-
摘要:
本文阐述CD81的结构和功能,探讨其在恶性实体肿瘤和血液系统肿瘤中的作用及价值,介绍CD81相关的抗体和小分子化合物,分析抗CD81治疗在临床应用中面临的优势与挑战,并对其在未来治疗恶性血液病的应用前景进行展望。
Abstract:This article elaborates on the structure and function of CD81, explores its role and value in malignant solid and hematological tumors, introduces CD81-related antibodies and small-molecule compounds, analyzes the advantages and challenges faced by anti-CD81 therapy in clinical applications, and looks forward to the future application prospects of anti-CD81 therapy in the treatment of malignant hematological diseases.
-
Key words:
- CD81 /
- Tumor /
- Role /
- Anti-CD81 therapy
-
0 引言
CD81是一种四跨膜蛋白家族的成员,它参与了体内多种重要的细胞生物学过程,如膜结构组成、蛋白质合成转运、细胞融合及细胞-细胞相互作用[1]。在免疫系统中,CD81可调控免疫突触、受体聚集和信号转导,同时它还能介导适应性和先天性免疫抑制。另外,人们发现CD81不仅是肝细胞中病原体(如丙型肝炎病毒和疟原虫)的一个重要通道,还会增加李斯特菌的易感性[2-3]。这些不同的生物学作用是由于CD81易与其他四跨膜蛋白和细胞特异性伴侣蛋白结合,并为细胞提供信号转导平台。同时CD81被证明可以调节细胞的迁移和侵袭,与癌症的进展相关。事实上,越来越多的证据表明CD81的确有助于肿瘤的生长和转移[4]。CD81在包括乳腺癌、肺癌、前列腺癌、黑色素瘤、脑癌和淋巴瘤等多种肿瘤中都有表达,该分子的过表达或下调与预后是密切相关的。在此,本文将详细讨论CD81在肿瘤中的作用及其作为肿瘤靶点的潜在治疗用途。
1 CD81的分子结构和动态构架
CD81最初被命名为抗增殖抗体-1(Anti-proliferative antibody-1, TAPA-1)的靶点,它是通过筛选能够抑制B细胞淋巴瘤增殖的不同单克隆抗体时被发现的[5]。CD81的基因组DNA由8个外显子和7个内含子组成,位于11p15.5~11.2位点上。而它的蛋白分子由236个氨基酸残基组成,分子量25.8 kD。CD81是一种经典的四跨膜蛋白,其标准的跨膜部分两侧是细胞内和胞外结构域,见图1。这种蛋白质作为一种膜受体,可以在培养的细胞中过表达,并通过免疫亲和力纯化。在结构水平上,CD81的四个跨膜片段(Transmembrane, TM1-TM4)形成了一个漏斗,胆固醇分子可在其中结合。漏斗由一个四股左螺旋旋线圈构成,一个较大的细胞外环(Extracellular loop, EC2)盖住漏斗,作为外膜小叶的盖子,见图1。这个大环EC2(或large loop EC2, LEL)与邻近的较小环EC1(或smaller loop EC1, SEL)相互作用,以调节蛋白的构象[6]。EC1含有一个小的疏水β链,该链包裹在EC2的保守疏水沟槽中,EC2本身由两个亚结构域组成,包括δ环[7-8]。CD81高分辨率的晶体结构证实了其EC2结构域存在双二硫桥和高度保守的Cys-Cys-Gly基序。纯化的全长蛋白为单体形式,而CD81的胞外EC2结构域(236个残基中的88个)的晶体结构为二聚体[9]。二聚体形式可以指引不同的四跨膜结合蛋白在细胞表面的聚集[10]。CD81的结构域在四跨膜蛋白中高度保守,特别是跨膜结构域结构,从而实现了分子内和分子间的相互作用,并形成了所谓的“四跨膜蛋白网”[11]。
![]() 图 1 CD81结构域的组织和结构[4]Figure 1 Domain organization and structure of CD81[4]A: schematic representation of CD81 structure, with four transmembrane (TM) segments embedded in the membrane bilayer, which sequestering a molecule of cholesterol and two extracellular (EC) loops, the small loop EC1 (or SEL) and large loop EC2 (or SEL). The disulfur bridges between the cysteine residue in EC2, as well as the palmitoylated cysteine residue contribute to the anchorage of the protein in the membrane bilayer; B: model of CD81 with EC2 in closed conformation (based on PDB: 5TCX).
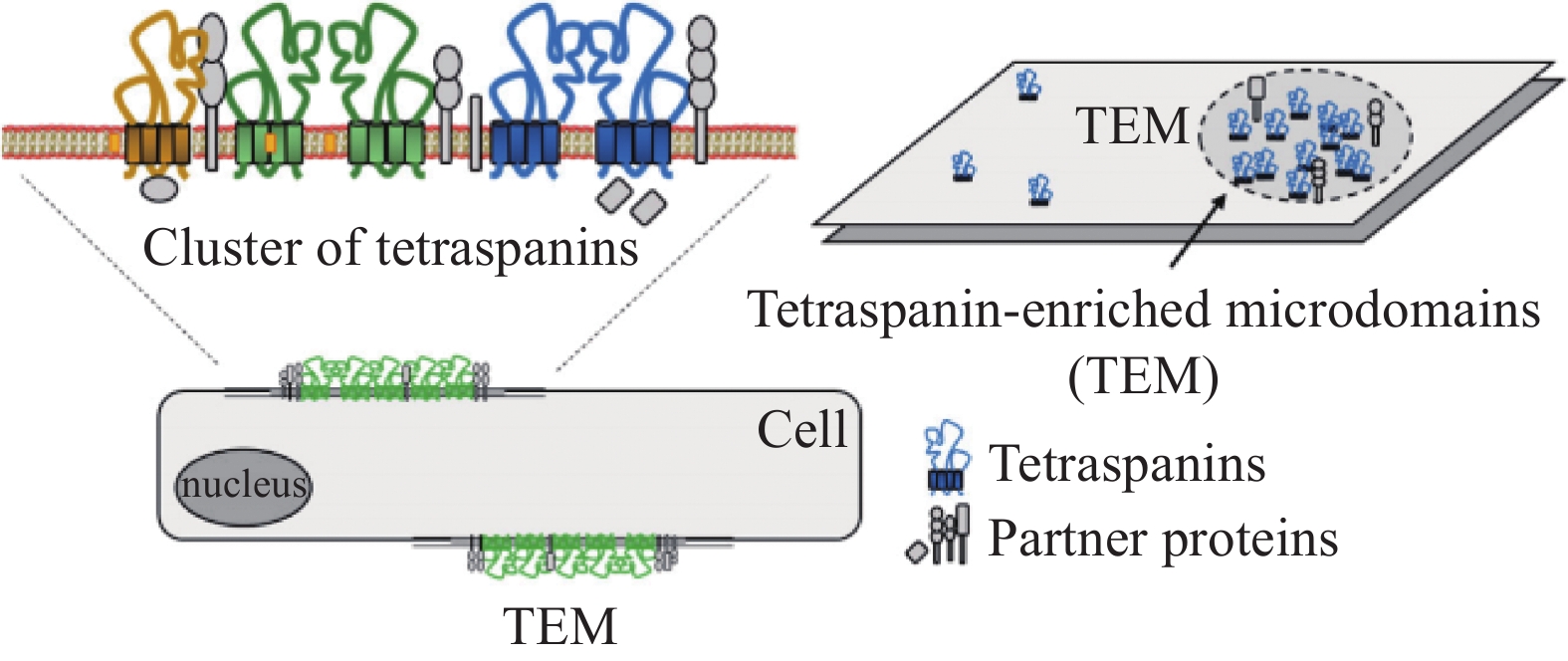
图 1 CD81结构域的组织和结构[4]Figure 1 Domain organization and structure of CD81[4]A: schematic representation of CD81 structure, with four transmembrane (TM) segments embedded in the membrane bilayer, which sequestering a molecule of cholesterol and two extracellular (EC) loops, the small loop EC1 (or SEL) and large loop EC2 (or SEL). The disulfur bridges between the cysteine residue in EC2, as well as the palmitoylated cysteine residue contribute to the anchorage of the protein in the membrane bilayer; B: model of CD81 with EC2 in closed conformation (based on PDB: 5TCX).CD81能够结合胆固醇是其一大特点,它固有的胆固醇结合袋对其调整蛋白质构象和某些病毒进入细胞来说非常重要。CD81是多种病毒的共同受体,包括丙型肝炎病毒(Hepatitis C virus, HCV)、人类免疫缺陷病毒1型(Human immunodeficiency virus, HIV-1)、单纯疱疹病毒1型(Herpes simplex virus, HSV-1)、甲型流感病毒(Influenza A virus, IAV)、基孔肯雅病毒等;偶尔也可作为某些细菌的共同受体,如单核增生李斯特菌、热带寄生虫和约氏疟原虫[4]。CD81在HCV感染中的作用已被大量研究。最近人们发现了胆固醇结合调控CD81构象的一种潜在的变构机制。与胆固醇结合的封闭构象相比,CD81的无胆固醇开放形式表现出HCV受体活性的降低,反之表现出HCV进入时活性的增强。这两种形式之间的构象转换阐明了CD81的胆固醇感应机制[12]。因此,CD81是一种高度动态的蛋白,它的构象变化会带来受体功能及它在细胞中活性的变化。比如当其处于封闭构象时,CD81细胞的外环EC2会与EC1分离,进一步导致其构象改变,从而阻止CD81与其主要伴侣CD19的结合[13]。四跨膜蛋白与不同的蛋白伴侣和膜中的胆固醇相结合,从而在膜微域形成蛋白簇,称为四跨膜蛋白网或富含四跨膜蛋白的微域,见图2。这些结构域对应于膜区域,四跨膜蛋白通过相互作用在局部形成功能性高阶蛋白复合物。
![]() 图 2 四跨膜蛋白的微域[4]Figure 2 Microdomains of tetraspanins[4]Representation of the clustering of tetraspanins to form microdomains at the plasma membrane, associating diverse tetraspanins and partner proteins. The tetraspanin web is a dynamic structural organization at the cell surface and includes promiscuous and specific interactions.
图 2 四跨膜蛋白的微域[4]Figure 2 Microdomains of tetraspanins[4]Representation of the clustering of tetraspanins to form microdomains at the plasma membrane, associating diverse tetraspanins and partner proteins. The tetraspanin web is a dynamic structural organization at the cell surface and includes promiscuous and specific interactions.2 CD81的分子互作、运输和信号转导
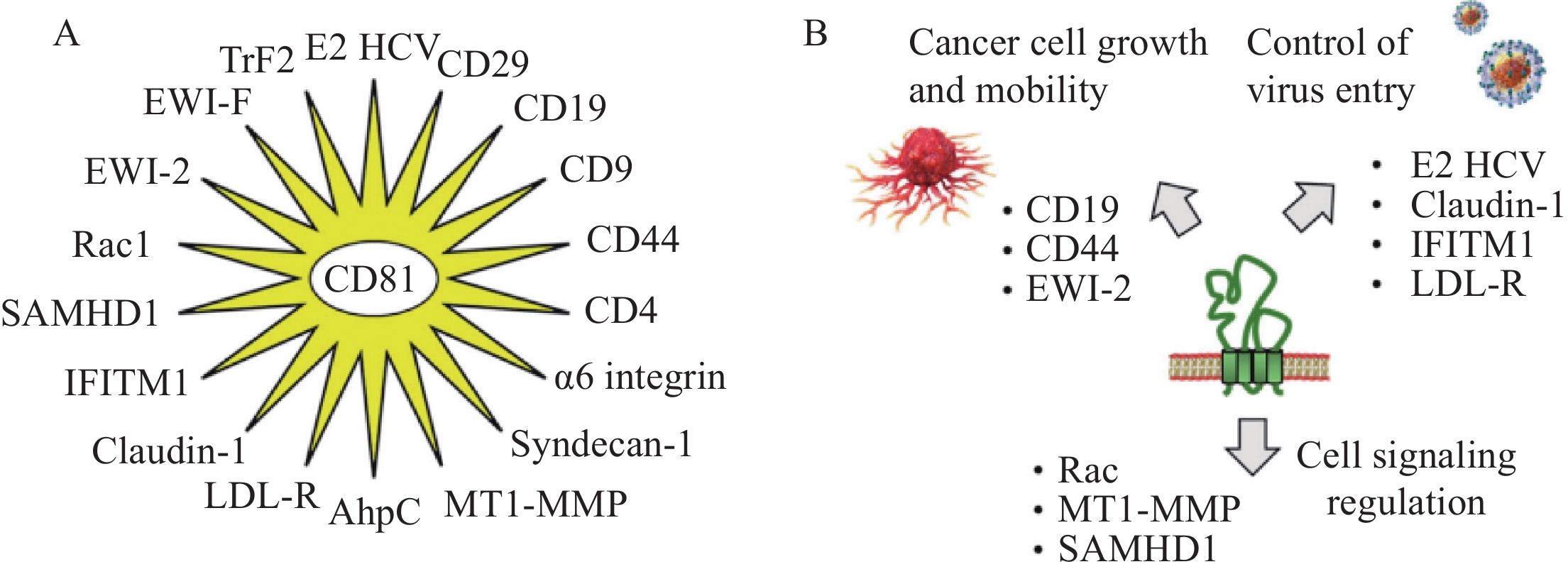
四跨膜蛋白在细胞中具有多种功能。在质膜上,它们可促进与其他细胞膜和细胞内蛋白质及脂质的相互作用,以形成膜结构域组织,并被认为是连接细胞外和细胞质信号元件的“分子促进剂”[14]。CD81的外环EC2(LEL)可与各类蛋白质互作,以促进细胞间相互作用和捕获;因此,CD81有利于病毒感染框架内的细胞黏附或融合。人们已经鉴定出了18种可以直接与CD81相互作用的蛋白,见表1和图3。
表 1 与CD81相互作用的蛋白质Table 1 Proteins interacting with CD81Proteins Types Interactions and Effects E2 HCV Viral protein The transmembrane E2 glycoprotein of HCV utilizes CD81 as a coreceptor for cell entry. Claudin-1 Tight junction protein The interaction of CD81-Claudin1 contributes to HCV infection. IFITM1 Tight junction protein IFITM1 interacts with CD81 to limit HCV entry. LDL-R Membrane receptor CD81, LDL-R, and PCSK9 interact with each other to control HCV entry into hepatic cells. CD19 Membrane receptor CD81 and CD19 are the core subunits of the B cell coreceptor complex. CD81 controls CD19 export activity via a dynamically regulated process upon B cell activation. EWI-2 (IgSF8, PGRL, and CD316) Signaling protein The CD81-EWI-2 interaction contributes to the tetraspanin web and plays a role in the growth and motility of cancer cells and entry of HCV. EWI-F
(FPRP and CD9P-1)Signaling protein Complexes formed between EWI-F and CD81 (and CD9) participate in the fusion of myotubes, which are essential elements of muscle structure. CD44 Adhesion molecule The interaction between CD81 and CD44 through their extracellular regions promotes the formation of tumor cell clusters and lung metastasis of triple negative breast cancer. ɑ6 Integrin Adhesion molecule In male germ cells, CD81 interacts with the α6 integrin subunit, which forms a dimer with β4 integrin. The complex plays a role in sperm maturation. βRac1 integrin (CD29) Adhesion molecule Radiation was found to induce CD29-CD81 complex formation, thereby increasing the cellular uptake of exosomes. Rac1 Small GTPase Rac interacts with the C-terminal cytoplasmic portion of CD81 to regulate cell motility and plays a role in bacterial infection. CD4 Cell surface antigen CD81 interacts with the CD4 dimers concentrated in tetraspanin-enriched microdomains. CD9 Tetraspanin CD9 and CD81 are involved in tetraspanin web formation in sperm. Molecular modeling suggests the occurrence of protein-protein interactions during sperm-egg membrane fusion. SAMHD1 Enzyme CD81 interacts with the deoxynucleoside triphosphate phosphohydrolase SAMHD1 and regulates its expression. This interaction promotes the proteasome-dependent degradation of SAMHD1. It is a metabolic regulator of HIV-1 replication. MT1-MMP Enzyme Several tetraspanins, including CD81, associate with the membrane-type 1 matrix metalloproteinase to regulate its cell surface localization and its function (notably its capacity to activate pro-MMP-2). Syndecan-1 Proteoglycan The knockdown of syndecan-1 and CD81 inhibits HCV infection, suggesting their cooperative action. AhpC Enzyme The mycobacterial enzyme alkyl hydroperoxide reductase C interacts with CD81-LEL to promote the uptake of pathogens by host cells. TfR2 Membrane receptor Transferrin receptor 2 is a binding partner for CD81. The interaction triggers RfR2 degradation by the ubiquitin E3 ligase GRAIL. ![]() 图 3 CD81的蛋白伴侣[4]Figure 3 CD81 protein partners[4]A: eighteen CD81-interacting proteins have been identified. These proteins include 10 that were defined with the support of STRING database (https://cn.string-db.org/) and other proteins discovered through our own analysis of the scientific literature; B: partners include proteins that interact with the extracellular loops of CD81, such as those implicated in virus entry into cells, involved in cancer cell growth and mobility, and interacting with the intracellular portions of CD81 for cell signaling.
图 3 CD81的蛋白伴侣[4]Figure 3 CD81 protein partners[4]A: eighteen CD81-interacting proteins have been identified. These proteins include 10 that were defined with the support of STRING database (https://cn.string-db.org/) and other proteins discovered through our own analysis of the scientific literature; B: partners include proteins that interact with the extracellular loops of CD81, such as those implicated in virus entry into cells, involved in cancer cell growth and mobility, and interacting with the intracellular portions of CD81 for cell signaling.3 CD81与非血液系统肿瘤
现已发现一些四跨膜蛋白在介导肿瘤细胞生长、侵袭和迁移中的关键作用。比如CD151和Tspan8发挥肿瘤启动子的作用,然而CD63和CD82具有抑制肿瘤的功能。这些相反的功能也在不同的肿瘤模型和临床样本中得到证实[15]。然而有趣的是CD9和CD81在不同的肿瘤模型中却表现出促进和抑制肿瘤生长和转移的双重作用,但确切的作用机制尚未完全阐明。例如,在肝癌细胞株中低表达CD81的肝癌细胞比高表达的更具转移潜能,研究发现CD81与Ⅱ型磷酸肌醇4-激酶(PI4KⅡ)之间的相互作用是通过促进富含CD81的囊泡的形成来抑制肝癌细胞运动的,而且这些囊泡可隔离肌动蛋白-4,并重塑肌动蛋白细胞骨架[16]。在胃癌中CD81表达失活可能为肿瘤带来更多的生长和生存优势,研究发现CD81的转录沉默可能与异常高甲基化密切相关,CD81下调能增强肿瘤细胞的集落形成能力,同时CD81可抑制p38(但不抑制ERK、JNK和AKT)磷酸化,其生长抑制作用也可通过p38的上调和下调来消除[17]。此外,在膀胱癌中,CD81的低表达是一种不良的预后标志,CD81低表达患者可能需要更密切的监测和更积极的治疗[18]。相反,在黑色素瘤细胞株中过表达CD81,通过上调1型膜基质金属蛋白酶(MT1-MMP)显著促进肿瘤的生长和转移;而且与正常皮肤组织相比,黑色素瘤患者样本中CD81和MT1-MMP的表达呈正相关[19]。另外,在骨肉瘤中发现CD81抑制后,AKT和ERK也受到抑制,肿瘤细胞的增殖、迁移和侵袭减少;乳腺癌细胞中下调CD81可以减少肿瘤的进展和扩散[20-21]。当原位注射小鼠乳腺癌肿瘤时,CD81缺失的小鼠更少发生肺转移[22]。近期发现CD81在胶质母细胞瘤中也高表达,且CD81的表达与DNA损伤反应密切相关,而同源重组修复(Homologous recombination repair, HRR)负责CD81介导的放射耐药,特别是核膜蛋白CD81协助Rad51的核转运,它是放射后参与HRR过程的关键蛋白[23]。再者,有研究表明循环表达CD81的细胞外囊泡可作为晚期非小细胞肺癌(Non-small cell lung cancer, NSCLC)中免疫检查点抑制剂PD-1应答的生物标志物,结果显示对PD-1有反应者的细胞外囊泡表达四跨膜蛋白(CD9、CD81、CD63)的水平高于无反应者,且多因素分析表明CD81表达水平与总体反应率密切相关[24]。
4 CD81与血液系统肿瘤
CD81在造血系统的许多细胞中表达,包括B细胞、T细胞、单核细胞和树突状细胞[25]。CD81的一个重要伴侣是B淋巴细胞共受体CD19,它是PI3K通路的关键激活因子,也是一种重要的肿瘤相关抗原。CD19是B/浆细胞谱系标志物,目前抗CD19嵌合抗原受体(Chimeric antigen receptor, CAR)-T细胞疗法,是对抗B细胞恶性肿瘤的一种有效方式[26]。CD19和CD81结合诱导外域开放和跨膜螺旋重组,可导致胆固醇结合袋闭塞[27]。CD81-CD19相互作用在B细胞活化时受到动态调节,这种动态与B细胞功能的调节也是有关的[28]。研究发现CD81表达缺失和CD81基因突变会阻断高尔基体中CD19的加工和成熟,从而导致CD19在胞内聚集,无法在细胞表面表达[22,29]。CD19复合体由CD19、CD81、CD21(也称补体受体2,CR2)和CD225组成,是B细胞受体(B cell receptor, BCR)的共同受体。当CD81突变时,该复合物不能正常形成,并导致原发性免疫性缺陷疾病,但CD81突变在人类中极为罕见。
虽然早在2010年就有研究者分析了800多例肿瘤组织样本的CD81表达,但目前针对CD81在血液系统肿瘤中的研究并不多。在非霍奇金淋巴瘤中,有85%的滤泡性淋巴瘤、63%的弥漫性大B细胞淋巴瘤、100%的Burkitt淋巴瘤、67%的套细胞淋巴瘤和100%的脾边缘区淋巴瘤表达CD81蛋白;相比之下,只有17%的霍奇金淋巴瘤和13%的多发性骨髓瘤(Multiple myeloma, MM)样本表达CD81[30]。发表在Leukemia上的一项研究表明,45%(103/230)的MM患者用流式细胞术检测到CD81的表达,且CD81阳性是无进展生存(Progression-free survival, PFS)和总体生存(Overall survival, OS)不良的独立预后因素;这种不良影响在另外325例移植候选MM患者队列中得到验证。此外研究发现,CD81表达阳性的冒烟型MM进展为MM的时间明显缩短,这说明CD81可能在MM的发病机制中发挥相关作用[31]。Paiva等[32]研究表明,在健康个体中,浆细胞表型存在从CD19+CD81+向CD19-CD81+和CD19-CD81-的逐渐分化增加;随后在225例新诊断的MM患者中发现,59%的患者具有完全分化(CD19-CD81-)克隆,38%的患者具有中分化(CD19-CD81+)克隆,3%的患者具有低分化(CD19+CD81+)克隆,低分化克隆的患者预后最差。此外,研究对浆细胞系中CpG甲基化进行了分析,发现CD81的表达受表观遗传学调控。在急性髓系白血病(Acute myelocytic leukemia, AML)的研究中也同样证明了CD81的表达与不良预后相关。有研究检测了134例初治的AML患者细胞膜上CD81的表达情况,发现有69%(92/134)的患者CD81表达阳性,CD81的表达不仅与AML患者白细胞偏高有关,更与细胞遗传学的中等风险或不良风险相关;再者,CD81表达对无事件生存(Event-free survival, EFS)、OS、无复发生存(Relapse-free survival, RFS)都有不良影响,而且对于伴NPM1突变患者及欧洲白血病网(European leukmia net, ELN)认为的预后良好组患者的OS也有不良影响[33]。近期,研究者发现在急性B淋巴细胞白血病(B cell-acute lymphoblastic leukemia, B-ALL)中敲除CD81可减少细胞黏附,同时可破坏B-ALL在体内骨髓的归巢和植入,并诱导化学增敏改变;这种化学增敏化是通过控制布鲁顿酪氨酸激酶(Bruton’s tyrosine kinase, BTK)信号转导和诱导p53介导的细胞死亡来介导的[34];另外此研究还发现,表观遗传药物组合对CD81细胞表面表达的调节介导了阿扎胞苷/帕比司他诱导的ALL细胞的化学增敏。
Braig等[35]观察到4例经CD19-CD3双特异性T细胞衔接器(Bispecific T cell engager, BiTE)贝林妥欧单抗治疗后发生CD19阴性复发的B-ALL患者中有1例CD19无突变,表达全长CD19 mRNA但不表达CD81(CD81与内质网中的CD19结合,对CD19向细胞表面的转运至关重要)。因此,CD19阴性免疫逃逸是由于内质网和(或)高尔基体中缺乏CD81表达,随后CD19的转运和(或)成熟缺陷所致。BiTE的这一耐药机制也可能与患者经CD19 CAR-T细胞治疗后抗原阴性复发[36]的情况相似,但目前尚未报道。此外,最近研究者发现利用CD81作为一种共刺激分子,联合CD3/CD28不仅可增强初始T细胞的活化,还可以增强初始T细胞中的CAR转导效率,并且在CAR基因转导后,CD81介导的T细胞活化显著增强了从初始T细胞到记忆T细胞的表型转变[37]。
5 抗CD81单抗
特异性靶向CD81的小鼠IgG1抗体5A6,见图4。早在30多年前就已经被发现,早期该单抗主要被用于证明CD81在T细胞活化、IL-2的产生和增殖中的作用[38]。5A6在淋巴瘤细胞中可通过激活caspase-3,直接发挥细胞毒性作用。除了直接诱导凋亡,5A6可通过NK细胞促进抗体依赖的细胞毒作用(Antibody-dependent cell cytotoxicity, ADCC),通过巨噬细胞促进抗体依赖的细胞吞噬作用(Antibody-dependent cellular phagocytosis, ADCP)。小鼠IgG2a和人类IgG1 5A6变体也显示出很强的补体依赖的细胞毒作用(Complement-dependent cytotoxicity, CDC)。正常的淋巴细胞对抗CD81抗体的敏感性要低得多。这可能部分是由于与淋巴瘤B细胞相比,正常淋巴细胞上CD81的表达水平较低,而补体抑制剂CD55的表达水平较高[25]。在异种移植模型中,5A6被证明是安全的(对正常外周血单个核细胞没有细胞毒性),并且与利妥昔单抗(抗CD20)一样能够有效地抑制B细胞淋巴瘤的生长。5A6单抗的人源化形式已被开发为治疗B细胞淋巴瘤的候选药物[39]。5A6识别CD81外结构域上的一个构象表位,当CD19结合在B细胞表面时,该表位被掩盖[28]。在三阴性乳腺癌(Triple-negative breast cancer, TNBC)的异种移植模型中,mAb 5A6在抑制TNBC细胞的侵袭和转移方面具有非常有效的抑制作用[40]。
![]() 图 4 抗CD81抗体(单克隆抗体)[4]Figure 4 Anti-CD81 antibodies (mAbs)[4]Anti-CD81 antibodies are used to study CD81 expression and/or function specifically to characterize membrane-bound CD81 on exosomes. Several anti-CD81 mAbs, such as QV-6A8-F2-C4, MT81, K04, K21, and JS-81, can be used to control virus entry into cells. The mAbs 2F7, Eat1, and Eat2 have been essentially used to study autoimmune encephalomyelitis and colitis, whereas mAb DSP-8250 has been tested against intestinal bowel disease. The lead mAb 5A6 displays marked antitumor activities in murine models of B cell lymphoma and triple-negative breast cancer. A molecular model of the Fab fragment of 5A6 bound to CD81-EC2 is shown (PDB:6U9S). 5A6 recognizes a conformational epitope on CD81 that is masked when CD81 is bound to CD19.
图 4 抗CD81抗体(单克隆抗体)[4]Figure 4 Anti-CD81 antibodies (mAbs)[4]Anti-CD81 antibodies are used to study CD81 expression and/or function specifically to characterize membrane-bound CD81 on exosomes. Several anti-CD81 mAbs, such as QV-6A8-F2-C4, MT81, K04, K21, and JS-81, can be used to control virus entry into cells. The mAbs 2F7, Eat1, and Eat2 have been essentially used to study autoimmune encephalomyelitis and colitis, whereas mAb DSP-8250 has been tested against intestinal bowel disease. The lead mAb 5A6 displays marked antitumor activities in murine models of B cell lymphoma and triple-negative breast cancer. A molecular model of the Fab fragment of 5A6 bound to CD81-EC2 is shown (PDB:6U9S). 5A6 recognizes a conformational epitope on CD81 that is masked when CD81 is bound to CD19.其他抗CD81单克隆抗体也在研发中,如单克隆抗体DSP-8250,它识别一个独特的表位,并具有同样强大的减弱T细胞迁移的能力,但与5A6单抗相比,没有细胞因子增强活性[41]。这种单抗来自日本,已被用于肠道疾病(Intestinal bowel disease, IBD)的临床前测试,但没有进一步发展。还有其他的小鼠抗CD81单克隆抗体,如2F7、Eat1和Eat2的克隆抗体,用于自身免疫性脑脊髓炎和(或)结肠炎的实验性研究。有研究发现2F7对小鼠结肠炎有持久的治疗效果[42]。QV-6A8-F2-C4、MT81、K04、K21和JS-81是另外五种靶向人CD81的单抗,用于阻断HCV E2蛋白结合。也有其他针对中和CD81-EC2的单克隆抗体被用于研究CD81的功能,这些研究成果优化了CD81单抗对CD81-EC2的高亲和力,并显著增强了预防HCV感染的能力[43]。然而,目前临床上还没有抗CD81的药物,这些单克隆抗体都用于研究目的,例如通过亲和层析分离和表征CD81外泌体[44],CD81是外泌体和其他类型的细胞外囊泡最常用的生物标志物之一[45]。
6 CD81小分子结合物
由于四跨膜蛋白的混杂性质和结构功能的冗余,设计与CD81选择性相互作用的小分子结合物是具有挑战的。然而,考虑到它们的特定动态特征以及相同的生物学行为,选择性靶向特定的四跨膜蛋白也可行[46]。CD81与伴侣蛋白的相互作用可以被非特异性的蛋白-蛋白相互作用抑制剂阻断,如甲基蛋白[47]。然而,研究的目标是发现对CD81有选择性的小分子。目前多数分子通过噬菌体展示肽库技术或者模拟表位肽方式,主要针对HCV-E2蛋白的CD81结合位点来获取,包括九肽ATWVCGPCT、基序SPQYWTGPA、E2肽710-725等[4]。最近,研究者又发现了一系列能够结合并稳定CD81的喹啉衍生物[48]。该系列的先导化合物是苯并噻唑-喹啉衍生物6(3-(苯并噻唑-2-喹啉酮)),主要是通过与谷氨酸、天冬氨酸、丝氨酸和赖氨酸残基的相互作用与CD81结合。
有趣的是还存在一种天然的化合物“络石苷元”,它是一种抗肿瘤的二苄基丁内酯型木脂素,可以从多种药用植物(长节藤、橙焰风车子)中提取[49]。它的作用机制与喹啉衍生物类似,也是通过与CD81 EC2上的苏氨酸、天冬酰胺、赖氨酸和谷氨酸残基相互作用。然而,CD81并不是这种天然产物的唯一目标,它还可针对其他的蛋白质靶点包括中枢谷氨酸受体,但这并不一定会限制它的应用,因为靶向谷氨酸受体可能还存在神经保护作用[50]。
另外,可以考虑从已知的与其他四跨膜蛋白结合的分子来设计与CD81结合的化合物。例如,近期发现了针对四跨膜蛋白CD151的大细胞外环(LEL)的小分子,邻苯二酚和抗癌药物5-Fu被认为是CD151-LEL结合物和CD151表达抑制剂[51]。同样,一种短环肽(L-亮氨酸-L-脯氨酸)也被证明可以结合CD151,并阻断其与表皮生长因子受体(Epidermal growth factor receptor, EGFR)的相互作用,从而发挥抗癌作用[52]。这些可能是可转座以靶向CD81的LEL的替代选择。最后,也可以通过上游靶向CD81 mRNA来调控CD81的表达。已有研究发现多酚表没食子儿茶素没食子酸酯(EGCG)可以增加CD81 mRNA 3’UTR区域的miR-548m的表达,从而下调HCV感染的Huh7人肝癌细胞中的CD81蛋白和mRNA的水平[53]。同样,EGCG也已被证明可以增强miR-194的表达,从而导致CD81表达下调[54]。
7 总结与展望
CD81不仅与病毒及致病菌感染有关,更是肿瘤细胞生长和迁移的重要调节因子,是对抗不同类型的实体肿瘤和淋巴恶性肿瘤的有效靶点。小分子靶向CD81为晚期实体瘤的口服治疗和转移预防提供了一个选择。然而,以上讨论的小分子药物还处于临床前研究,距离临床应用还较远。此领域快速发展的可能还是通过单抗,但无论在阳性病例中的比例还是在淋巴细胞的表达水平上,CD81在B细胞淋巴瘤的表达都呈现出高度异质性特征。因此,确定哪些类型的B细胞淋巴瘤可以通过抗CD81抗体进行治疗,以及何种CD81的表达水平对于有效消除淋巴瘤细胞至关重要。目前白血病和淋巴瘤的早期临床中已开发出抗CD37 BI 836826的四跨膜蛋白单抗,提示CD81也可能成为二线治疗联合靶向治疗的药物。目前还有一些问题值得关注,比如CD81由多种类型的免疫细胞表达,特别是在生发中心B细胞中表达最高,若长期应用,这些细胞可能被消除,导致在CD81抗体治疗期间体液免疫反应受损。此外,CD81在调节性T细胞和髓系来源的抑制细胞调节肿瘤生长和转移中发挥免疫抑制功能,抗CD81抗体是否对这些细胞带有刺激作用或细胞毒性作用尚未知,这些潜在的副作用可能会支持或削弱治疗的效果。期待未来有更多针对CD81的研究能够破解这些疑问。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:赵 茜:文章撰写、修改李 锋:文章主题设定、撰写及修改 -
表 1 与CD81相互作用的蛋白质
Table 1 Proteins interacting with CD81
Proteins Types Interactions and Effects E2 HCV Viral protein The transmembrane E2 glycoprotein of HCV utilizes CD81 as a coreceptor for cell entry. Claudin-1 Tight junction protein The interaction of CD81-Claudin1 contributes to HCV infection. IFITM1 Tight junction protein IFITM1 interacts with CD81 to limit HCV entry. LDL-R Membrane receptor CD81, LDL-R, and PCSK9 interact with each other to control HCV entry into hepatic cells. CD19 Membrane receptor CD81 and CD19 are the core subunits of the B cell coreceptor complex. CD81 controls CD19 export activity via a dynamically regulated process upon B cell activation. EWI-2 (IgSF8, PGRL, and CD316) Signaling protein The CD81-EWI-2 interaction contributes to the tetraspanin web and plays a role in the growth and motility of cancer cells and entry of HCV. EWI-F
(FPRP and CD9P-1)Signaling protein Complexes formed between EWI-F and CD81 (and CD9) participate in the fusion of myotubes, which are essential elements of muscle structure. CD44 Adhesion molecule The interaction between CD81 and CD44 through their extracellular regions promotes the formation of tumor cell clusters and lung metastasis of triple negative breast cancer. ɑ6 Integrin Adhesion molecule In male germ cells, CD81 interacts with the α6 integrin subunit, which forms a dimer with β4 integrin. The complex plays a role in sperm maturation. βRac1 integrin (CD29) Adhesion molecule Radiation was found to induce CD29-CD81 complex formation, thereby increasing the cellular uptake of exosomes. Rac1 Small GTPase Rac interacts with the C-terminal cytoplasmic portion of CD81 to regulate cell motility and plays a role in bacterial infection. CD4 Cell surface antigen CD81 interacts with the CD4 dimers concentrated in tetraspanin-enriched microdomains. CD9 Tetraspanin CD9 and CD81 are involved in tetraspanin web formation in sperm. Molecular modeling suggests the occurrence of protein-protein interactions during sperm-egg membrane fusion. SAMHD1 Enzyme CD81 interacts with the deoxynucleoside triphosphate phosphohydrolase SAMHD1 and regulates its expression. This interaction promotes the proteasome-dependent degradation of SAMHD1. It is a metabolic regulator of HIV-1 replication. MT1-MMP Enzyme Several tetraspanins, including CD81, associate with the membrane-type 1 matrix metalloproteinase to regulate its cell surface localization and its function (notably its capacity to activate pro-MMP-2). Syndecan-1 Proteoglycan The knockdown of syndecan-1 and CD81 inhibits HCV infection, suggesting their cooperative action. AhpC Enzyme The mycobacterial enzyme alkyl hydroperoxide reductase C interacts with CD81-LEL to promote the uptake of pathogens by host cells. TfR2 Membrane receptor Transferrin receptor 2 is a binding partner for CD81. The interaction triggers RfR2 degradation by the ubiquitin E3 ligase GRAIL. -
[1] Becic A, Leifeld J, Shaukat J, et al. Tetraspanins as Potential Modulators of Glutamatergic Synaptic Function[J]. Front Mol Neurosci, 2022, 14: 801882. doi: 10.3389/fnmol.2021.801882
[2] New C, Lee ZY, Tan KS, et al. Tetraspanins: Host Factors in Viral Infections[J]. Int J Mol Sci, 2021, 22(21): 11609. doi: 10.3390/ijms222111609
[3] Karam J, Méresse S, Kremer L, et al. The roles of tetraspanins in bacterial infections[J]. Cell Microbiol, 2020, 22(12): e13260.
[4] Bailly C, Thuru X. Targeting of Tetraspanin CD81 with Monoclonal Antibodies and Small Molecules to Combat Cancers and Viral Diseases[J]. Cancers (Basel), 2023, 15(7): 2186. doi: 10.3390/cancers15072186
[5] Oren R, Takahashi S, Doss C, et al. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins[J]. Mol Cell Biol, 1990, 10(8): 4007-4015.
[6] Zimmerman B, Kelly B, McMillan BJ, et al. Crystal Structure of a Full-Length Human Tetraspanin Reveals a Cholesterol-Binding Pocket[J]. Cell, 2016, 167(4): 1041-1051. e11.
[7] Seigneuret M, Delaguillaumie A, Lagaudrière-Gesbert C, et al. Structure of the tetraspanin main extracellular domain. A partially conserved fold with a structurally variable domain insertion[J]. J Biol Chem, 2001, 276(43): 40055-40064. doi: 10.1074/jbc.M105557200
[8] Seigneuret M. Complete predicted three-dimensional structure of the facilitator transmembrane protein and hepatitis C virus receptor CD81: conserved and variable structural domains in the tetraspanin superfamily[J]. Biophys J, 2006, 90(1): 212-227. doi: 10.1529/biophysj.105.069666
[9] Kitadokoro K, Ponassi M, Galli G, et al. Subunit association and conformational flexibility in the head subdomain of human CD81 large extracellular loop[J]. Biol Chem, 2002, 383(9): 1447-1452.
[10] Kitadokoro K, Bordo D, Galli G, et al. CD81 extracellular domain 3D structure: insight into the tetraspanin superfamily structural motifs[J]. EMBO J, 2001, 20(1-2): 12-18.
[11] van Deventer SJ, Dunlock VE, van Spriel AB. Molecular interactions shaping the tetraspanin web[J]. Biochem Soc Trans, 2017, 45(3): 741-750. doi: 10.1042/BST20160284
[12] Palor M, Stejskal L, Mandal P, et al. Cholesterol sensing by CD81 is important for hepatitis C virus entry[J]. J Biol Chem, 2020, 295(50): 16931-16948. doi: 10.1074/jbc.RA120.014761
[13] Yang Y, Liu XR, Greenberg ZJ, et al. Open conformation of tetraspanins shapes interaction partner networks on cell membranes[J]. EMBO J, 2020, 39(18): e105246. doi: 10.15252/embj.2020105246
[14] Orinska Z, Hagemann PM, Halova I, et al. Tetraspanins in the regulation of mast cell function[J]. Med Microbiol Immunol, 2020, 209(4): 531-543. doi: 10.1007/s00430-020-00679-x
[15] Vences-Catalán F, Duault C, Kuo CC, et al. CD81 as a tumor target[J]. Biochem Soc Trans, 2017, 45(2): 531-535. doi: 10.1042/BST20160478
[16] Mazzocca A, Liotta F, Carloni V. Tetraspanin CD81-regulated cell motility plays a critical role in intrahepatic metastasis of hepatocellular carcinoma[J]. Gastroenterology, 2008, 135(1): 244-256. e1.
[17] Yoo TH, Ryu BK, Lee MG, et al. CD81 is a candidate tumor suppressor gene in human gastric cancer[J]. Cell Oncol (Dordr), 2013, 36(2): 141-153. doi: 10.1007/s13402-012-0119-z
[18] Lee MS, Kim JH, Lee JS, et al. Prognostic Significance of CREB-Binding Protein and CD81 Expression in Primary High Grade Non-Muscle Invasive Bladder Cancer: Identification of Novel Biomarkers for Bladder Cancer Using Antibody Microarray[J]. PLoS One, 2015, 10(4): e0125405. doi: 10.1371/journal.pone.0125405
[19] Hong IK, Byun HJ, Lee J, et al. The tetraspanin CD81 protein increases melanoma cell motility by up-regulating metalloproteinase MT1-MMP expression through the pro-oncogenic Akt-dependent Sp1 activation signaling pathways[J]. J Biol Chem, 2014, 289(22): 15691-15704. doi: 10.1074/jbc.M113.534206
[20] Mizoshiri N, Shirai T, Terauchi R, et al. The tetraspanin CD81 mediates the growth and metastases of human osteosarcoma[J]. Cell Oncol (Dordr), 2019, 42(6): 861-871.
[21] Uretmen Kagiali ZC, Sanal E, Karayel Ö, et al. Systems-level Analysis Reveals Multiple Modulators of Epithelial-mesenchymal Transition and Identifies DNAJB4 and CD81 as Novel Metastasis Inducers in Breast Cancer[J]. Mol Cell Proteomics, 2019, 18(9): 1756-1771. doi: 10.1074/mcp.RA119.001446
[22] Vences-Catalán F, Rajapaksa R, Srivastava MK, et al. Tetraspanin CD81 promotes tumor growth and metastasis by modulating the functions of T regulatory and myeloid-derived suppressor cells[J]. Cancer Res, 2015, 75(21): 4517-4526. doi: 10.1158/0008-5472.CAN-15-1021
[23] Zheng W, Chen Q, Liu H, et al. CD81 Enhances Radioresistance of Glioblastoma by Promoting Nuclear Translocation of Rad51[J]. Cancers (Basel), 2021, 13(9): 1998. doi: 10.3390/cancers13091998
[24] Signorelli D, Ghidotti P, Proto C, et al. Circulating CD81-expressing extracellular vesicles as biomarkers of response for immune-checkpoint inhibitors in advanced NSCLC[J]. Front Immunol, 2022, 13: 987639. doi: 10.3389/fimmu.2022.987639
[25] Küppers R. CD81 as target for B cell lymphomas[J]. J Exp Med, 2019, 216(7): 1469-1470. doi: 10.1084/jem.20190733
[26] Sermer D, Elavalakanar P, Abramson JS, et al. Targeting CD19 for diffuse large B cell lymphoma in the era of CARs: Other modes of transportation[J]. Blood Rev, 2023, 57: 101002. doi: 10.1016/j.blre.2022.101002
[27] Susa KJ, Rawson S, Kruse AC, et al. Cryo-EM structure of the B cell co-receptor CD19 bound to the tetraspanin CD81[J]. Science, 2021, 371(6526): 300-305. doi: 10.1126/science.abd9836
[28] Susa KJ, Seegar TC, Blacklow SC, et al. A dynamic interaction between CD19 and the tetraspanin CD81 controls B cell co-receptor trafficking[J]. Elife, 2020, 9: e52337. doi: 10.7554/eLife.52337
[29] Velasquez MP, Gottschalk S. Targeting CD19: the good, the bad, and CD81[J]. Blood, 2017, 129(1): 9-10. doi: 10.1182/blood-2016-11-749143
[30] Luo RF, Zhao S, Tibshirani R, et al. CD81 protein is expressed at high levels in normal germinal center B cells and in subtypes of human lymphomas[J]. Hum Pathol, 2010, 41(2): 271-280. doi: 10.1016/j.humpath.2009.07.022
[31] Paiva B, Gutiérrez NC, Chen X, et al. Clinical significance of CD81 expression by clonal plasma cells in high-risk smoldering and symptomatic multiple myeloma patients[J]. Leukemia, 2012, 26(8): 1862-1869. doi: 10.1038/leu.2012.42
[32] Paiva B, Puig N, Cedena MT, et al. Differentiation stage of myeloma plasma cells: biological and clinical significance[J]. Leukemia, 2017, 31(2): 382-392. doi: 10.1038/leu.2016.211
[33] Boyer T, Guihard S, Roumier C, et al. Tetraspanin CD81 is an adverse prognostic marker in acute myeloid leukemia[J]. Oncotarget, 2016, 7(38): 62377-62385. doi: 10.18632/oncotarget.11481
[34] Quagliano A, Gopalakrishnapillai A, Kolb EA, et al. CD81 knockout promotes chemosensitivity and disrupts in vivo homing and engraftment in acute lymphoblastic leukemia[J]. Blood Adv, 2020, 4(18): 4393-4405. doi: 10.1182/bloodadvances.2020001592
[35] Braig F, Brandt A, Goebeler M, et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking[J]. Blood, 2017, 129(1): 100-104. doi: 10.1182/blood-2016-05-718395
[36] Ruella M, Korell F, Porazzi P, et al. Mechanisms of resistance to chimeric antigen receptor-T cells in haematological malignancies[J]. Nat Rev Drug Discov, 2023, 22(12): 976-995. doi: 10.1038/s41573-023-00807-1
[37] Schultz LM, Czerwinski DK, Levy R, et al. CD81 costimulation skews CAR transduction toward naive T cells[J]. Proc Natl Acad Sci U S A, 2022, 119(5): e1910844119. doi: 10.1073/pnas.1910844119
[38] Van Compernolle SE, Levy S, Todd SC. Anti-CD81 activates LFA-1 on T cells and promotes T cell-B cell collaboration[J]. Eur J Immunol, 2001, 31(3): 823-831. doi: 10.1002/1521-4141(200103)31:3<823::AID-IMMU823>3.0.CO;2-D
[39] Vences-Catalán F, Kuo CC, Rajapaksa R, et al. CD81 is a novel immunotherapeutic target for B cell lymphoma[J]. J Exp Med, 2019, 216(7): 1497-1508. doi: 10.1084/jem.20190186
[40] Vences-Catalán F, Rajapaksa R, Kuo CC, et al. Targeting the tetraspanin CD81 reduces cancer invasion and metastasis[J]. Proc Natl Acad Sci U S A, 2021, 118(24): e2018961118. doi: 10.1073/pnas.2018961118
[41] Hasezaki T, Yoshima T, Mattsson M, et al. A monoclonal antibody recognizing a new epitope on CD81 inhibits T-cell migration without inducing cytokine production[J]. J Biochem, 2020, 167(4): 399-409. doi: 10.1093/jb/mvz103
[42] Hasezaki T, Yoshima T, Mine Y. Anti-CD81 antibodies reduce migration of activated T lymphocytes and attenuate mouse experimental colitis[J]. Sci Rep, 2020, 10(1): 6969. doi: 10.1038/s41598-020-64012-5
[43] Nelson B, Adams J, Kuglstatter A, et al. Structure-Guided Combinatorial Engineering Facilitates Affinity and Specificity Optimization of Anti-CD81 Antibodies[J]. J Mol Biol, 2018, 430(14): 2139-2152. doi: 10.1016/j.jmb.2018.05.018
[44] Burkova EE, Dmitrenok PS, Bulgakov DV, et al. Exosomes from human placenta purified by affinity chromatography on sepharose bearing immobilized antibodies against CD81 tetraspanin contain many peptides and small proteins[J]. IUBMB Life, 2018, 70(11): 1144-1155. doi: 10.1002/iub.1928
[45] Tan KL, Chia WC, How CW, et al. Benchtop Isolation and Characterisation of Small Extracellular Vesicles from Human Mesenchymal Stem Cells[J]. Mol Biotechnol, 2021, 63(9): 780-791. doi: 10.1007/s12033-021-00339-2
[46] Fernandez L, Malrieu M, Bénistant C, et al. CD82 and Gangliosides Tune CD81 Membrane Behavior[J]. Int J Mol Sci, 2021, 22(16): 8459. doi: 10.3390/ijms22168459
[47] Chuang ST, Papp H, Kuczmog A, et al. Methylene Blue Is aNonspecific Protein-Protein Interaction Inhibitor with Potential for Repurposing as an Antiviral for COVID-19[J]. Pharmaceuticals, 2022, 15(5): 621. doi: 10.3390/ph15050621
[48] Anand K, Khan FI, Singh T, et al. Green Synthesis, Experimental and Theoretical Studies to Discover Novel Binders of Exosomal Tetraspanin CD81 Protein[J]. ACS Omega, 2020, 5(29): 17973-17982. doi: 10.1021/acsomega.0c01166
[49] Moura AF, Lima KSB, Sousa TS, et al. In vitro antitumor effect of a lignan isolated from Combretum fruticosum, trachelogenin, in HCT-116 human colon cancer cells[J]. Toxicol In Vitro, 2018, 47: 129-136. doi: 10.1016/j.tiv.2017.11.014
[50] Koech PK, Jócsák G, Boldizsár I, et al. Anti-glutamatergic Effects of Three Lignan Compounds: Arctigenin, Matairesinol and Trachelogenin-An ex vivo Study on Rat Brain Slices[J]. Planta Med, 2023, 89(9): 879-889. doi: 10.1055/a-2005-5497
[51] Akella M, Malla R. Molecular modeling and in vitro study on pyrocatechol as potential pharmacophore of CD151 inhibitor[J]. J Mol Graph Model, 2020, 100: 107681. doi: 10.1016/j.jmgm.2020.107681
[52] Kgk D, Kumari S, G S, et al. Marine natural compound cyclo(L-leucyl-L-prolyl) peptide inhibits migration of triple negative breast cancer cells by disrupting interaction of CD151 and EGFR signaling[J]. Chem Biol Interact, 2020, 315: 108872. doi: 10.1016/j.cbi.2019.108872
[53] Mekky RY, El-Ekiaby N, El Sobky SA, et al. Epigallocatechin gallate (EGCG) and miR-548m reduce HCV entry through repression of CD81 receptor in HCV cell models[J]. Arch Virol, 2019, 164(6): 1587-1595. doi: 10.1007/s00705-019-04232-x
[54] Mekky RY, El-Ekiaby NM, Hamza MT, et al. Mir-194 is a hepatocyte gate keeper hindering HCV entry through targeting CD81 receptor[J]. J Infect, 2015, 70(1): 78-87. doi: 10.1016/j.jinf.2014.08.013




 下载:
下载: