Discrimination Models for Helicobacter Pylori Infection by Multi-Serological Line Assay in Chinese Population
-
摘要:目的
筛选血清特异性抗体,建立适用于中国人群胃癌筛查、非侵入性判别幽门螺杆菌感染状态的抗体组合和模型。
方法基于胃癌高发区胃镜筛查队列,纳入300例幽门螺杆菌现况感染、既往感染和感染阴性受试者。采用血清免疫印迹试剂盒对10种特异性抗体进行比较和筛选。
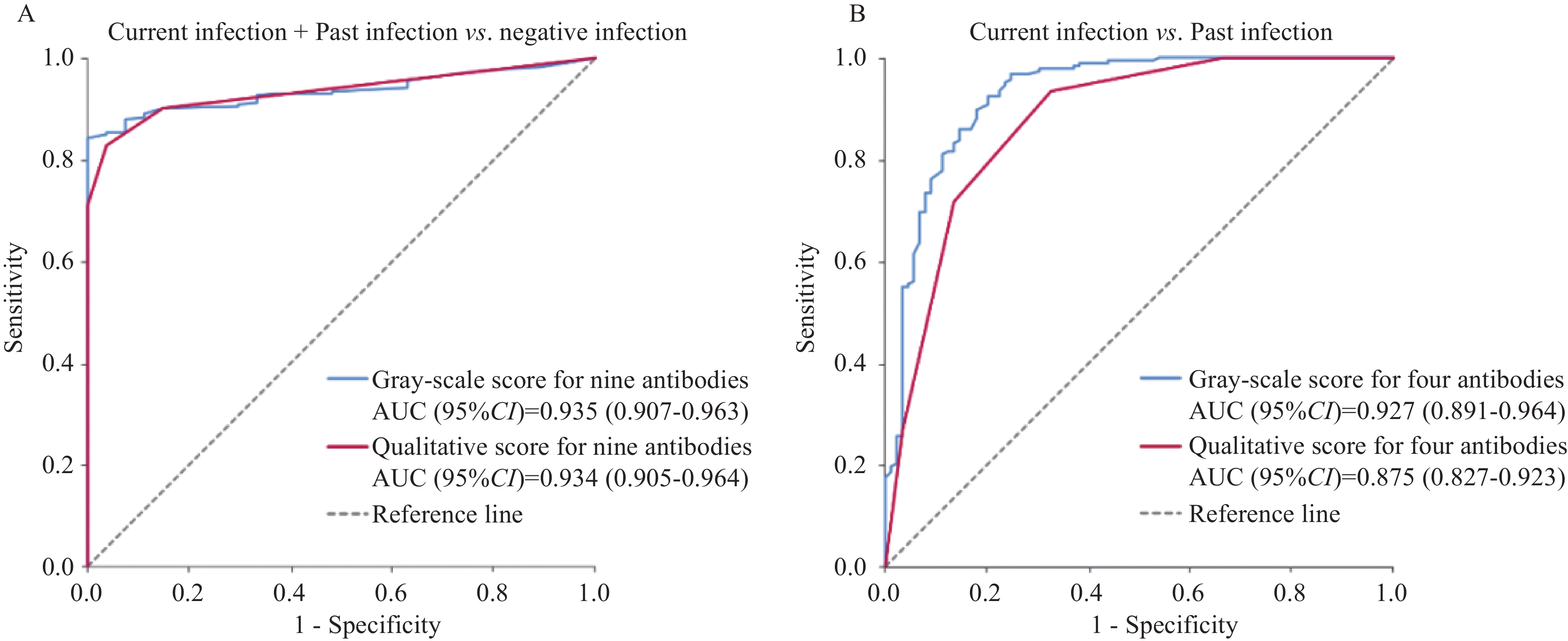
结果9种特异性抗体的阳性率在不同组间差异显著,均P<0.05。利用9种抗体灰度值得分建立判别模型,能区分感染暴露(现况+既往感染)和阴性病例,曲线下面积(AUC)为0.935(95%CI:0.907~0.963)。CagA、GroEL、FliD和gGT抗体灰度值得分能区分现况和既往感染病例(AUC=0.927,95%CI:0.891~0.964)。
结论本研究筛选和建立的幽门螺杆菌感染相关抗体组合和判别模型为制定精准化胃癌预防策略提供了一种潜在、非侵入性筛查方法。
Abstract:ObjectiveTo screen specific antibodies to Helicobacter pylori (H. pylori) in serum, and establish antibody panels and discrimination models for different infection status, which are non-invasive and suitable for gastric cancer screening in Chinese population.
MethodsA total of 300 subjects with different H. pylori statuses were enrolled depending on an endoscopy screening cohort in a high-risk area of gastric cancer, including current, past, and negative infections. The recomLine Helicobacter IgG 2.0 immunoblotting assay was used to analyze and screen 10 H. pylori specific antibodies in serum samples.
ResultsA total of nine antibody reactivity against CagA, VacA, GroEL, FliD, HpaA, gGT, HtrA, NapA, and CtkA showed significant differences among different H. pylori infection status groups (all P<0.05). A panel comprising the nine antibodies distinguished exposure subjects to H. pylori (current and past infections) from negatives, with an area under the curve (AUC) of 0.935 (95%CI: 0.907–0.963). The combination of four antibodies (CagA, GroEL, FliD, and gGT) may help to discriminate current and past infection subjects, with an AUC of 0.927 (95%CI: 0.891–0.964).
ConclusionThe antibody panels and discriminant models for H. pylori infection status established in the present study may provide a potential and non-invasive screening method for the development of precise gastric cancer prevention strategies.
-
Key words:
- Helicobacter pylori /
- Specific antibody /
- Infection exposure /
- Current infection /
- Past infection
-
0 引言
幽门螺杆菌(Helicobacter pylori,H. pylori)感染是胃癌Ⅰ类致癌物,约89%的非贲门胃癌可归因于H. pylori感染[1- 2]。我国是胃癌高发国家,全球约37%的新发和39%的死亡病例在我国[1],防控形势十分严峻。本课题组前期在我国特有的胃癌高发区山东省临朐县开展了大规模干预研究,经过长期随访证实根除H. pylori感染是预防胃癌的有效措施[3]。此外,既往H. pylori的暴露情况也可能与胃黏膜病变及胃癌显著相关,具有风险评估参考价值。因此,在我国胃癌高发地区精准筛查H. pylori感染状态,实施有针对性的干预和预防策略具有重要意义。
目前用于人群H. pylori感染筛查的主要方法包括13C-尿素呼气试验(13C-UBT)和血清学检测。13C-UBT是最常用的非侵入性检测方法之一,主要利用H. pylori的尿素酶活性,通过测定标记13C的CO2产物,判定是否存在活动性感染。研究发现,抑制胃酸分泌的质子泵抑制剂(PPI)或胃黏膜萎缩可能影响13C-UBT检测的准确性[4-5]。传统的血清学方法(例如ELISA)更加简便易行、成本较低,但仅能测定H. pylori的总抗体水平,无法区分活动性感染及抗体水平尚未降至阈值以下的既往感染状态。因此,开发一种新型非侵入性检测方法,能够简便、精准判定H. pylori感染状态,对高发人群的胃癌防控具有重要意义。
近年来,多项研究对特定H. pylori毒力因子的血清特异性抗体开展了定量或定性分析,发现其可能与胃癌风险密切相关,并在根除治疗后具有不同的动态变化规律。综合不同抗体的标志物组合可能具有预测胃癌进展风险,以及鉴别H. pylori感染状态的潜在临床应用价值[6-8]。一项基于德国医院病例的研究采用recomLine Helicobacter IgG2.0试剂盒对10种H. pylori特异性抗体进行分析,发现包括5种抗体组合的模型能够帮助区分活动性感染和根除治疗后病例[8]。但中国与欧洲人群的H. pylori菌株可能具有较大差异,有必要进一步在中国人群中建立适用于早期筛查、感染状态判定的血清特异性抗体组合。
本研究利用课题组前期在山东省临朐县胃癌高发区已建立的胃镜筛查队列收集的血清样本,采用recomLine Helicobacter IgG2.0试剂盒分析感染状态相关的差异性抗体,并初步建立适用于中国人群、可简便快速鉴别H. pylori感染状态的抗体组合和判别模型。
1 资料与方法
1.1 研究对象和样本收集
山东省临朐县是我国胃癌高发地区之一[3]。本研究依托在临朐县开展的国家上消化道癌早诊早治筛查项目[9],纳入2014—2016年参加胃镜筛查、同时接受了13C-UBT检测,并提供了血清样本以及胃黏膜活检组织样本的300例受试者,研究对象均通过问卷调查表收集年龄、性别、吸烟、饮酒等完整的流行病学信息。本研究对胃黏膜活检组织样本进行病理学诊断,判定胃黏膜病变类型及组织学感染状态;对血清样本采用RecomLine Helicobacter IgG2.0试剂盒进行H. pylori特异性抗体定性和定量检测。本研究获得北京大学临床肿瘤学院伦理委员会批准,且所有研究对象均已签署知情同意书。
1.2 上消化道内镜检查和组织病理学
上消化道内镜检查由两位经验丰富的内镜医师采用高清白光内镜(Olympus GIF-H260或H290)进行,并从胃窦和胃体处至少取两个活检组织样本用于组织学诊断。根据Padova国际分类[10]、中国慢性胃炎共识意见(2017)[11]以及新悉尼标准(2001)[12],两位病理学家对胃黏膜样本进行了独立评估。此外,德国拜罗伊特医院病理研究所所长、国际知名病理学专家Michael Vieth教授在访华期间对病理切片进行了复核。胃黏膜病变类型分为正常、浅表性胃炎、慢性萎缩性胃炎、肠上皮化生、异型增生和胃癌。并且根据是否存在H. pylori细菌、活动性胃炎、慢性非活动性胃炎、基底淋巴滤泡残留等,将所有受试者诊断分为组织学活动性感染、既往感染和未感染状态。
1.3 13C-尿素呼气试验
所有受试者在清晨空腹或禁食2 h状态下,收集基线呼出的CO2气体样本后,给予30~40 ml含有13C-尿素和柠檬酸溶液口服。在服药后的10~15 min内,采用鼻管反复收集呼出的气体。使用气体同位素比质谱仪(BreathID Hp Lab System®,Exalenz Bioscience Ltd,以色列)测定基线样本和服药后反复收集样本中的13CO2丰度值,计算超基准值(DOB)。服药后15 min内DOB值不低于5‰,则认为该受试者13C-UBT检测结果为阳性[13]。
1.4 血清H. pylori特异性抗体的检测
RecomLine Helicobacter IgG2.0(Mikrogen,德国)是一种基于高度纯化重组H. pylori抗原的免疫印记检测方法,可简便、快速识别10种针对特定H. pylori抗原(CagA、VacA、GroEL、FliD、HpaA、gGT、HtrA、NapA、HP0231和CtkA)的特异性抗体[6]。当每个特异性抗体条带灰度大于内对照条带灰度时,该抗体被判定为血清阳性。根据不同H. pylori毒力因子与胃黏膜病变风险的关联程度,为每个相应抗体的血清阳性赋值:CagA和VacA各4分,GroEL、FliD、HpaA、gGT和HtrA各2分,NapA、HP0231和CtkA各1分。当各抗体评分总和≥4时,判定该个体为H. pylori血清学阳性,并结合13C-UBT检测结果和组织学诊断结果共同进行感染状态综合判定。
此外,本研究还采用灰度扫描仪和recomScan软件对每种特异性抗体条带灰度值进行定量扫描,并与内对照条带灰度值比较进行定性判定,将阳性赋值1、阴性赋值0,用于感染状态相关差异性抗体筛选、灰度值得分和定性得分计算以及判别模型构建。
1.5 H. pylori感染状态的综合判定
结合血清学、13C-UBT以及组织学检测结果,对所有受试者的H. pylori感染状态进行综合判定和分组。判定和分组标准如下:(1)当受试者的13C-UBT检测结果为阳性,即被诊断为现况感染病例;(2)当非现况感染病例具有血清学总抗体评分阳性,或者组织学诊断为既往感染状态,即被判定为既往感染病例;(3)当受试者为非现况感染和非既往感染状态时,判定为感染阴性病例。
1.6 数据与统计学方法
使用Pearson卡方检验或Kruskal-Wallis检验对不同H. pylori感染状态组之间的基本特征进行比较。采用多因素非条件Logistic回归分析后退法筛选与H. pylori感染状态相关的特异性抗体标志物,并利用比值比(Odd ratio, OR)和95%置信区间(Confidence interval, CI)量化特异性抗体与感染状态间的关联强度。结合筛选出的感染状态相关差异性抗体标志物组合,分别构建H. pylori感染暴露判别模型,区分感染暴露(现况感染+既往感染)和未感染病例;现况感染判别模型,区分现况感染和既往感染病例。绘制受试者工作特征曲线(ROC)以评估不同抗体组合及模型的判别能力。上述所有统计分析均采用SPSS 27.0(IBM)完成。
2 结果
2.1 研究对象基本资料
本研究纳入300例研究对象,根据H. pylori感染状态综合判定标准,可分为现况感染组184例(61.3%)、既往感染组89例(29.7%)、感染阴性组27例(9.0%),三组间平均年龄差异无统计学意义(P=0.742)。男性、吸烟者、饮酒者所占比例在现况感染组(58.7%、27.2%、36.4%)、既往感染组(38.2%、10.1%、19.1%)和感染阴性组(51.9%、22.2%、33.3%)具有显著差异,P值分别为0.006、0.008和0.013。现况感染组中,慢性萎缩性胃炎和重度胃黏膜病变(肠上皮化生/异型增生/胃癌)所占比例(40.2%、46.7%)显著高于既往感染(28.1%、22.5%)和感染阴性组(25.9%、11.1%),P<0.001,见表1。
表 1 不同H. pylori感染状态组间的基本特征Table 1 General characteristics of different H. pylori infection status groupsCharacteristics Negative
group
(n = 27)Past infection group
(n = 89)Current infection group
(n = 184)P Age (years) a 55. 3 ± 7.9 54.3 ± 7.0 55.1 ± 8.56 0.742 Gender b 0.006 Female 13 (48.1%) 55 (61.8%) 76 (41.3%) Male 14 (51.9%) 34 (38.2%) 108 (58.7%) Smoking b 0.008 No 21 (77.8%) 80 (89.9%) 134 (72.8%) Yes 6 (22.2%) 9 (10.1%) 50 (27.2%) Drinking b 0.013 No 18 (66.7%) 72 (80.9%) 117 (63.6%) Yes 9 (33.3%) 17 (19.1%) 67 (36.4%) Gastric
lesions b<0.001 Normal /SG 17 (63.0%) 44 (49.4%) 24 (13.0%) CAG 7 (25.9%) 25 (28.1%) 74 (40.2%) IM/DYS/GC 3 (11.1%) 20 (22.5%) 86 (46.7%) Notes: SG: superficial gastritis; CAG: chronic atrophic gastritis; IM: intestinal metaplasia; DYS: dysplasia; GC: gastric cancer. a: ANOVA. b: Pearson χ2 Test. 2.2 不同H. pylori感染状态组间特异性抗体比较
本研究在不同感染状态组间对10种特异性抗体进行比较,发现9种抗体的血清学阳性率具有显著差异,均P<0.05。其中,CagA、VacA、GroEL、FliD、HpaA、gGT、HtrA、NapA和CtkA在现况感染组的血清阳性率显著高于既往感染组。感染阴性组中仅有GroEL、HtrA、HpaA和NapA抗体具有较低的血清阳性率(7.4%、3.7%、3.7%和3.7%),其他5种抗体阳性率均为0。此外,HP231抗体阳性率在三组中均较低,差异无统计学意义,P=0.057,见表2。
表 2 不同H. pylori感染状态组间特异性抗体比较Table 2 Comparison of specific antibodies among groups with different H. pylori infection statusesAntibodies a Negative
group
(n=27)Past
infection
group
(n=89)Current
infection
group
(n=184)P CagA <0.001 Negative 27 (100.0%) 32 (36.0%) 7 (3.8%) Positive 0 (0.0%) 57 (64.0%) 177 (96.2%) VacA <0.001 Negative 27 (100.0%) 69 (77.5%) 107 (58.2%) Positive 0 (0.0%) 20 (22.5%) 77 (41.8%) GroEL <0.001 Negative 25 (92.6%) 71 (79.8%) 54 (29.3%) Positive 2 (7.4%) 18 (20.2%) 130 (70.7%) FliD <0.001 Negative 27 (100.0%) 65 (73.0%) 29 (15.8%) Positive 0 (0.0%) 24 (27.0%) 155 (84.2%) HpaA <0.001 Negative 26 (96.3%) 69 (77.5%) 51 (27.7%) Positive 1 (3.7%) 20 (22.5%) 133 (72.3%) gGT <0.001 Negative 27 (100.0%) 84 (94.4%) 109 (59.2%) Positive 0 (0.0%) 5 (5.6%) 75 (40.8%) HtrA 0.016 Negative 26 (96.3%) 81 (91.0%) 148 (80.4%) Positive 1 (3.7%) 8 (9.0%) 36 (19.6%) NapA <0.001 Negative 26 (96.3%) 86 (96.6%) 148 (80.4%) Positive 1 (3.7%) 3 (3.4%) 36 (19.6%) HP231 0.057 Negative 27 (100.0%) 87 (97.8%) 168 (91.3%) Positive 0 (0.0%) 2 (2.2%) 16 (8.7%) CtkA 0.002 Negative 27 (100.0%) 86 (96.6%) 157 (85.3%) Positive 0 (0.0%) 3 (3.4%) 27 (14.7%) Notes: CagA: cytotoxin-associated gene A; VacA: vacuolating cytotoxin A; GroEL: H. pylori chaperone; FliD: flagella hook-associated protein 2 homologue; HpaA: H. pylori adhesin A; gGT: γ-glutamyl transpeptidase; HtrA: high temperature requirement A; NapA: neutrophil-activating protein A; HP231: oxidoreductase 231; CtkA: cell translocating kinase A. a: Pearson χ2 Test. 2.3 H. pylori感染暴露相关特异性抗体标志物筛选及模型构建
为了区分H. pylori感染暴露(现况感染+既往感染)和感染阴性病例,本研究采用非条件Logistic回归调整性别、吸烟、饮酒、胃黏膜病变,纳入9种差异显著的抗体,采用后退法在感染暴露(现况感染+既往感染)和阴性组间进行筛选,结果发现,仅有CagA、GroEL和HpaA保留在回归模型中,P值分别为0.994、0.762和0.442,见表3。因此我们利用9种特异性抗体的灰度值或定性赋值相加获得的灰度值得分或定性得分,分别建立了H. pylori感染暴露判别模型,AUC为0.935(95%CI: 0.907~0.963)和0.934(95%CI: 0.905~0.964),见图1A。
表 3 H. pylori感染状态相关特异性抗体筛选Table 3 Selection of specific antibodies associated with H. pylori infection statusVariables H. pylori exposure model Current H. pylori infection model Negative Current
infection+Past
infectionOR (95%CI) a P a Past
infectionCurrent
infectionOR (95%CI) a P a CagA (+/−) 0/27 234/39 5.5×108 (0-∞) 0.994 57/32 177/7 6.5 (1.5–27.0) 0.011 GroEL (+/−) 2/25 148/125 1.3 (0.2–8.9) 0.762 18/71 130/54 3.1 (1.4–6.6) 0.004 FliD (+/−) 0/27 179/94 - - 24/65 155/29 7.9 (3.6–17.5) <0.001 HpaA (+/−) 1/26 153/120 2.6 (0.2–28.7) 0.442 3/86 36/148 - - gGT (+/−) 0/27 80/193 - - 5/84 75/109 5.5 (1.9–16.1) 0.002 Notes: a: unconditional multivariable logistic regression by backward selection was performed for H. pylori antibody reactivities against CagA, VacA, GroEL, FliD, HpaA, gGT, HtrA, NapA and CtkA as initial variables, adjusted for gender, smoking, drinking, and gastric lesions. - : no data. 本研究综合考虑约登指数、敏感度、特异度,选择最优的9种抗体灰度值得分(2.2)和定性得分(1.0)作为阈值。当灰度值得分≥2.2时,判定为感染暴露,敏感度为87.9%,特异度为92.6%,kappa值为0.531,P<0.001;当定性得分≥1.0时,判定为感染暴露,敏感度为90.1%,特异度为85.2%,kappa值为0.544,P<0.001,见表4。
表 4 H. pylori特异性抗体得分判别感染暴露状态的准确性评价Table 4 Accuracy of H. pylori-specific antibodies in identifying infection exposureAntibody score classification H. pylori exposure (n(%)) Kappa P Current infection+past infection Negative Classification of gray-scale score for nine antibodies a 0.531 <0.001 Current infection + past infection (≥2.2) 240 (87.9%) 2 (7.4%) Negative (<2.2) 33 (12.1%) 25 (92.6%) Classification of qualitative score for nine antibodies b 0.544 <0.001 Current infection + past infection (≥1.0) 246 (90.1%) 4 (14.8%) Negative (<1.0) 27 (9.9%) 23 (85.2%) Total 273 (100.0%) 27 (100.0%) Notes: a: the gray-scale score for nine antibodies is the sum of the gray-scale values of the antibody bands for CagA, VacA, GroEL, FliD, HpaA, gGT, HtrA, NapA, and CtkA; b: the qualitative score for nine antibodies is the sum of the qualitative values for the nine antibodies. 2.4 H. pylori现况感染状态相关特异性抗体标志物筛选及模型构建
为了进一步从H. pylori感染暴露病例中精准筛选出在临床诊疗过程中需要重点关注及干预的现况感染病例,本研究采用非条件Logistic回归调整性别、吸烟、饮酒、胃黏膜病变因素,纳入初筛获得的9种特异性抗体,采用后退法在现况感染和既往感染病例间进行筛选,结果发现4个特异性抗体阳性率与现况感染显著相关,包括CagA、GroEL、FliD和gGT,见表3。利用4种抗体的灰度值得分和定性得分能够构建出较好的判别模型区分现况感染和既往感染病例,AUC分别为0.927(95%CI: 0.891~0.964)和0.875(95%CI: 0.827~0.923),见图1B。
本研究综合考虑约登指数、灵敏度、特异度,选择最优的4种抗体灰度值得分(5.5)和定性得分(2.0)作为阈值。当灰度值得分≥5.5时,判定为现况感染,敏感度为96.7%,特异度为75.3%,Kappa值为0.755,P<0.001;当定性得分≥2.0时,判定为现况感染,敏感度为93.5%,特异度为67.4%,Kappa值为0.641,P<0.001,见表5。
表 5 H. pylori特异性抗体得分判别现况感染状态的准确性评价Table 5 Accuracy of scores of H. pylori-specific antibodies in identifying current infectionAntibody score classification H. pylori infection (n(%)) Kappa P Current infection Past infection Classification of gray-scale score for four antibodies a 0.755 <0.001 Current infection (≥5.5) 178 (96.7) 22 (24.7) Past infection (<5.5) 6 (3.3) 67 (75.3) Classification of qualitative score for four antibodies b 0.641 <0.001 Current infection (≥2.0) 172 (93.5) 29 (32.6) Past infection (<2.0) 12 (6.5) 60 (67.4) Total 184 (100.0) 89 (100.0) Notes: a: the gray-scale score for four antibodies is the sum of the gray-scale values of the antibody bands for CagA, GroEL, FliD and gGT; b: the qualitative score for four antibodies is the sum of the qualitative values for the four antibodies. 3 讨论
H. pylori感染状态的准确判定对胃癌及癌前病变的防控及干预策略实施具有重要意义。胃癌筛查监测不仅仅需要关注现况感染者,还应充分了解既往H. pylori的暴露情况,以便于评估胃癌发生风险,制定更加精准化的防控策略。传统的血清学方法仅能够检测H. pylori总抗体,无法区分现况和既往感染状态;而现况感染的诊断金标准13C-UBT也无法判定既往的暴露情况。因此,往往需要结合血清学、13C-UBT、组织学诊断等多种方法才能够综合判断现况、既往和未感染状态。目前尚缺乏一种简便、易行、非侵入性的精准筛查技术。本研究依托中国胃癌高发区人群,利用recomLine Helicobacter IgG 2.0试剂盒,定量和定性分析10种血清特异性抗体水平与H. pylori感染状态的关系,分别建立能够较好区分感染暴露状态(现况+既往感染 vs. 感染阴性)和现况感染状态(现况感染 vs. 既往感染)的抗体组合模型。
本研究采用的商品化recomLine Helicobacter IgG 2.0试剂盒可检测血清中10种H. pylori特异性抗体,并通过与试纸条预设的内对照条带进行定性分析,或依靠recomScan软件进行抗体条带灰度值的定量分析。既往已有多项研究利用多种特异性血清抗体,发现不同H. pylori毒力因子可能与胃黏膜病变及胃癌发生风险具有密切关系[7,14-15]。近期,本课题组联合德国慕尼黑工业大学的合作者,在德国医院病例中发现,可利用该试剂盒中的CagA、GroEL和FliD抗体组合,以及VacA、GroEL、FliD、HpaA和gGT抗体组合分别鉴别H. pylori感染暴露者和现况感染者[9]。但本课题组的前期研究结果提示,中国人群中常见的H. pylori菌株可能与欧洲人群菌株具有不同的毒力因子[7, 16],因此有必要在中国人群中进一步开展血清特异性抗体与H. pylori感染状态的关系研究,开发适用于中国人群的感染状态筛查血清抗体组合及判别模型。
本研究在具有代表性的中国临朐胃癌高发区人群中发现,9种抗体水平(CagA、VacA、GroEL、FliD、HpaA、gGT、HtrA、NapA和CtkA)与H. pylori感染状态密切相关,依托9种抗体条带灰度值得分建立的模型能够显著区分感染暴露(现况感染+既往感染)病例和感染阴性病例(AUC=0.935)。当9种抗体灰度值得分≥2.2时,模型判别感染暴露病例的敏感度和特异度分别为87.9%和92.6%。进一步筛选发现,综合4种抗体(CagA、GroEL、FliD和gGT)灰度值得分的模型,能够显著区分现况和既往感染病例(AUC=0.927)。当4种抗体灰度值得分≥5.5时,模型判别现况感染病例的敏感度和特异度分别为96.7%和75.3%。上述在中国胃癌高发区人群初步筛选和建立的H. pylori感染状态判别模型,具有简便、快速区分现况、既往和未感染病例的潜力。
本研究在中国人群中初步建立的感染状态相关抗体组合,与欧洲人群的研究结果具有一定差异。例如在中国人群中对感染暴露和现况感染状态的区分均具有重要作用的CagA抗体水平,并未被纳入欧洲人群现况和既往感染判别抗体组合(主要包括VacA、GroEL、FliD、HpaA 和 gGT)[8]。大量研究提示,不同地区常见H. pylori菌株间可能存在显著的抗原表达和致病性差异。其中,在中国、日本、韩国等东亚人群中流行的H. pylori菌株约90%以上为CagA蛋白表达阳性,远高于欧美国家人群中约60%~70%的表达阳性率[17]。不同人群的遗传背景差异可能影响宿主对病原体的免疫识别和应答机制,从而造成宿主对H. pylori菌株的易感性差异。例如亚洲人群中HLA基因多态性与H. pylori感染风险显著相关,与欧洲人群存在差异[18]。此外,生活方式、饮食习惯和卫生条件等环境因素,也可能影响不同地区H. pylori感染风险[19]。因此需要优化和大样本验证适用于中国人群的H. pylori感染状态判别抗体组合和模型。
基于欧洲和美国人群的前期研究发现,根除H. pylori治疗后,不同血清特异性抗体水平下降的速度可能具有较大差异,因此可以帮助鉴别现况和既往感染状态[20-21]。针对H. pylori重要的致病相关毒力因子CagA的特异性抗体水平,在根除治疗后下降缓慢,部分根除成功病例可能在较长时间内,表现为单一CagA抗体阳性[20]。本研究在中国胃癌高发区人群中发现,H. pylori现况感染病例中96.2%为CagA血清抗体阳性,既往感染组中阳性率虽有明显下降,但仍高达64.0%,与前期报道一致。此外,在我们筛选获得的现况感染判别模型中,GroEL、FliD和gGT抗体水平在现况感染组中也显著升高,可能反映出其已知的诱导炎性反应、功能性鞭毛组装、维持细菌的运动性、定植能力、免疫逃逸等重要作用[15, 22-24]。上述3种抗体水平在中国人群既往感染组中迅速降低,能够有效帮助判别现况和既往感染病例,3种抗体水平的变化趋势与欧洲人群的筛选结果相似[8]。
本研究采用了新开发的抗体条带灰度扫描仪及recomScan软件,对血清特异性抗体水平能够进行定量检测和分析,发现使用9种抗体灰度值得分的感染暴露模型的判别能力与纳入定性得分的模型相似(AUC为0.935和0.934)。而使用3种抗体灰度值得分的现况感染模型的判别能力则略高于纳入定性得分的模型(AUC为0.927和0.875)。结果提示,对抗体标志物条带进行灰度定量分析可能会提高模型的判别能力。但在缺乏相应的扫描设备和分析软件的条件下,定性得分模型仍可能对H. pylori感染状态进行较好的判别。
本研究为首次在中国胃癌高发区人群中,利用涵盖10种H. pylori特异性抗体的重组免疫印迹试剂盒,筛选与感染状态密切相关的差异性抗体,并初步建立可区分感染暴露和现况感染的判别模型。但历史队列中,同时具有13C-UBT、活检组织感染状态病理诊断和血清学结果的病例样本量较小。因此本研究尚有待于进一步扩大样本验证。在结合了13C-UBT、血清学及组织学检测结果综合判定现况、既往和未感染状态时,本研究未能获得既往感染病例血清样本采集时点与H. pylori根除的时间间隔信息。因此为了对中国人群常见H. pylori菌株的感染状态特征进行精准识别,建立了准确、适用的抗体组合和模型。本研究结果还有待于进一步在设计严谨、长期规律随访的大规模干预队列研究中进行验证,并充分评估根除治疗后的时间间隔对既往感染状态判别准确性的可能影响。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:张 丽:研究实施、数据分析、论文撰写张婧莹、周彤、李文庆:数据收集、文章审阅游伟程:研究设计指导潘凯枫、张阳:研究设计指导、文章审阅及修改 -
表 1 不同H. pylori感染状态组间的基本特征
Table 1 General characteristics of different H. pylori infection status groups
Characteristics Negative
group
(n = 27)Past infection group
(n = 89)Current infection group
(n = 184)P Age (years) a 55. 3 ± 7.9 54.3 ± 7.0 55.1 ± 8.56 0.742 Gender b 0.006 Female 13 (48.1%) 55 (61.8%) 76 (41.3%) Male 14 (51.9%) 34 (38.2%) 108 (58.7%) Smoking b 0.008 No 21 (77.8%) 80 (89.9%) 134 (72.8%) Yes 6 (22.2%) 9 (10.1%) 50 (27.2%) Drinking b 0.013 No 18 (66.7%) 72 (80.9%) 117 (63.6%) Yes 9 (33.3%) 17 (19.1%) 67 (36.4%) Gastric
lesions b<0.001 Normal /SG 17 (63.0%) 44 (49.4%) 24 (13.0%) CAG 7 (25.9%) 25 (28.1%) 74 (40.2%) IM/DYS/GC 3 (11.1%) 20 (22.5%) 86 (46.7%) Notes: SG: superficial gastritis; CAG: chronic atrophic gastritis; IM: intestinal metaplasia; DYS: dysplasia; GC: gastric cancer. a: ANOVA. b: Pearson χ2 Test. 表 2 不同H. pylori感染状态组间特异性抗体比较
Table 2 Comparison of specific antibodies among groups with different H. pylori infection statuses
Antibodies a Negative
group
(n=27)Past
infection
group
(n=89)Current
infection
group
(n=184)P CagA <0.001 Negative 27 (100.0%) 32 (36.0%) 7 (3.8%) Positive 0 (0.0%) 57 (64.0%) 177 (96.2%) VacA <0.001 Negative 27 (100.0%) 69 (77.5%) 107 (58.2%) Positive 0 (0.0%) 20 (22.5%) 77 (41.8%) GroEL <0.001 Negative 25 (92.6%) 71 (79.8%) 54 (29.3%) Positive 2 (7.4%) 18 (20.2%) 130 (70.7%) FliD <0.001 Negative 27 (100.0%) 65 (73.0%) 29 (15.8%) Positive 0 (0.0%) 24 (27.0%) 155 (84.2%) HpaA <0.001 Negative 26 (96.3%) 69 (77.5%) 51 (27.7%) Positive 1 (3.7%) 20 (22.5%) 133 (72.3%) gGT <0.001 Negative 27 (100.0%) 84 (94.4%) 109 (59.2%) Positive 0 (0.0%) 5 (5.6%) 75 (40.8%) HtrA 0.016 Negative 26 (96.3%) 81 (91.0%) 148 (80.4%) Positive 1 (3.7%) 8 (9.0%) 36 (19.6%) NapA <0.001 Negative 26 (96.3%) 86 (96.6%) 148 (80.4%) Positive 1 (3.7%) 3 (3.4%) 36 (19.6%) HP231 0.057 Negative 27 (100.0%) 87 (97.8%) 168 (91.3%) Positive 0 (0.0%) 2 (2.2%) 16 (8.7%) CtkA 0.002 Negative 27 (100.0%) 86 (96.6%) 157 (85.3%) Positive 0 (0.0%) 3 (3.4%) 27 (14.7%) Notes: CagA: cytotoxin-associated gene A; VacA: vacuolating cytotoxin A; GroEL: H. pylori chaperone; FliD: flagella hook-associated protein 2 homologue; HpaA: H. pylori adhesin A; gGT: γ-glutamyl transpeptidase; HtrA: high temperature requirement A; NapA: neutrophil-activating protein A; HP231: oxidoreductase 231; CtkA: cell translocating kinase A. a: Pearson χ2 Test. 表 3 H. pylori感染状态相关特异性抗体筛选
Table 3 Selection of specific antibodies associated with H. pylori infection status
Variables H. pylori exposure model Current H. pylori infection model Negative Current
infection+Past
infectionOR (95%CI) a P a Past
infectionCurrent
infectionOR (95%CI) a P a CagA (+/−) 0/27 234/39 5.5×108 (0-∞) 0.994 57/32 177/7 6.5 (1.5–27.0) 0.011 GroEL (+/−) 2/25 148/125 1.3 (0.2–8.9) 0.762 18/71 130/54 3.1 (1.4–6.6) 0.004 FliD (+/−) 0/27 179/94 - - 24/65 155/29 7.9 (3.6–17.5) <0.001 HpaA (+/−) 1/26 153/120 2.6 (0.2–28.7) 0.442 3/86 36/148 - - gGT (+/−) 0/27 80/193 - - 5/84 75/109 5.5 (1.9–16.1) 0.002 Notes: a: unconditional multivariable logistic regression by backward selection was performed for H. pylori antibody reactivities against CagA, VacA, GroEL, FliD, HpaA, gGT, HtrA, NapA and CtkA as initial variables, adjusted for gender, smoking, drinking, and gastric lesions. - : no data. 表 4 H. pylori特异性抗体得分判别感染暴露状态的准确性评价
Table 4 Accuracy of H. pylori-specific antibodies in identifying infection exposure
Antibody score classification H. pylori exposure (n(%)) Kappa P Current infection+past infection Negative Classification of gray-scale score for nine antibodies a 0.531 <0.001 Current infection + past infection (≥2.2) 240 (87.9%) 2 (7.4%) Negative (<2.2) 33 (12.1%) 25 (92.6%) Classification of qualitative score for nine antibodies b 0.544 <0.001 Current infection + past infection (≥1.0) 246 (90.1%) 4 (14.8%) Negative (<1.0) 27 (9.9%) 23 (85.2%) Total 273 (100.0%) 27 (100.0%) Notes: a: the gray-scale score for nine antibodies is the sum of the gray-scale values of the antibody bands for CagA, VacA, GroEL, FliD, HpaA, gGT, HtrA, NapA, and CtkA; b: the qualitative score for nine antibodies is the sum of the qualitative values for the nine antibodies. 表 5 H. pylori特异性抗体得分判别现况感染状态的准确性评价
Table 5 Accuracy of scores of H. pylori-specific antibodies in identifying current infection
Antibody score classification H. pylori infection (n(%)) Kappa P Current infection Past infection Classification of gray-scale score for four antibodies a 0.755 <0.001 Current infection (≥5.5) 178 (96.7) 22 (24.7) Past infection (<5.5) 6 (3.3) 67 (75.3) Classification of qualitative score for four antibodies b 0.641 <0.001 Current infection (≥2.0) 172 (93.5) 29 (32.6) Past infection (<2.0) 12 (6.5) 60 (67.4) Total 184 (100.0) 89 (100.0) Notes: a: the gray-scale score for four antibodies is the sum of the gray-scale values of the antibody bands for CagA, GroEL, FliD and gGT; b: the qualitative score for four antibodies is the sum of the qualitative values for the four antibodies. -
[1] Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2024, 74(3): 229-263. doi: 10.3322/caac.21834
[2] de Martel C, Georges D, Bray F, et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis[J]. Lancet Glob Health, 2020, 8(2): e180-e190. doi: 10.1016/S2214-109X(19)30488-7
[3] Guo Y, Zhang Y, Gerhard M, et al. Effect of Helicobacter pylori on gastrointestinal microbiota: a population-based study in Linqu, a high-risk area of gastric cancer[J]. Gut, 2020, 69(9): 1598-1607. doi: 10.1136/gutjnl-2019-319696
[4] Logan RP. Urea breath tests in the management of Helicobacter pylori infection[J]. Gut, 1998, 43 Suppl 1 (Suppl 1): S47-50.
[5] Patel SK, Pratap CB, Jain AK, et al. Diagnosis of Helicobacter pylori: what should be the gold standard?[J]. World J Gastroenterol, 2014, 20(36): 12847-12859. doi: 10.3748/wjg.v20.i36.12847
[6] Formichella L, Romberg L, Bolz C, et al. A novel line immunoassay based on recombinant virulence factors enables highly specific and sensitive serologic diagnosis of Helicobacter pylori infection[J]. Clin Vaccine Immunol, 2013, 20(11): 1703-1710. doi: 10.1128/CVI.00433-13
[7] Pan KF, Formichella L, Zhang L, et al. Helicobacter pylori antibody responses and evolution of precancerous gastric lesions in a Chinese population[J]. Int J Cancer, 2014, 134(9): 2118-2125. doi: 10.1002/ijc.28560
[8] Li ZX, Bronny K, Formichella L, et al. A multiserological line assay to potentially discriminate current from past Helicobacter pylori infection[J]. Clin Microbiol Infect, 2024, 30(1): 114-121. doi: 10.1016/j.cmi.2023.10.006
[9] Kadeerhan G, Gerhard M, Gao JJ, et al. Microbiota alteration at different stages in gastric lesion progression: a population-based study in Linqu, China[J]. Am J Cancer Res, 2021, 11(2): 561-575.
[10] Rugge M, Correa P, Dixon MF, et al. Gastric dysplasia: the Padova international classification[J]. Am J Surg Pathol, 2000, 24(2): 167-176. doi: 10.1097/00000478-200002000-00001
[11] Fang JY, Du YQ, Liu WZ, et al. Chinese consensus on chronic gastritis (2017, Shanghai)[J]. J Dig Dis, 2018, 19(4): 182-203. doi: 10.1111/1751-2980.12593
[12] Stolte M, Meining A. The updated Sydney system: classification and grading of gastritis as the basis of diagnosis and treatment[J]. Can J Gastroenterol, 2001, 15(9): 591-598. doi: 10.1155/2001/367832
[13] Coelho LGV, Trindade OR, Leao LA, et al. Prospective Study for Validation of a Single Protocol for the 13C-Urea Breath Test Using Two Different Devices in the Diagnosis of H. Pylori Infection[J]. Arq Gastroenterol, 2019, 56(2): 197-201. doi: 10.1590/s0004-2803.201900000-38
[14] Cai H, Ye F, Michel A, et al. Helicobacter pylori blood biomarker for gastric cancer risk in East Asia[J]. Int J Epidemiol, 2016, 45(3): 774-781. doi: 10.1093/ije/dyw078
[15] Gao L, Michel A, Weck MN, et al. Helicobacter pylori infection and gastric cancer risk: evaluation of 15 H. pylori proteins determined by novel multiplex serology[J]. Cancer Res, 2009, 69(15): 6164-6170. doi: 10.1158/0008-5472.CAN-09-0596
[16] Li ZX, Huang LL, Liu C, et al. Cut-off optimization for (13)C-urea breath test in a community-based trial by mathematic, histology and serology approach[J]. Sci Rep, 2017, 7(1): 2072. doi: 10.1038/s41598-017-02180-7
[17] Yamaoka Y. Helicobacter pylori typing as a tool for tracking human migration[J]. Clin Microbiol Infect, 2009, 15(9): 829-834. doi: 10.1111/j.1469-0691.2009.02967.x
[18] Mahmud MT, Ahmed F, Rana MJ, et al. Association of HLA gene polymorphisms with Helicobacter pylori related gastric cancer-a systematic review[J]. HLA, 2024, 103(2): e15394. doi: 10.1111/tan.15394
[19] Balendra V, Amoroso C, Galassi B, et al. High-Salt Diet Exacerbates H. pylori Infection and Increases Gastric Cancer Risks[J]. J Pers Med, 2023, 13(9): 1325.
[20] Formichella L, Romberg L, Meyer H, et al. Validation of a Novel Immunoline Assay for Patient Stratification according to Virulence of the Infecting Helicobacter pylori Strain and Eradication Status[J]. J Immunol Res, 2017, 2017: 8394593.
[21] Butt J, Blot WJ, Shrubsole MJ, et al. Performance of multiplex serology in discriminating active vs past Helicobacter pylori infection in a primarily African American population in the southeastern United States[J]. Helicobacter, 2020, 25(1): e12671. doi: 10.1111/hel.12671
[22] Carlsohn E, Nystrom J, Bolin I, et al. HpaA is essential for Helicobacter pylori colonization in mice. Infect Immun[J], 2006, 74 (2): 920-926.
[23] Khalifeh Gholi M, Kalali B, Formichella L, et al. Helicobacter pylori FliD protein is a highly sensitive and specific marker for serologic diagnosis of H. pylori infection[J]. Int J Med Microbiol, 2013, 303(8): 618-623. doi: 10.1016/j.ijmm.2013.08.005
[24] Bergonzelli GE, Granato D, Pridmore RD, et al. GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori[J]. Infect Immun, 2006, 74(1): 425-434. doi: 10.1128/IAI.74.1.425-434.2006



 下载:
下载: