 10.3322/caac.21834
[2] Wang X, Liu S, Xue Y. Clinicopathological features and prognosis of male breast cancer[J]. J Int Med Res, 2021, 49(10): 3000605211049977.
[3] Kelsall IR, Langenick J, Mackay C, et al. The Fanconi anaemia components UBE2T and FANCM are functionally linked to nucleotide excision repair[J]. PLoS One, 2012, 7(5): e36970. doi: 10.1371/journal.pone.0036970
[4] Matthew JC, Graeme JT, Julia C, et al. Small-Molecule Inhibition of UBE2T/FANCL-Mediated Ubiquitylation in the Fanconi Anemia Pathway[J]. ACS Chem Biol, 2019, 14(10): 2148-2154.
[5] Zhu ZR, Cao CH, Zhang DY, et al. UBE2T-mediated Akt ubiquitination and Akt/β-catenin activation promotes hepatocellular carcinoma development by increasing pyrimidine metabolism[J]. Cell Death Dis, 2022, 13(2): 154. doi: 10.1038/s41419-022-04596-0
[6] Wu MQ, Li XL, Huang WW, et al. Ubiquitin-conjugating enzyme E2T(UBE2T) promotes colorectal cancer progression by facilitating ubiquitination and degradation of p53[J]. Clin Res Hepatol Gastroenterol, 2020, 45(2): 101493.
[7] Rossi L, Mazzara C, Pagani O. Diagnosis and Treatment of Breast Cancer in Young Women[J]. Curr Treat Options Oncol, 2019, 20(12): 86. doi: 10.1007/s11864-019-0685-7
[8] Du XD, Song HY, Shen HY, et al. The Molecular Basis of Ubiquitin-Conjugating Enzymes (E2s) as a Potential Target for Cancer Therapy[J]. Int J Mol Sci, 2021, 22(7): 3440. doi: 10.3390/ijms22073440
[9] Arno FA, Viduth C, Helen W. Mechanism and disease association of E2-conjugating enzymes: lessons from UBE2T and UBE2L3[J]. Biochem J, 2016, 473(20): 3401-3419. doi: 10.1042/BCJ20160028
[10] Zhang Y, Zhou X, Huang P. Fanconi anemia and ubiquitination[J]. J Genet Genomics, 2007, 34(7): 573-580. doi: 10.1016/S1673-8527(07)60065-4
[11] Hu W, Xiao LS, Cao CH, et al. UBE2T promotes nasopharyngeal carcinoma cell proliferation, invasion, and metastasis by activating the AKT/GSK3β/β-catenin pathway[J]. Oncotarget, 2016, 7(12): 15161-15172. doi: 10.18632/oncotarget.7805
[12] Luo C, Yao Y, Yu Z, et al. UBE2T knockdown inhibits gastric cancer progression[J]. Oncotarget, 2017, 8(20): 32639-32654. doi: 10.18632/oncotarget.15947
[13] Liu LL, Zhu JM, Yu XN, et al. UBE2T promotes proliferation via G2/M checkpoint in hepatocellular carcinoma[J]. Cancer Manag Res, 2019, 11: 8359-8370. doi: 10.2147/CMAR.S202631
[14] Wen MX, Kwon YW, Wang YS, et al. Elevated expression of UBE2T exhibits oncogenic properties in human prostate cancer[J]. Oncotarget, 2015, 6(28): 25226-25239. doi: 10.18632/oncotarget.4712
[15] Cao K, Ling XD, Jiang XY, et al. Pan-cancer analysis of UBE2T with a focus on prognostic and immunological roles in lung adenocarcinoma[J]. Respir Res, 2022, 23(1): 306. doi: 10.1186/s12931-022-02226-z
[16] Hao J, Xu A, Xie X, et al. Elevated expression of UBE2T in lung cancer tumors and cell lines[J]. Tumour Biol, 2008, 29(3): 195-203. doi: 10.1159/000148187
[17] 杨豪帅, 赖慧娜, 饶怡茹, 等. 基于生物信息学途径评估UBE2家族及UBE2T基因在肺腺癌中的表达及临床意义[J]. 中山大学学报(医学科学版), 2020, 41(3): 445-451. [Yang HS, Lai HN, Rao YR, et al. Expressions and Clinical Implication of UBE2 Family and UBE2T Gene in Lung Adenocarcinoma Based on Bioinformatics[J]. Zhongshan Da Xue Xue Bao (Yi Xue Ke Xue Ban), 2020, 41(3): 445-451.] Yang HS, Lai HN, Rao YR, et al. Expressions and Clinical Implication of UBE2 Family and UBE2T Gene in Lung Adenocarcinoma Based on Bioinformatics[J]. Zhongshan Da Xue Xue Bao (Yi Xue Ke Xue Ban), 2020, 41(3): 445-451.
[18] Qiao L, Dong C, Ma BL. UBE2T promotes proliferation, invasion and glycolysis of breast cancer cells by regualting the PI3K/AKT signaling pathway[J]. J Recept Signal Transduct Res, 2021, 42(2): 151-159.
[19] Li LN, Liu J, Huang W. E2F5 promotes proliferation and invasion of gastric cancer through directly upregulating UBE2T transcription[J]. Dig Liver Dis, 2021, 54(7): 937-945.
[20] Fanfone D, Wu Z, Mammi J, et al. Confined migration promotes cancer metastasis through resistance to anoikis and increased invasiveness[J]. Elife, 2022, 11: e73150. doi: 10.7554/eLife.73150
[21] Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis[J]. Annu Rev Pathol, 2018, 13: 395-412. doi: 10.1146/annurev-pathol-020117-043854
[22] Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis[J]. Trends Cell Biol, 2019, 29(3): 212-226. doi: 10.1016/j.tcb.2018.12.001
[23] Lim B, Woodward WA, Wang X, et al. Inflammatory breast cancer biology: the tumour microenvironment is key[J]. Nat Rev Cancer, 2018, 18(8): 485-499. doi: 10.1038/s41568-018-0010-y
[24] Huang W, Huang HY, Xiao YZ, et al. UBE2T is upregulated, predicts poor prognosis, and promotes cell proliferation and invasion by promoting epithelial-mesenchymal transition via inhibiting autophagy in an AKT/mTOR dependent manner in ovarian cancer[J]. Cell Cycle, 2022, 21(8): 780-791. doi: 10.1080/15384101.2022.2031426
[25] Padmanaban V, Krol I, Suhail Y, et al. E-cadherin is required for metastasis in multiple models of breast cancer[J]. Nature, 2019, 573(7774): 439-444. doi: 10.1038/s41586-019-1526-3
[26] Dong HM, Liu G, Hou YF, et al. Dominant-negative E-cadherin inhibits the invasiveness of inflammatory breast cancer cells in vitro[J]. J Cancer Res Clin Oncol, 2007, 133(2): 83-92.




 下载:
下载: