Effect of CCNA2 on Prognosis of Colon Cancer by Regulating Immune Microenvironment of Tumor Cells
-
摘要:目的
从免疫浸润角度探讨细胞周期蛋白A2(CCNA2)与结肠癌预后的关系及可能机制。
方法从癌症基因组图谱数据库下载结肠癌患者转录组数据。根据CCNA2表达情况进行临床病理特征分析及生存分析。收集75例结肠癌患者术后肿瘤组织和正常组织标本,免疫组织化学法分析CCNA2表达水平,多因素分析其与临床病理特征的关系。基因富集分析(GSEA)评估结肠癌中CCNA2潜在分子功能。CIBERSORT算法计算CCNA2与结肠癌免疫细胞浸润的相关程度。
结果数据库分析及免疫组织化学结果提示,CCNA2在结肠癌肿瘤组织中表达程度高于正常组织(P<0.001)。CCNA2高表达组的总体生存期、疾病特异生存期、无进展间隔期均长于低表达组(均P<0.05)。在肿瘤组织中,CCNA2的表达水平随病理分期及TNM分期的升高而降低(P<0.05)。正常组织的CCNA2表达程度均小于各个病理分期的肿瘤组织(均P<0.001)。GSEA提示,CCNA2低表达时有Wnt/β-Catenin、KRAS等信号通路富集。CIBERSORT分析显示,调节性T细胞和巨噬细胞M0等免疫细胞在CCNA2低表达时浸润增加。
结论CCNA2在结肠癌中高表达,与病理分期、TNM分期密切相关,其可能通过KRAS、Wnt/β-Catenin通路募集调节性T细胞,减少免疫细胞浸润,促进结肠癌进展导致不良预后。
Abstract:ObjectiveTo investigate the relationship between cyclin A2 (CCNA2) and the prognosis of colon cancer, and its possible mechanism from the perspective of immune infiltration.
MethodsWe downloaded the transcriptome data of colon cancer patients from The Cancer Genome Atlas database. Clinicopathological feature analysis and survival analysis were performed based on the expression levels of CCNA2. A total of 75 specimens of colon cancer and normal tissues were collected, and the expression level of CCNA2 was analyzed using immunohistochemical methods. Multivariate analysis was conducted to explore its relationship with clinicopathological features. Gene Set Enrichment Analysis (GSEA) was used to assess the potential molecular functions of CCNA2 in colon cancer. CIBERSORT algorithm was applied to calculate the correlation between CCNA2 and immune-cell infiltration in colon cancer.
ResultsDatabase and immunohistochemical analyses indicated that CCNA2 was expressed at a significantly higher level in colon cancer tissues than normal tissues (P<0.001). The overall survival, disease-specific survival, and progression-free interval were all longer in the group with high CCNA2 expression than the group with low expression (all P<0.05). In tumor tissues, the expression level of CCNA2 decreased with increased pathological and TNM stages (P<0.05). The expression level of CCNA2 in normal tissues was consistently lower than that in colon cancer tissues across all clinical stages (all P<0.001). GSEA suggested that Wnt/β-catenin, KRAS, and other signaling pathways were enriched when CCNA2 was lowly expressed. CIBERSORT analysis revealed an increase in the infiltration of immune cells such as regulatory T cells and macrophages M0 when CCNA2 expression was low.
ConclusionCCNA2 is highly expressed in colon cancer and closely associated with grade of pathology and TNM stage. It may recruit regulatory T cells through the KRAS and Wnt/β-catenin pathways, thereby reducing immune-cell infiltration and promoting colon cancer progression, leading to poor prognosis.
-
Key words:
- CCNA2 /
- Colon cancer /
- Tumor microenvironment /
- Immune infiltration /
- Prognosis /
- Clinical features /
- Bioinformatics analysis
-
0 引言
结肠癌是消化道常见的恶性肿瘤之一,是人类癌症死亡的第二大常见病因,发病率在所有癌症中排第三位,趋于年轻化[1-3]。探索与结肠癌相关的基因标志物,研究结肠癌发展过程中的分子调控机制,是当前结肠癌学术研究中的主要方向。细胞周期蛋白A2(Cyclin A2, CCNA2)是细胞周期蛋白家族成员,其可以通过结合cdk2和cdc2,调控细胞周期从S期向G2期转变[4]。CCNA2具有多种生物学功能,例如细胞骨架动力学和细胞运动等[5]。同时,CCNA2影响恶性肿瘤的免疫浸润,与淋巴瘤、乳腺癌、前列腺癌、结直肠癌、甲状腺癌等多种癌症相关[6]。CCNA2与多种肿瘤预后关系的研究在国内外均有报道。CCNA2与多种肿瘤的免疫浸润相关,但CCNA2影响结肠癌免疫微环境的机制尚不明确,为进一步探索CCNA2与结肠癌免疫微环境及患者预后的关系,本研究从免疫浸润的角度探讨CCNA2导致结肠癌患者预后不佳的潜在分子机制,为结肠癌的诊治提供新思路。
1 资料与方法
1.1 数据整理及分析
从TCGA数据库(https://portal.gdc.cancer.gov/)中下载494例患者(其中肿瘤组织453例,非肿瘤患者正常组织41例)基因表达(RNA-seq)数据和临床病理资料。收集2019年7月—2024年7月湖南师范大学附属岳阳医院75例结肠手术标本(肿瘤组织标本52例,正常组织标本23例)。纳入标准:(1)经病理诊断确诊为结肠癌患者;(2)术前未经放疗、化疗,未服用过靶向药物;(3)临床资料完整。排除标准:(1)转移性肿瘤,合并其他部位恶性肿瘤患者;(2)合并其他严重疾病患者。所有研究对象均签署知情同意书,本研究经湖南师范大学附属岳阳医院(岳阳市人民医院医学伦理审查委员会)批准(科研文2024015)。
1.2 免疫组织化学检测
采集手术患者的结肠组织,免疫组织化学法处理标本:石蜡包埋切片、二甲苯脱蜡、梯度乙醇水化、PBS修复液抗原修复、过氧化物酶温室孵育、滴加CCNA2抗体(英国Abcam公司,1:250)孵育过夜、加入HRP二抗免疫显色试剂、DAB染色、中性树胶封片。染色强度评分:不着色(0分)、弱(1分)、中(2分)、强(3分);阳性细胞比例评分:<20%(1分),≥20%~30%(2分),≥30%~40%(3分),≥40%(4分)。CCNA2蛋白表达总体评分值=阳性细胞比例评分×染色强度评分;最终评判:<6分为低表达,≥6分为高表达。
1.3 CCNA2表达差异分析
根据湖南师范大学附属岳阳医院搜集到的75例手术标本及TCGA数据库中494例患者组织基因表达数据,分析肿瘤组织与正常组织中CCNA2表达水平并绘制箱式图。同时配对化分析来自同一患者的正常和肿瘤样本中的CCNA2表达情况并绘制配对连线散点图。
1.4 生存分析
使用Survival和survmine函数进行生存分析,记录生存时间跨度为0~11.69年,surv_cutpoint函数确定最佳截断值,将453例肿瘤组织样本根据最佳截断值分为高表达组和低表达组。比较两组间总体生存期、疾病特异生存期、无进展间隔期的差别。
1.5 临床病理特征分析
根据TCGA数据库453例肿瘤组织及湖南师范大学附属岳阳医院52例结肠手术肿瘤组织的临床病理特征数据(包括性别、年龄、肿瘤原发部位、肿瘤大小、分化程度、病理分期、T、N、M分期)进行差异分析,评估CCNA2表达程度与临床病理特征间关系。
1.6 免疫浸润分析
采用“CIBERSORT”包量化分析结肠癌患者中的免疫细胞评分,并分析22种免疫细胞(LM22)的免疫浸润程度与CCNA2的表达相关性。根据CCNA2的表达程度绘制箱式图及线性相关图。
1.7 基因富集分析
从GSEA官网下载基因集“Molecular Signatures Database”并进行GSEA基因富集分析,以Hallmark基因集作为GSEA的目标集合,用enrichplot包绘制富集曲线图。
1.8 统计学方法
使用RStudio(Version: 2023.12.0+369)、SPSS 26.0进行统计学分析及图片处理绘制。使用Wilcoxon秩和检验、配对样本T检验比较正常组织与肿瘤组织CCNA2表达差异。使用Kruskal-Wallis检验、Mann-Whitney U检验和χ2检验分析CCNA2表达水平与临床病理特征之间的关系。使用Log rank检验进行生存分析。P<0.05为差异有统计学意义。
2 结果
2.1 CCNA2在结肠癌组织中的表达
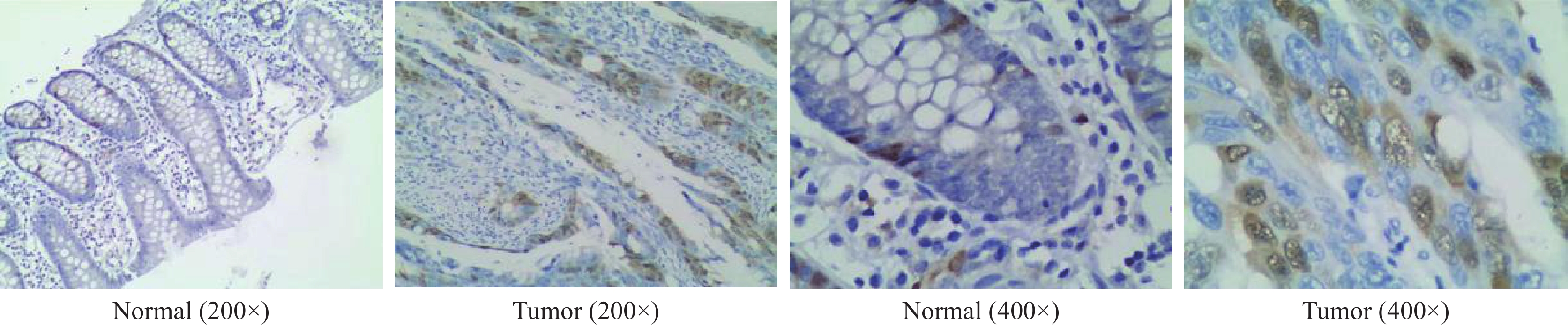
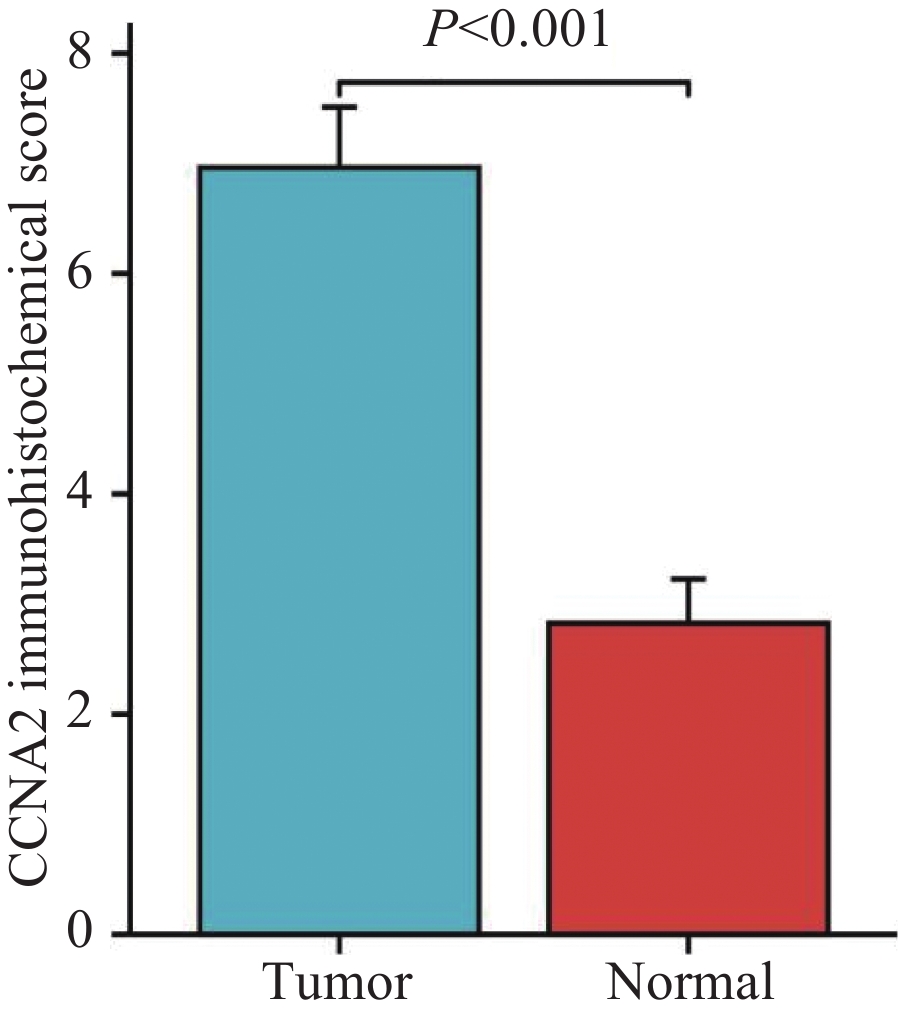
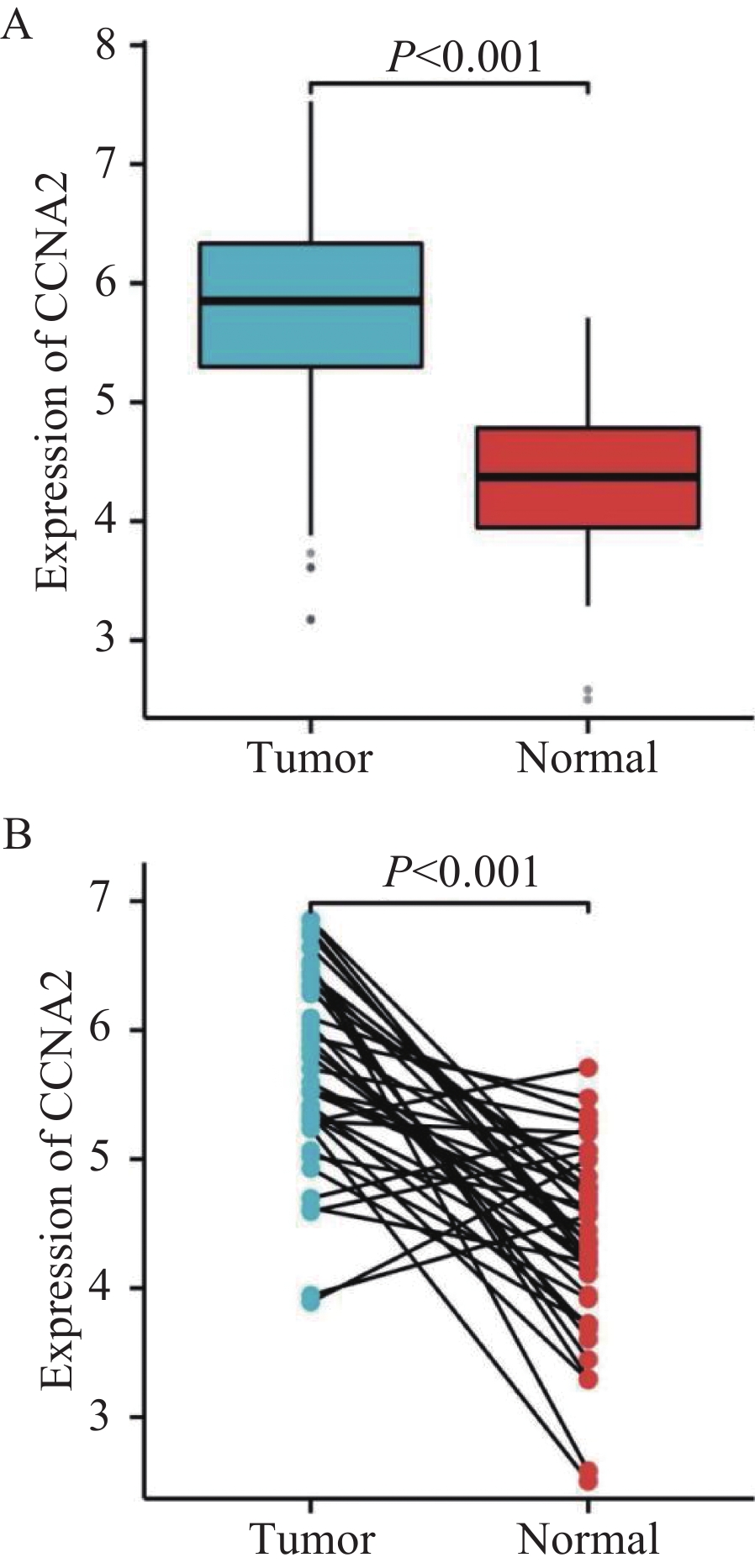
在TCGA数据库494例数据中,共有肿瘤组织453例,正常组织41例。与正常组织对比,CCNA2在肿瘤组织中的表达明显升高(P<0.001),见图1A。配对样本分析提示,CCNA2在结肠癌组织中的表达程度显著高于与之配对的正常组织(P<0.001),见图1B。为验证上述结果,采用免疫组织化学法检测湖南师范大学附属岳阳医院结肠术后52例肿瘤组织及23例正常组织中CCNA2表达情况。结果显示,结肠癌组织中部分肿瘤细胞呈核染色及弱胞质染色,且结肠癌组织中CCNA2蛋白表达明显高于正常组织(P<0.001),见图2、3。
2.2 CCNA2与结肠癌患者临床病理特征的关系
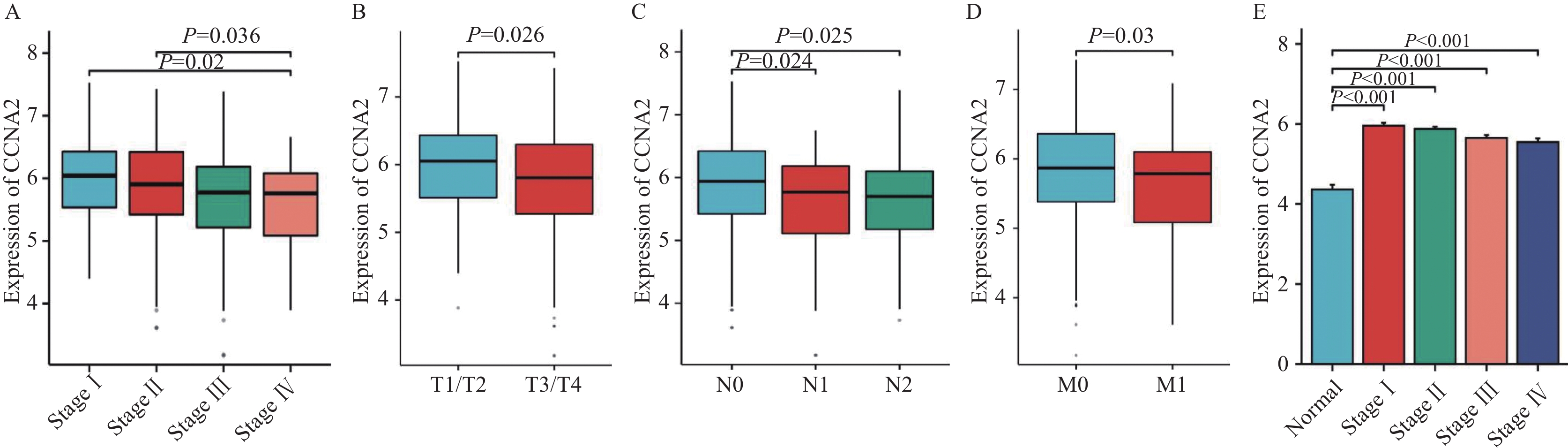
结果显示,病理分期Ⅰ期(P=0.036)、Ⅱ期(P=0.02)结肠癌组织中的CCNA2表达程度显著高于Ⅳ期,见图4A。T1/T2期结肠癌组织的CCNA2表达程度显著高于T3/T4期(P=0.026),见图4B。N0期结肠癌组织中CCNA2表达程度显著高于N1期(P=0.024)、N2期(P=0.025),见图4C。M0期结肠癌组织的CCNA2表达程度显著高于M1期(P=0.03),见图4D。同时,CCNA2在正常组织中表达程度均显著低于所有病理分期的结肠癌组织(P<0.001),见图4E。湖南师范大学附属岳阳医院52例手术患者的肿瘤组织标本相关性分析结果显示,CCNA2表达程度与性别、年龄、肿瘤原发部位、肿瘤大小、分化程度、N分期差异均无统计学意义(P>0.05),而与病理分期(P=0.023)、T分期(P=0.019)、M分期(P=0.048)差异具有统计学意义,见表1。
![]() 图 4 CCNA2与结肠癌临床病理特征关系图Figure 4 Relationship between CCNA2 and clinicopathological features of colon cancer patientsA: relationship between CCNA2 expression level and tumor pathological stage; B: relationship between CCNA2 expression level and T stage; C: relationship between CCNA2 expression level and N stage; D: relationship between CCNA2 expression level and M stage; E: differences in the expression of CCNA2 between normal tissues and colon cancer tissues at different pathological stages.表 1 52例结肠癌患者CCNA2表达水平与临床病理特征的关系Table 1 Relationship between CCNA2 expression level and clinicopathological features of 52 colon cancer patients
图 4 CCNA2与结肠癌临床病理特征关系图Figure 4 Relationship between CCNA2 and clinicopathological features of colon cancer patientsA: relationship between CCNA2 expression level and tumor pathological stage; B: relationship between CCNA2 expression level and T stage; C: relationship between CCNA2 expression level and N stage; D: relationship between CCNA2 expression level and M stage; E: differences in the expression of CCNA2 between normal tissues and colon cancer tissues at different pathological stages.表 1 52例结肠癌患者CCNA2表达水平与临床病理特征的关系Table 1 Relationship between CCNA2 expression level and clinicopathological features of 52 colon cancer patientsClinicopathological
featuresNumber
of casesHigh CCNA2
expression
group (n=32)Low CCNA2
expression
group (n=20)χ2 P Gender 0.600 0.439 Male 37 24(64.9%) 13(35.1%) Female 15 8(53.3%) 7(46.7%) Age (years) 0.002 0.693 > 65 34 21(61.8%) 13(38.2%) ≤65 18 11(61.1%) 7(38.9%) Primary site 1.300 0.254 Left semicolon 26 18(69.2%) 8(30.8%) Right semicolon 26 14(53.8%) 12(46.2%) Tumor size (cm) 0.033 0.855 ≥5 19 12(63.2%) 7(36.8%) <5 33 20(60.6%) 13(39.4%) Differentiation 0.068 0.795 Well 12 7(58.3%) 5(41.7%) Moderate and poor 40 25(62.5%) 15(37.5%) Pathologic stage 5.200 0.023 Ⅰ-Ⅱ 26 20(76.9%) 6(23.1%) Ⅲ-Ⅳ 26 12(46.2%) 14(53.8%) T stage 5.525 0.019 T1-T2 18 15(83.3%) 3(16.7%) T3-T4 34 17(50.0%) 17(50.0%) N stage 0.123 0.746 N0 27 16(59.3%) 11(40.7%) N1-N2 25 16(64.0%) 9(36.0%) M stage 3.900 0.048 M0 39 27(69.2%) 12(30.8%) M1 13 5(38.5%) 8(61.5%) 2.3 CCNA2与结肠癌患者生存的关系
根据计算出的最佳截断值(5.95)为分界点,将453例结肠癌肿瘤样本分为低表达组与高表达组。发现CCNA2高表达组的总生存期(OS)(P=
0.0056 )、无进展间隔期(PFI)(P=0.0039 )、疾病特异性生存期(DSS)(P=0.013)均显著长于低表达组,见图5。2.4 结肠癌中CCNA2相关基因信号通路分析
将TCGA数据库中453例结肠癌样本根据CCNA2中位表达水平分为高表达组和低表达组(中位表达水平5.850)。GSEA分析显示,雄激素响应元、IL6-JAK-STAT3信号、干扰素α、MYC-V2靶点、胰腺β细胞等基因通路在CCNA2高表达时富集(均P<0.05),见图6A,而顶端连接复合物、胆汁酸代谢、凝血、上皮间充质转化、Hedgehog、Wnt/β-Catenin、KRAS等基因通路在CCNA2低表达时富集(均P<0.05),见图6B。
2.5 结肠癌CCNA2免疫浸润相关性分析
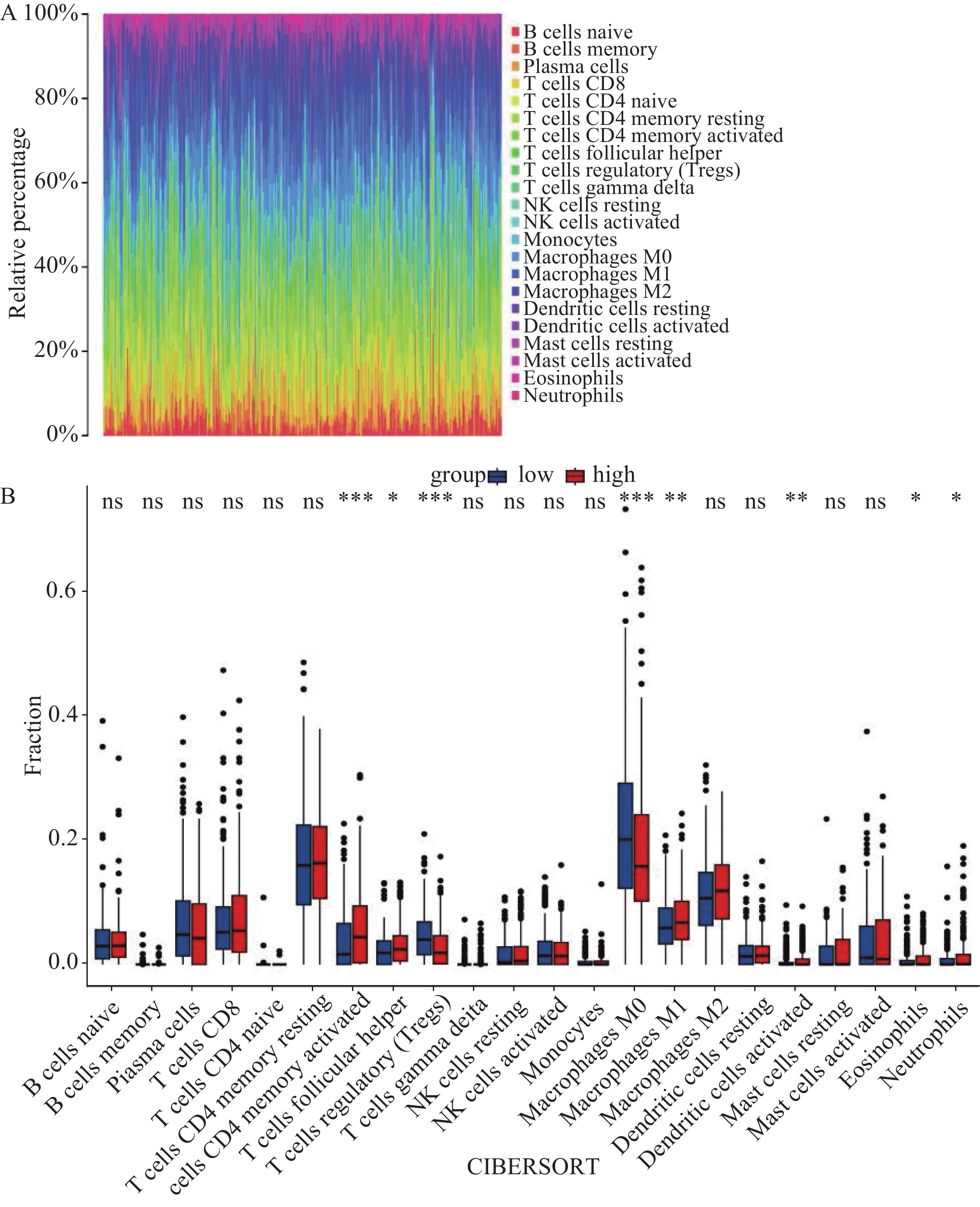
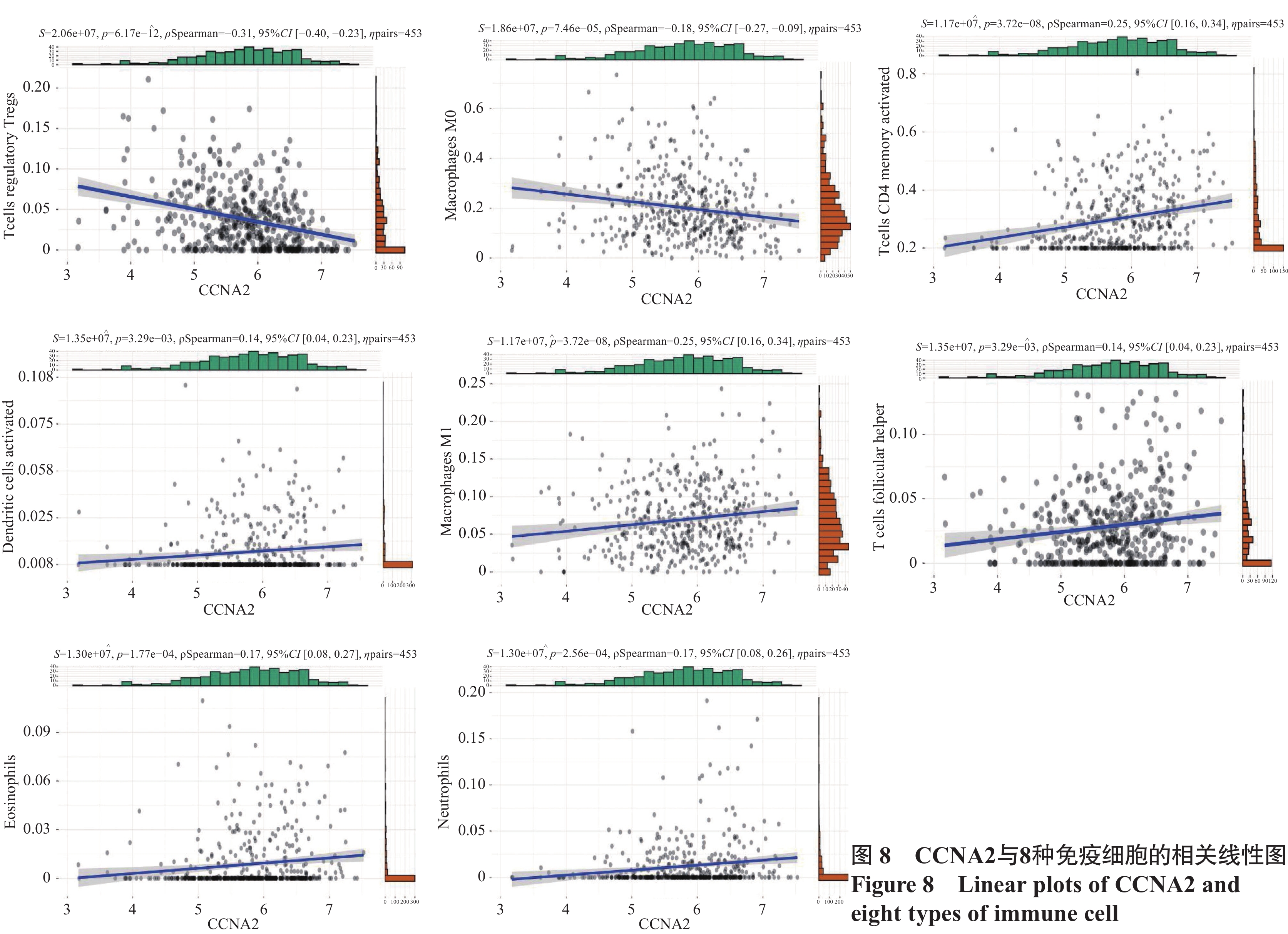
通过LM22矩阵及CIBERSORT算法分析,计算出了结肠癌中22种免疫细胞比例谱,见图7A。箱式图显示,记忆CD4 T细胞、滤泡辅助T细胞、巨噬细胞M1、树突细胞、嗜酸性粒细胞、中性粒细胞等免疫细胞数量在CCNA2高表达组中显著升高(均P<0.05),调节性T细胞、巨噬细胞M0等免疫细胞数量在CCNA2低表达组中显著升高(均P<0.05),见图7B。线性相关图显示,有8种免疫细胞浸润数量与CCNA2表达程度呈线性相关:CCNA2的表达与调节性T细胞和巨噬细胞M0的浸润呈负相关(均P<0.05),与活化树突状细胞、巨噬细胞M1、记忆CD4 T细胞、滤泡辅助T细胞、嗜酸性粒细胞、中性粒细胞的浸润呈正相关(均P<0.05),见图8。
3 讨论
CCNA2属于细胞周期蛋白家族成员,最早发现于非洲爪蟾的组织培养细胞系中[7]。其通过激活细胞周期蛋白依赖性激酶(Cyclin-dependent kinases, CDK)促进细胞周期进入G1期,并调节S期DNA的合成,从而保证细胞周期的有序进行[8],CCNA2转录起始点的遗传变异与肺癌、肝癌、结直肠癌的侵袭、转移、复发、化疗耐药相关,导致患者不良预后[9-10]。
TCGA数据库分析得出,CCNA2在肿瘤组织中表达程度显著高于正常组织,同一患者间肿瘤与癌旁组织对比,也得到同样结论。免疫组织化学实验与TCGA数据库实验结果一致。生存分析提示CCNA2低表达组患者预后不良。Liu等的研究也取得了类似的结论,CCNA2在结肠癌组织中表达显著高于正常组织,且CCNA2表达上调与结肠癌患者存活率增加相关[11]。
临床病理特征分析提示,结肠癌中CCNA2的表达与病理分期、TNM分期相关,且Ⅰ期、Ⅱ期及早期T、N、M分期的结肠癌组织中CCNA2表达程度更高。本研究进一步分析了湖南师范大学附属岳阳医院52例结肠癌患者数据,结果显示CCNA2表达与病理分期、T分期、M分期相关(P<0.05),但与N分期无显著相关性,这可能与样本量小有关。结合TCGA数据库与临床免疫组织化学分析,CCNA2与结肠癌的肿瘤大小、淋巴结转移及远处转移相关。
GSEA分析得出,CCNA2低表达时有顶端连接复合物、上皮间充质转化、Hedgehog信号、Wnt/β-Catenin、KRAS等信号通路富集。顶端连接复合物、Hedgehog信号通路、上皮间充质转化通路活化时可促进癌症的发生及侵袭[12-14]。在结肠癌中,APC等位基因缺失可激活Wnt/β-Catenin信号通路,促进肿瘤细胞的自我更新及生长[15]。Wnt/β-Catenin作为致癌信号通路,可减少免疫细胞浸润,促进肿瘤免疫逃逸[16]。研究表明,CCNA2可稳定axin/GSK3破坏复合体促进β−连环蛋白降解,且CCNA2表达降低时,β−连环蛋白积累增加[17-18]。结合本实验结果,CCNA2表达降低时,Wnt/β-Catenin信号通路活跃促进免疫逃逸,增加肿瘤侵袭性。KRAS信号通路可以影响免疫细胞募集、激活、分化,使肿瘤细胞逃避免疫监视,促进肿瘤的发展[19]。
调节性T细胞在肿瘤微环境中阻碍抗肿瘤免疫,促进肿瘤发展,且其抑制过度免疫反应的功能成为抗肿瘤免疫的主要障碍[20]。研究表明,KRAS突变结肠癌患者CD4 T细胞的浸润程度降低,调节性T细胞浸润升高[21]。Zdanov等的研究证明,KRAS通路活跃情况下可以促进常规T细胞向调节性T细胞转化[22]。同时,在胃癌中Wnt/β-Catenin激活可增加调节性T细胞浸润,阻断此通路能减少调节性T细胞数量并抑制胃癌进展[23]。本研究结果提示,CCNA2低表达时KRAS、Wnt/β-Catenin通路表达活跃,且调节性T细胞浸润增加。CCNA2可能通过上述通路促使肿瘤组织逃避免疫识别。巨噬细胞M0可极化成有抗肿瘤作用的M1型和促肿瘤作用的M2型[24]。免疫浸润分析结果提示,CCNA2高表达时,M1型巨噬细胞浸润增加。随着CCNA2表达下降,M1型巨噬细胞逐渐减少,M0型巨噬细胞增多,可能使结肠癌组织的侵袭性进一步增加。
随着病理分期增加,CCNA2的表达逐渐下降,但正常组织中CCNA2表达仍远低于Ⅳ期肿瘤组织。CCNA2表达活跃能加快细胞周期进程,促进细胞从间期向有丝分裂期转变,促进细胞增殖分裂[25]。在正常组织向肿瘤组织发展的过程中,CCNA2的表达升高,其可能影响细胞周期,促进细胞过度异常增殖分裂,导致肿瘤的发生。而随着病理分期的增加,肿瘤恶性程度升高,CCNA2表达降低,其可能通过KRAS、Wnt/β-Catenin通路影响免疫浸润,募集调节性T细胞,促进肿瘤免疫逃逸。因此,CCNA2表达升高可能促进正常组织向肿瘤组织转变,同时其表达程度降低可进一步影响免疫浸润从而增加结肠癌的侵袭性,导致不良预后。
综上,本研究证实了CCNA2在结肠癌组织中高表达,其表达缺失是结肠癌预后的不良因素。随着CCNA2表达程度降低,其通过KRAS、Wnt/β-Catenin通路募集调节T细胞,减少免疫细胞浸润,使结肠癌细胞逃避免疫识别,导致肿瘤恶性程度及侵袭性增加。CCNA2通过调节免疫浸润影响结肠癌患者预后,有望成为结肠癌患者诊断及治疗的分子标志物。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:杨 鹏:研究设计与操作、数据采集与分析、统计学分析、论文撰写与修改邱子奕、王灵灵:数据分析和整理胡园、陈峥桢:论文修改、数据整理钟美珍:统计学分析余飞跃:研究设计与指导、论文修改邱荣元:研究设计与指导、实验操作指导、论文修改 -
表 1 52例结肠癌患者CCNA2表达水平与临床病理特征的关系
Table 1 Relationship between CCNA2 expression level and clinicopathological features of 52 colon cancer patients
Clinicopathological
featuresNumber
of casesHigh CCNA2
expression
group (n=32)Low CCNA2
expression
group (n=20)χ2 P Gender 0.600 0.439 Male 37 24(64.9%) 13(35.1%) Female 15 8(53.3%) 7(46.7%) Age (years) 0.002 0.693 > 65 34 21(61.8%) 13(38.2%) ≤65 18 11(61.1%) 7(38.9%) Primary site 1.300 0.254 Left semicolon 26 18(69.2%) 8(30.8%) Right semicolon 26 14(53.8%) 12(46.2%) Tumor size (cm) 0.033 0.855 ≥5 19 12(63.2%) 7(36.8%) <5 33 20(60.6%) 13(39.4%) Differentiation 0.068 0.795 Well 12 7(58.3%) 5(41.7%) Moderate and poor 40 25(62.5%) 15(37.5%) Pathologic stage 5.200 0.023 Ⅰ-Ⅱ 26 20(76.9%) 6(23.1%) Ⅲ-Ⅳ 26 12(46.2%) 14(53.8%) T stage 5.525 0.019 T1-T2 18 15(83.3%) 3(16.7%) T3-T4 34 17(50.0%) 17(50.0%) N stage 0.123 0.746 N0 27 16(59.3%) 11(40.7%) N1-N2 25 16(64.0%) 9(36.0%) M stage 3.900 0.048 M0 39 27(69.2%) 12(30.8%) M1 13 5(38.5%) 8(61.5%) -
[1] Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024[J]. CA Cancer J Clin, 2024, 74(1): 12-49. doi: 10.3322/caac.21820
[2] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[3] Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults[J]. Gut, 2019, 68(12): 2179-2185. doi: 10.1136/gutjnl-2019-319511
[4] Loukil A, Cheung CT, Bendris N, et al. Cyclin A2: At the crossroads of cell cycle and cell invasion[J]. World J Biol Chem, 2015, 6(4): 346-350.
[5] Zhang QH, Yuen WS, Adhikari D, et al. Cyclin A2 modulates kinetochore-microtubule attachment in meiosis Ⅱ[J]. J Cell Biol, 2017, 216(10): 3133-3143.
[6] Chen S, Zhao Z, Wang X, et al. The Predictive Competing Endogenous RNA Regulatory Networks and Potential Prognostic and Immunological Roles of Cyclin A2 in Pan-Cancer Analysis[J]. Front Mol Biosci, 2022, 9: 809509. doi: 10.3389/fmolb.2022.809509
[7] Blanchard JM. Cyclin A2 transcriptional regulation: modulation of cell cycle control at the G1/S transition by peripheral cues[J]. Biochem Pharmacol, 2000, 60(8): 1179-1184.
[8] Hydbring P, Malumbres M, Sicinski P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases[J]. Nat Rev Mol Cell Biol, 2016, 17(5): 280-292. doi: 10.1038/nrm.2016.27
[9] Jiang A, Zhou Y, Gong W, et al. CCNA2 as an Immunological Biomarker Encompassing Tumor Microenvironment and Therapeutic Response in Multiple Cancer Types[J]. Oxid Med Cell Longev, 2022, 2022: 5910575.
[10] Wu SQ, Huang SH, Lin QW, et al. FDI-6 and olaparib synergistically inhibit the growth of pancreatic cancer by repressing BUB1, BRCA1 and CDC25A signaling pathways[J]. Pharmacol Res, 2022, 175: 106040.
[11] Liu X, Liu X, Qiao T, et al. Identification of crucial genes and pathways associated with colorectal cancer by bioinformatics analysis[J]. Oncol Lett, 2020, 19(3): 1881-1889.
[12] González-Mariscal L, Miranda J, Gallego-Gutiérrez H, et al. Relationship between apical junction proteins, gene expression and cancer[J]. Biochim Biophys Acta Biomembr, 2020, 1862(9): 183278. doi: 10.1016/j.bbamem.2020.183278
[13] Jiang J. Hedgehog signaling mechanism and role in cancer[J]. Semin Cancer Biol, 2022, 85: 107-122. doi: 10.1016/j.semcancer.2021.04.003
[14] Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition[J]. Nat Rev Mol Cell Biol, 2014, 15(3): 178-196. doi: 10.1038/nrm3758
[15] Liu J, Xiao Q, Xiao J, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities[J]. Signal Transduct Target Ther, 2022, 7(1): 3. doi: 10.1038/s41392-021-00762-6
[16] Pai SG, Carneiro BA, Mota JM, et al. Wnt/beta-catenin pathway: modulating anticancer immune response[J]. J Hematol Oncol, 2017, 10(1): 101. doi: 10.1186/s13045-017-0471-6
[17] Kim SI, Park CS, Lee MS, et al. Cyclin-dependent kinase 2 regulates the interaction of Axin with beta-catenin[J]. Biochem Biophys Res Commun, 2004, 317(2): 478-483. doi: 10.1016/j.bbrc.2004.03.065
[18] Guo Y, Gabola M, Lattanzio R, et al. Cyclin A2 maintains colon homeostasis and is a prognostic factor in colorectal cancer[J]. J Clin Invest, 2021, 131(4): e131517. doi: 10.1172/JCI131517
[19] Dias Carvalho P, Guimarães CF, Cardoso AP, et al. KRAS Oncogenic Signaling Extends beyond Cancer Cells to Orchestrate the Microenvironment[J]. Cancer Res, 2018, 78(1): 7-14. doi: 10.1158/0008-5472.CAN-17-2084
[20] Yan Y, Huang L, Liu Y, et al. Metabolic profiles of regulatory T cells and their adaptations to the tumor microenvironment: implications for antitumor immunity[J]. J Hematol Oncol, 2022, 15(1): 104. doi: 10.1186/s13045-022-01322-3
[21] Liu J, Huang X, Liu H, et al. Immune landscape and prognostic immune-related genes in KRAS-mutant colorectal cancer patients[J]. J Transl Med, 2021, 19(1): 27. doi: 10.1186/s12967-020-02638-9
[22] Zdanov S, Mandapathil M, Abu Eid R, et al. Mutant KRAS Conversion of Conventional T Cells into Regulatory T Cells[J]. Cancer Immunol Res, 2016, 4(4): 354-365. doi: 10.1158/2326-6066.CIR-15-0241
[23] Ji L, Qian W, Gui L, et al. Blockade of β-Catenin-Induced CCL28 Suppresses Gastric Cancer Progression via Inhibition of Treg Cell Infiltration[J]. Cancer Res, 2020, 80(10): 2004-2016. doi: 10.1158/0008-5472.CAN-19-3074
[24] Miao X, Leng X, Zhang Q. The Current State of Nanoparticle-Induced Macrophage Polarization and Reprogramming Research[J]. Int J Mol Sci, 2017, 18(2): 336. doi: 10.3390/ijms18020336
[25] Zhang S, Tischer T, Barford D. Cyclin A2 degradation during the spindle assembly checkpoint requires multiple binding modes to the APC/C[J]. Nat Commun, 2019, 10(1): 3863. doi: 10.1038/s41467-019-11833-2



 下载:
下载: