Clinical Observation of Pirfenidone in Prevention and Treatment of Radiation–Induced Lung Injury in Esophageal Cancer
-
摘要:目的
观察吡非尼酮防治食管癌放射性肺损伤的有效性和安全性。
方法回顾性分析103例食管癌患者资料,其中联合组53例行同步放化疗联合吡非尼酮治疗、对照组50例仅行同步放化疗;随访3年,观察患者疗效、不良反应和生存情况,以及放疗后1年内放射性肺损伤发生率、肺功能和肺损伤细胞因子水平变化。
结果联合组患者的有效率(86.8%)、2年生存率(84.9%)、3年生存率(71.7%)均高于对照组(70.0%、68.0%和52.0%)(P<0.05),联合组1、2年和3年无病生存率分别为86.8%、67.9%和47.2%,均高于对照组的62.0%、46.0%和28.0%(P<0.05);放疗后3个月放射性肺炎、放疗后6个月和1年肺纤维化发生率分别为22.6%、13.2%和14.0%,均低于同期对照组的42.0%、30.0%和31.8%(P<0.05);放疗结束时、放疗后3个月和6个月及1年肺功能指标水平均高于同期对照组,而肺损伤相关细胞因子水平均低于同期对照组(P<0.05);除了皮疹发生率联合组高于对照组(18.9% vs. 2.0%,P<0.05),其余不良反应发生率及严重反应率两组间差异均无统计学意义(P>0.05)。
结论吡非尼酮不仅可以有效降低食管癌同步放化疗患者的放射性肺损伤,改善肺功能,还有助于提高肿瘤控制率和患者生存率,且安全性良好。
Abstract:ObjectiveTo observe the effectiveness and safety of pirfenidone in the prevention and treatment of radiation-induced lung injury in esophageal cancer.
MethodsWe retrospectively analyzed the data of 103 patients with esophageal cancer, of whom 53 in the combined group were treated with simultaneous chemoradiotherapy combined with pirfenidone and 50 in the control group were treated with simultaneous chemoradiotherapy only. The patients were followed up for three years to observe the treatment effects, adverse effects, and survival, as well as the incidence of radiation-induced lung injury, lung function, and changes in lung injury cytokine levels within one year after radiotherapy.
ResultsTreatment efficiency in the combined group was higher than that in the control group (86.8% vs. 70.0%, P<0.05). The two- and three-year survival rates in the combined group were 84.9% and 71.7%, respectively, which were higher than those (68.0% and 52.0%) in the control group (P<0.05). The one-, two-, and three-year disease-free survival rates in the combined group were 86.8%, 67.9%, and 47.2%, respectively, which were higher than those (62.0%, 46.0%, and 28.0%) in the control group (P<0.05). The incidence rates of radiation pneumonitis at three months, pulmonary fibrosis at six months, and one year after radiotherapy in the combined group were 22.6%, 13.2%, and 14.0%, respectively, which were lower than those (42.0%, 30.0%, and 31.8%) in the control group at the same time (P<0.05). At the end of radiotherapy and at three months, six months and one year after radiotherapy, the combined group showed higher levels of lung function indicators but lower levels of lung injury-related cytokines than the control group (P<0.05). The incidence of rash in the combined group was 18.9%, which was higher than that (2.0%) in the control group (P<0.05). However, no statistically significant difference in the incidence and severity of other adverse reactions was found between the two groups (P>0.05).
ConclusionPirfenidone not only effectively reduces radiation-induced lung injury and improves lung function in esophageal cancer patients undergoing simultaneous chemoradiotherapy, but also helps improve tumor control rates and patient survival with a good safety profile.
-
Key words:
- Radiation-induced lung injury /
- Esophageal cancer /
- Pirfenidone /
- Efficacy /
- Safety
-
0 引言
食管癌(Esophageal cancer, EC)是我国常见的恶性肿瘤,放射治疗是其非手术治疗的主要方法。但由于肺组织对射线敏感,当食管癌放疗总剂量大于40 Gy时就会造成不同程度的放射性肺损伤(Radiation-induced lung injury, RILI),包括急性放射性肺炎(Radiation pneumonitis, RP)和慢性放射性肺纤维化(Radiation-induced lung fibrosis, RILF)。RILI的发展是一个渐进性过程,RP通常持续时间短,多发生于放疗后6个月内,病理表现为肺泡壁充血、水肿、炎性细胞渗出和肺泡膜破坏;RILF多发生于放疗后6~12个月,以不可逆的肺泡壁或间质纤维化为特征[1]。RILI不仅严重影响患者肺功能和生活质量,还限制了放疗最大剂量,继而影响肿瘤疗效[2]。目前主要给予糖皮质激素,辅以吸氧、止咳平喘和抗感染等对症治疗,尚无特效防治方法[1]。研究表明RILI的发生是多种受损细胞相互作用并受相应细胞因子调控的一系列病理生理反应[3]。吡非尼酮(Pirfenidone, PFD)是一种多效吡啶化合物,具有广谱的抗炎、抗纤维化和抗氧化作用[4],可以降低多种炎性反应因子和纤维化因子的释放,减轻炎性反应、组织纤维化和重塑,在防治RILI方面已受到关注[5-6]。然而,关于PFD在RILI中的作用研究目前仍处于探索阶段。因此,我们观察53例采用PFD防治食管癌患者同步放化疗RILI的临床疗效和安全性,旨在为临床用药提供参考。
1 资料与方法
1.1 临床资料
回顾性分析2016年9月至2021年6月厦门大学附属成功医院收治的行同步放化疗食管癌患者的临床资料。本研究经本院伦理委员会批准(编号73JYY2024155670)。纳入标准:(1)经胃镜活检病理学初次确诊为食管鳞癌;(2)不宜或不愿手术治疗;(3)年龄20~75岁;(4)卡氏评分(Karnofsky performance score, KPS)≥60分;(5)无食管瘘或重度食管梗阻;(6)患者知情同意。剔除标准:(1)有远处转移;(2)胸部放疗或手术史;(3)既往有矽肺、间质性肺纤维化;(4)合并心肝肾等其他系统严重疾病;(5)联合靶向或免疫治疗等其他抗肿瘤治疗;(6)临床资料不完整者。患者筛选流程见图1。本研究最终共纳入103例患者,其中53例行同步放化疗联合PFD治疗作为联合组,50例单纯行同步放化疗作为对照组。两组患者一般资料均衡可比(P>0.05),见表1。
表 1 食管癌联合组和对照组患者临床基线特征比较Table 1 Comparison of clinical baseline characteristics between combined and control groups of esophageal cancer patientsCharacteristics Combined group
(n=53)Control group
(n=50)t/χ2 P Median age
(years)64.17 ± 21.38 63.21 ± 22.47 0.222 0.825 Gender 0.278 0.598 Male 28 29 Female 25 21 KPS score 0.734 0.392 60-70 21 24 ≥80 32 26 Tumor location 0.279 0.870 Cervical and
upper10 8 Middle 26 27 Lower and
abdominal17 15 Tumor length (cm) 0.018 0.893 <5 9 8 ≥5 44 42 TNM stage 0.737 0.692 Ⅱ 5 3 Ⅲ 29 31 Ⅳa 19 16 Average dose
(cGy)In left lung 1251.62 ± 41.331247.48 ± 41.510.4525 0.6521 In right lung 1202.27 ± 40.781198.65 ± 40.630.4027 0.6883 1.2 治疗方法
1.2.1 同步放化疗
两组均给予食管癌根治性放疗,采用东芝Alexion TSX-032A CT模拟定位,3 mm薄层增强扫描,勾画靶区,肿瘤靶区(Gross target volume, GTV)为食管病灶和局部肿大淋巴结(直径≥1 cm);临床靶区(Clinical target volume, CTV)为GTV向四周均放0.8 cm、上下均放3~5 cm,并包括转移率较高的淋巴引流区;计划靶区(Planning target volume, PTV)为CTV三维方向外放0.5~1.0 cm;采用医科达Synergy System直线加速器,调强放疗(Intensity-modulated radiation therapy, IMRT)技术,处方剂量PTV-GTV 60 Gy/33 f、PTV-CTV 50.4 Gy/28 f,覆盖95% PTV;危及器官剂量限制双肺V20≤35%、V30≤20%、平均剂量≤15 Gy,心脏V40≤40%、平均剂量≤25 Gy,脊髓最大剂量≤45 Gy/6周。两组放疗期间均同步化疗2个周期,放疗结束后继续巩固2个周期,共4个周期。化疗采用FP方案(5-氟尿嘧啶750 mg/m2持续24 h静脉泵入 d1~4;顺铂25 mg/m2静脉滴注d1~3,28天为1周期)。
1.2.2 联合治疗
联合组在同步放化疗基础上加用PFD治疗(每粒100 mg,北京康蒂尼药业,国药准字H20133376),从放疗开始前3天起服药,每次400 mg,3次/天,餐后30分钟服用,连用12周,若出现RILI则继续服用12周;对照组仅行同步放化疗。两组除出现放射性食管炎需短时间口服小剂量地塞米松配置液处理外,均未联用尼达尼布、氨磷汀、沙利度胺、中药制剂等其他可能具有防治纤维化作用的药物。
1.3 评价指标
两组均于治疗前及治疗期间每周查血生化、血常规,按美国国家癌症研究所不良事件常用术语(National Cancer Institute Common Terminology Criteria for Adverse Events, NCI-CTC AE)5.0版标准[7],记录治疗相关不良反应情况。按修改后实体瘤疗效评价标准(Modified response evaluation criteria in solid tumors, mRECIST)[8]比较两组放疗后3个月疗效。疗效分为完全缓解(Complete response, CR)、部分缓解(Partial response, PR)、稳定(Stable disease, SD)和进展(Progressive disease, PD),以CR + PR计算总有效率(Objective response rate, ORR)。按NCI-CTC AE 5.0版标准评价RILI,比较两组放疗结束时和放疗后3个月RP发生率、放疗后6个月和1年RILF发生率。采用德国耶格Master-Screen肺功能仪测定患者一氧化碳弥散量(Diffusion capacity for carbon monoxide, DLCO)和用力肺活量(Forced vital capacity, FVC)。采用酶联免疫吸附试验法(Enzyme linked immunosorbent assay, ELISA)测定患者空腹外周血血清白细胞介素-6(Interleukin-6, IL-6)、肿瘤坏死因子-α(Tumor necrosis factor-α, TNF-α)、转化生长因子-β1(Transforming growth factor-β1, TGF-β1)和血管内皮生长因子(Vascular endothelial growth factor, VEGF),并比较两组放疗后1年内上述肺功能指标和肺损伤相关细胞因子表达水平的变化。
1.4 随访
以返院复查或电话方式随访3年,随访截至2024年5月31日。分别在放疗前、放疗结束时、放疗后3个月和6个月,继而每6个月复诊评价1次。自治疗之日计算两组患者的1、2、3年生存率(Survival rate)和无病生存率(Disease-free survival, DFS),随访终点为死亡、失访或生存3年。
1.5 统计学方法
应用SPSS 23.0统计软件包处理数据,计数资料以%表示,采用χ2检验;计量资料以${{\bar x}} \pm {{s}} $表示,采用t检验;Kaplan-Meier法绘制生存曲线,生存率差异采用Log rank检验;P<0.05为差异有统计学意义。
2 结果
2.1 肿瘤治疗疗效
两组患者均按计划完成放化疗,其中联合组PFD连服12周完成率为100%。联合组ORR(86.8%)明显高于对照组(P<0.05),见表2。
表 2 食管癌联合组和对照组疗效比较 (n)Table 2 Comparison of efficacy between combined and control groups of esophageal cancer patients (n)Groups N CR PR SD PD ORR (%) Combined group 53 21 25 4 3 46 (86.8)* Control group 50 15 20 9 6 35 (70.0) Note: *: χ2=4.319, P=0.038, compared with the control group. 2.2 生存情况
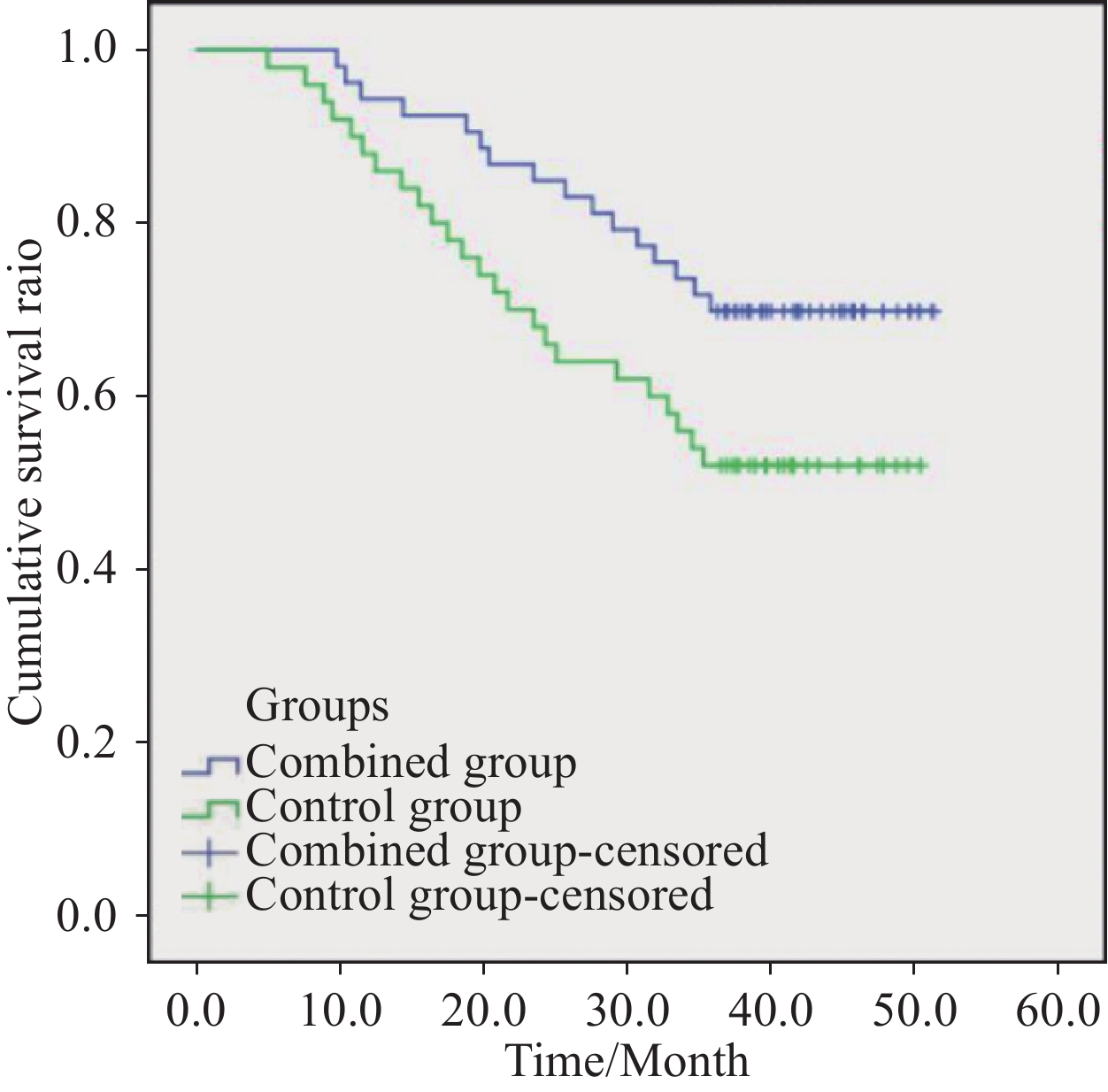
联合组中位随访时间为39.5(9.8~51.3)个月,对照组中位随访时间为36.7(5.0~50.4)个月。联合组1年生存率为94.3%,与对照组比较,差异无统计学意义(P>0.05),但2年和3年生存率分别为84.9%和71.7%,均明显高于对照组(均P<0.05);1、2和3年DFS分别为86.8%、67.9%和47.2%,均明显高于对照组(均P<0.05);Kaplan-Meier生存曲线分析χ2=4.015,P=0.045,见表3、图2。
表 3 联合组和对照组食管癌患者生存率及无病生存率比较 (n (%))Table 3 Comparison of survival and disease-free survival rates between combined and control groups of esophageal cancer patients (n(%))Groups N Six-month
disease-free
survivalSix-month
survival rateOne-year
disease-free
survivalOne-year
survival rateTwo-year
disease-free
survivalTwo-year
survival rateThree-year
disease-free
survivalThree-year
survival rateCombined group 53 53 (100.0) 53 (100.0) 46 (86.8) 50 (94.3) 36 (67.9) 45 (84.9) 25 (47.2) 38 (71.7) Control group 50 48 (96.0) 50 (100.0) 31 (62.0) 44 (88.0) 23 (46.0) 34 (68.0) 14 (28.0) 26 (52.0) χ2 0.529 8.380 0.624 5.054 4.114 4.019 4.243 P 0.467 0.004 0.430 0.025 0.043 0.045 0.039 2.3 肺损伤发生率
联合组53例患者放疗结束时RP发生率(18.9%)与对照组差异无统计学意义(P>0.05);但放疗后3个月RP发生率(22.6%)、放疗后6个月RILF发生率(13.2%)均明显低于同期对照组(均P<0.05);放疗后1年联合组中50例存活患者RILF发生率(14.0%)亦明显低于对照组44例存活患者的发生率(P<0.05),见表4、5。
表 4 联合组和对照组食管癌患者放射性肺炎发生率比较 (n)Table 4 Comparison of incidence of radiation pneumonitis between combined and control groups of esophageal cancer patients (n)Groups N Grade and incidence at the end of IMRT Grade and incidence at three months after IMRT Grade 1 Grade 2 Grade 3 Grade 4 Incidence (%) Grade 1 Grade 2 Grade 3 Grade 4 Incidence (%) Combined group 53 4 4 2 0 10 (18.9) 5 4 2 1 12 (22.6) Control group 50 4 6 3 1 14 (28.0) 7 7 5 2 21 (42.0) χ2 1.201 4.428 P 0.273 0.035 表 5 联合组和对照组食管癌患者放射性肺纤维化发生率比较 (n)Table 5 Comparison of incidence of radiation-induced pulmonary fibrosis between combined and control groups of esophageal cancer patients (n)Groups N Grade and incidence at six months after IMRT N Grade and incidence at one year after IMRT Grade 1 Grade 2 Grade 3 Grade 4 Incidence (%) Grade 1 Grade 2 Grade 3 Grade 4 Incidence (%) Combined group 53 3 3 1 0 7 (13.2) 50 4 2 1 0 7 (14.0) Control group 50 8 5 2 0 15 (30.0) 44 7 3 3 1 14 (31.8) χ2 4.319 4.283 P 0.038 0.038 2.4 肺功能水平
放疗前两组103例患者肺功能指标DLCO、FVC水平差异均无统计学意义(均P>0.05);放疗结束时和放疗后3、6个月两组103例患者以及放疗后1年两组94例存活患者DLCO和FVC水平均低于同组放疗前,且放疗结束时为最低值,但联合组水平均明显高于同期对照组(均P<0.05),见表6。
表 6 联合组和对照组食管癌患者放疗前后肺功能水平比较 (${{\bar x}} \pm {{s}} $)Table 6 Comparison of lung function levels before and after radiotherapy in combined and control groups of esophageal cancer patients (${{\bar x}} \pm {{s}} $)Groups Projects Pre-IMRT End of IMRT Time after IMRT Three months Six months One year Combined group (N=53) DLCO (%) 87.58±3.22 73.47±4.16 77.95±5.14 82.62±3.55 84.71±4.76 (n=50) FVC (L) 4.19±0.64 2.61±0.68 3.29±0.59 3.65±0.32 3.84±0.58 (n=50) Control group (N=50) DLCO (%) 88.61±3.26 69.54±3.28 73.83±4.63 78.87±2.59 80.53±3.64 (n=44) FVC (L) 4.25±0.62 2.03±0.35 2.57±0.67 2.85±0.37 3.11±0.43 (n=44) DLCO between groups t/P 1.6128 /0.1099 5.3032 /<0.0001 4.2656 /<0.0001 6.0939 /<0.0001 4.7322 /<0.0001 FVC between groups t/P 0.4828 /0.6303 5.3937 /<0.0001 5.7962 /<0.0001 11.7563 /<0.0001 6.8527 /<0.0001 Note: DLCO: diffusion capacity for carbon monoxide. 2.5 细胞因子水平
放疗前两组103例患者促炎细胞因子IL-6、TNF-α及促纤维化细胞因子TGF-β1、VEGF表达水平差异均无统计学意义(均P>0.05);放疗结束时两组上述细胞因子水平均出现先升高至峰值,而后随着时间推移逐渐回落;放疗后1年时两组94例存活患者上述细胞因子水平均继续下调;但放疗后联合组水平均低于同期对照组(均P<0.05),见表7、8。
表 7 联合组和对照组食管癌患者放疗前后促炎细胞因子水平比较 (pg/ml, ${{\bar x}} \pm {{s}} $)Table 7 Comparison of proinflammatory cytokine levels before and after radiotherapy in combined and control groups of esophageal cancer patients (pg/ml, ${{\bar x}} \pm {{s}} $)Groups Projects Pre-IMRT End of IMRT Time after IMRT Three months Six months One year Combined group (N=53) IL-6 25.76±7.63 85.52±12.13 51.65±11.57 27.16±9.41 26.25±7.13 (n=50) TNF-α 120.53±13.67 225.86±15.34 162.68±14.17 125.54±13.24 122.17±10.32 (n=50) Control group (N=50) IL-6 24.68±7.59 108.23±13.25 84.43±12.24 43.22±9.83 35.69±7.57 (n=44) TNF-α 117.64±13.78 247.16±16.34 188.75±15.38 140.56±14.53 134.24±11.41 (n=44) IL-6 between groups t/P 0.7198 /0.4733 9.0804 /<0.0001 13.9725 /<0.0001 8.4714 /<0.0001 6.2228 /<0.0001 TNF-α between groups t/P 1.0682 /0.2880 6.8237 /<0.0001 8.9533 /<0.0001 5.4886 /<0.0001 5.3852 /<0.0001 表 8 联合组和对照组食管癌患者放疗前后促纤维化细胞因子水平比较 (ng/ml, ${{\bar x}} \pm {{s}} $)Table 8 Comparison of profibrotic cytokine levels before and after radiotherapy in combined and control groups of esophageal cancer patients (ng/ml, ${{\bar x}} \pm {{s}} $)Groups Projects Pre-IMRT End of IMRT Time after IMRT Three months Six months One year Combined group (N=53) TGF-β1 23.16±3.35 59.53±5.87 50.25±5.24 31.27±4.43 25.15±3.17 (n=50) VEGF 83.44±14.86 156.57±23.38 127.91±17.65 96.93±15.54 87.13±11.63 (n=50) Control group (N=50) TGF-β1 22.09±3.71 76.46±8.14 62.52±6.55 42.11±5.08 34.24±4.16 (n=44) VEGF 82.16±13.54 178.44±25.71 144.73±18.39 116.87±16.63 98.26±18.35 (n=44) TGF-β1 between groups t/P 1.5378 /0.1272 12.1583 /<0.0001 10.5274 /<0.0001 11.5598 /<0.0001 11.9950 /<0.0001 VEGF between groups t/P 0.4561 /0.6493 4.5208 /<0.0001 4.7364 /<0.0001 6.2907 /<0.0001 3.5549 /0.0003 2.6 不良反应情况
两组患者不良反应中除了Ⅳ度骨髓抑制外,Ⅰ~Ⅱ度反应多见,Ⅲ度反应少见,均经对症处理后缓解,未出现不可耐受、影响放化疗或需减停PFD的严重不良反应;联合组皮疹发生率(18.9%)高于对照组(P<0.05);其余不良反应发生率及严重反应率组间差异均无统计学意义(均P>0.05),见表9。
表 9 联合组和对照组食管癌患者不良反应比较 (n)Table 9 Comparison of adverse reactions between combined and control groups of esophageal cancer patients (n)Adverse events Combined group (n=53) Control group (n=50) χ2 P Ⅰ Ⅱ Ⅲ Ⅳ Incidence (%) Ⅰ Ⅱ Ⅲ Ⅳ Incidence (%) Nausea and vomiting 13 9 2 0 45.3 14 4 3 0 42.0 0.113 0.737 Diminished appetite 17 12 6 0 66.0 17 8 5 0 60.0 0.403 0.526 Bloating 8 3 0 0 20.8 3 4 1 0 16.0 0.378 0.534 Constipation 7 2 0 0 17.0 6 4 0 0 20.0 0.156 0.693 Esophagitis 17 9 2 0 52.8 20 7 3 0 60.0 0.538 0.463 Fatigue 22 5 2 0 54.7 21 6 1 0 56.0 0.017 0.896 Myelosuppression 12 8 11 9 75.5 8 10 12 7 74.0 0.030 0.864 Rash 7 3 0 0 18.9 1 0 0 0 2.0 7.674 0.006 Peripheral neuritis 4 1 0 0 9.4 2 1 0 0 6.0 0.080 0.778 Hepatic impairment 3 1 0 0 7.6 3 2 0 0 10.0 0.008 0.927 3 讨论
RILI是食管癌患者放疗常见的一种严重并发症。近年来,随着放疗技术的进步和IMRT的临床应用,尽管食管癌放疗效果得到了提升,减少了对肿瘤周围正常组织的损伤,但RILI发生率并未发生明显改变[9]。RILI依然是严重制约食管癌近期疗效、长期存活和生活质量的重要因素。因此,防治RILI是迫切需要解决的临床难题。
随着对RILI的深入研究,目前认为当电离辐射作用于肺组织时,可直接导致肺泡上皮细胞和血管内皮细胞损伤,继而释放炎性反应介质,诱导中性粒细胞、巨噬细胞、淋巴细胞等炎性反应细胞在损伤部位聚集[10],产生多种细胞因子和趋化因子并激活其信号通路,启动机体损伤反应机制,放大炎性反应,更多的炎性反应细胞被募集,促进成纤维细胞的聚集和增殖,释放胶原纤维,刺激细胞外基质(Extracellular matrix, ECM)的合成并抑制其降解,导致ECM过度沉积,进一步引起细胞因子释放,从而形成RILF[2,11]。同时,电离辐射使肺组织内的水分子发生离子化反应,产生大量活性氧自由基,引起DNA、蛋白质及脂质的氧化损伤,并诱导一系列单核细胞的炎症反应和趋化性[1],最终导致RILI,进而产生干咳、气短、胸痛、发热等临床症状,甚至引起呼吸衰竭危及生命。
PFD是一种口服的小分子吡啶酮类药物。基础研究显示它可以减少炎性反应细胞和趋化因子的释放,以及通过抑制TGF-β1/Smad信号通路减少IL-6、TNF-α的分泌,从而发挥抗炎作用[12];可以下调纤维化过程中的TGF-β1、VEGF过表达,抑制成纤维细胞活性,减少胶原蛋白和ECM合成,从而发挥抗纤维化作用[13];还可以通过清除氧自由基,减少氧化应激反应对肺泡的损伤,从而发挥抗氧化作用[14];不仅可以改善急性肺损伤导致的肺纤维化[4],而且可以抑制心、肝、肾等多种器官纤维化并改善其功能[15-17],目前已获批用于特发型肺纤维化(Idiopathic pulmonary fibrosis, IPF)的临床治疗[18]。
由于RILI的病理机制和临床表现与IPF有许多相似之处[5],因此推测PFD可能是防治RILI的有效药物。Ying等[19]研究显示PFD可以通过抑制肺内趋化因子分泌、M2巨噬细胞浸润和TGF-β1/Smad信号通路激活,降低肺部胶原蛋白沉积和纤维化,从而改善X线照射后小鼠的RILF。Moustafa等[20]研究也显示小鼠单剂量14.5 Gy全胸照射16周后持续灌服PFD 8周,可以抑制其RILF发展后期的肺重构,具有抗纤维化活性。Mummudi等[21]在一项单臂Ⅱ期临床研究中显示PFD可以显著降低肺癌同步放化疗患者的RP发生率并改善肺功能。本研究采用PFD防治食管癌患者同步放化疗RILI,结果显示联合组放疗后3个月RP发生率、放疗后6个月和1年RILF发生率分别为22.6%、13.2%和14.0%,均明显低于同期对照组(均P<0.05);虽然放疗后1年内DLCO和FVC水平与对照组一样均低于放疗前,经历了放疗结束时降至最低水平,而后逐渐回升的波动过程,但联合组水平均明显高于同期对照组(均P<0.05),这与Chen等[6]报道的一项随机对照Ⅱ期临床研究结果相似,相比对照安慰剂组,PFD可以显著降低局部晚期食管癌同步放化疗患者≥2级RILI的发生率(10% vs. 25%, P<0.05)。表明联合组在同步放化疗的基础上加用PFD治疗,可以有效降低RILI的发生率,抑制肺纤维化,改善肺通气量和弥散功能,对肺功能改善或延缓肺功能下降有更积极的作用。
炎性反应是造成RILI的关键因素,IL-6和TNF-α均为体内启动炎性反应的主要细胞因子,可以诱导机体免疫应答,活化相关炎性反应细胞,促进胶原合成,并共同参与肺部炎性反应甚至纤维化的病理过程[3]。而纤维化是放射损伤的一种修复过程,其中TGF-β1介导的信号通路在纤维化过程中起着核心调控作用,其机制包括:募集并活化炎性反应细胞,促进炎性细胞因子分泌;诱导成纤维细胞增殖;促进ECM沉积;并诱导VEGF表达,促进内皮细胞增殖和肺微血管增生。这些级联反应最终导致慢性炎性反应的持续放大和组织纤维化的进行性加重[22]。本研究结果显示,两组患者IL-6、TNF-α及TGF-β1、VEGF表达水平均表现出放疗结束时先升高至峰值,而后逐渐回落,至放疗后1年时降至近放疗前水平的波动过程,表明因射线造成肺损伤后引起炎性反应,导致细胞因子合成迅速增加,而放疗后随时间延长,因机体免疫系统的自我调控和炎性反应的逐渐消退以及射线致肿瘤组织被破坏,进而使细胞因子生成减少而呈回落趋势。但联合组细胞因子的整体水平一直低于同期对照组(均P<0.05),表明联用PFD治疗可以进一步降低患者炎性反应细胞浸润和细胞因子释放,减轻炎性反应及其引起的浸润性损伤,抑制成纤维细胞活性、基质蛋白合成和ECM沉积,从而减轻RILI[4]。
炎性反应和纤维化过程是肿瘤发生发展的基础[23]。炎性反应可以形成支持肿瘤微环境(Tumor microenvironment, TME),从而促进肿瘤发展[24]。TME中的多种炎性反应细胞及IL-6、TNF-α在介导慢性炎性反应以及促进肿瘤细胞增殖、血管生成、转移和化疗耐药中发挥作用[25]。纤维化过程中的TGF-β1可以诱导肿瘤ECM的致瘤性改变及上皮间质转化,进而调节TME,促进肿瘤进展[26]。而VEGF可以介导肿瘤血管生成及调控肿瘤免疫微环境,促进食管癌的侵袭转移并影响预后[27]。目前已有多项研究发现PFD除了具有抗纤维化特性,还具有抗肿瘤作用。Wang等[28]研究显示PFD可以通过抑制TGF-β表达来阻止肿瘤细胞的EMT和髓系来源抑制性细胞的募集来塑造免疫抑制性TME,从而阻碍小鼠肾癌的进展。Miranda-Roblero等[29]研究显示PFD可以通过调节抗纤维化、抗氧化和抗增殖过程以及表观遗传标记来逆转整体DNA低甲基化,从而阻止大鼠肝癌的发展。Luo等[30]研究显示PFD可以通过抑制TGF-β诱导的Smad信号通路的激活和EMT诱导转录因子以及间充质基因的表达,从而抑制小鼠乳腺癌细胞的增殖和迁移。Zhang等[31]研究也显示PFD可以通过靶向TGF-β1抑制非小细胞肺癌EMT过程中的糖酵解,从而发挥抗肿瘤作用以及增强化疗敏感性。然而PFD对食管癌的影响尚未见相关报道。本研究结果初次发现联合组患者ORR率为86.8%,明显高于对照组,而且2和3年生存率分别为84.9%和71.7%,均明显高于对照组(均P<0.05);1、2和3年DFS分别为86.8%、67.9%和47.2%,也均明显高于对照组(均P<0.05),表明同步放化疗联合PFD治疗可以通过进一步降低患者血清肿瘤因子水平来改变TME和机体免疫状态,从而提高抗肿瘤疗效并延长患者生存期。但由于本研究属回顾性研究,样本量较少,下一步将继续扩大样本量,进行前瞻性随机对照研究。
我们进一步对PFD的药物安全性进行分析,结果显示两组患者均未出现不可耐受或影响放化疗的严重不良反应;联合组除了PFD特有的一过性Ⅰ~Ⅱ度皮疹反应发生率(18.9%)高于对照组(P<0.05),其余不良反应发生原因可能主要与放化疗相关,其发生率及严重反应率与对照组差异均无统计学意义(均P>0.05),表明PFD治疗具有较高的安全性。
综上所述,在食管癌患者同步放化疗时联合PFD治疗,临床效果满意,既能有效降低RILI发生率、改善患者肺功能,又有助于提高肿瘤控制效果、延长患者生存期,且安全性良好,值得临床进一步深入探讨和总结。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:邱国钦:项目申请、选题设计、资料与数据分析、论文撰写和修改陈肖珏、许英艺、陈金平:资料与数据收集、统计分析 -
表 1 食管癌联合组和对照组患者临床基线特征比较
Table 1 Comparison of clinical baseline characteristics between combined and control groups of esophageal cancer patients
Characteristics Combined group
(n=53)Control group
(n=50)t/χ2 P Median age
(years)64.17 ± 21.38 63.21 ± 22.47 0.222 0.825 Gender 0.278 0.598 Male 28 29 Female 25 21 KPS score 0.734 0.392 60-70 21 24 ≥80 32 26 Tumor location 0.279 0.870 Cervical and
upper10 8 Middle 26 27 Lower and
abdominal17 15 Tumor length (cm) 0.018 0.893 <5 9 8 ≥5 44 42 TNM stage 0.737 0.692 Ⅱ 5 3 Ⅲ 29 31 Ⅳa 19 16 Average dose
(cGy)In left lung 1251.62 ± 41.331247.48 ± 41.510.4525 0.6521 In right lung 1202.27 ± 40.781198.65 ± 40.630.4027 0.6883 表 2 食管癌联合组和对照组疗效比较 (n)
Table 2 Comparison of efficacy between combined and control groups of esophageal cancer patients (n)
Groups N CR PR SD PD ORR (%) Combined group 53 21 25 4 3 46 (86.8)* Control group 50 15 20 9 6 35 (70.0) Note: *: χ2=4.319, P=0.038, compared with the control group. 表 3 联合组和对照组食管癌患者生存率及无病生存率比较 (n (%))
Table 3 Comparison of survival and disease-free survival rates between combined and control groups of esophageal cancer patients (n(%))
Groups N Six-month
disease-free
survivalSix-month
survival rateOne-year
disease-free
survivalOne-year
survival rateTwo-year
disease-free
survivalTwo-year
survival rateThree-year
disease-free
survivalThree-year
survival rateCombined group 53 53 (100.0) 53 (100.0) 46 (86.8) 50 (94.3) 36 (67.9) 45 (84.9) 25 (47.2) 38 (71.7) Control group 50 48 (96.0) 50 (100.0) 31 (62.0) 44 (88.0) 23 (46.0) 34 (68.0) 14 (28.0) 26 (52.0) χ2 0.529 8.380 0.624 5.054 4.114 4.019 4.243 P 0.467 0.004 0.430 0.025 0.043 0.045 0.039 表 4 联合组和对照组食管癌患者放射性肺炎发生率比较 (n)
Table 4 Comparison of incidence of radiation pneumonitis between combined and control groups of esophageal cancer patients (n)
Groups N Grade and incidence at the end of IMRT Grade and incidence at three months after IMRT Grade 1 Grade 2 Grade 3 Grade 4 Incidence (%) Grade 1 Grade 2 Grade 3 Grade 4 Incidence (%) Combined group 53 4 4 2 0 10 (18.9) 5 4 2 1 12 (22.6) Control group 50 4 6 3 1 14 (28.0) 7 7 5 2 21 (42.0) χ2 1.201 4.428 P 0.273 0.035 表 5 联合组和对照组食管癌患者放射性肺纤维化发生率比较 (n)
Table 5 Comparison of incidence of radiation-induced pulmonary fibrosis between combined and control groups of esophageal cancer patients (n)
Groups N Grade and incidence at six months after IMRT N Grade and incidence at one year after IMRT Grade 1 Grade 2 Grade 3 Grade 4 Incidence (%) Grade 1 Grade 2 Grade 3 Grade 4 Incidence (%) Combined group 53 3 3 1 0 7 (13.2) 50 4 2 1 0 7 (14.0) Control group 50 8 5 2 0 15 (30.0) 44 7 3 3 1 14 (31.8) χ2 4.319 4.283 P 0.038 0.038 表 6 联合组和对照组食管癌患者放疗前后肺功能水平比较 (${{\bar x}} \pm {{s}} $)
Table 6 Comparison of lung function levels before and after radiotherapy in combined and control groups of esophageal cancer patients (${{\bar x}} \pm {{s}} $)
Groups Projects Pre-IMRT End of IMRT Time after IMRT Three months Six months One year Combined group (N=53) DLCO (%) 87.58±3.22 73.47±4.16 77.95±5.14 82.62±3.55 84.71±4.76 (n=50) FVC (L) 4.19±0.64 2.61±0.68 3.29±0.59 3.65±0.32 3.84±0.58 (n=50) Control group (N=50) DLCO (%) 88.61±3.26 69.54±3.28 73.83±4.63 78.87±2.59 80.53±3.64 (n=44) FVC (L) 4.25±0.62 2.03±0.35 2.57±0.67 2.85±0.37 3.11±0.43 (n=44) DLCO between groups t/P 1.6128 /0.1099 5.3032 /<0.0001 4.2656 /<0.0001 6.0939 /<0.0001 4.7322 /<0.0001 FVC between groups t/P 0.4828 /0.6303 5.3937 /<0.0001 5.7962 /<0.0001 11.7563 /<0.0001 6.8527 /<0.0001 Note: DLCO: diffusion capacity for carbon monoxide. 表 7 联合组和对照组食管癌患者放疗前后促炎细胞因子水平比较 (pg/ml, ${{\bar x}} \pm {{s}} $)
Table 7 Comparison of proinflammatory cytokine levels before and after radiotherapy in combined and control groups of esophageal cancer patients (pg/ml, ${{\bar x}} \pm {{s}} $)
Groups Projects Pre-IMRT End of IMRT Time after IMRT Three months Six months One year Combined group (N=53) IL-6 25.76±7.63 85.52±12.13 51.65±11.57 27.16±9.41 26.25±7.13 (n=50) TNF-α 120.53±13.67 225.86±15.34 162.68±14.17 125.54±13.24 122.17±10.32 (n=50) Control group (N=50) IL-6 24.68±7.59 108.23±13.25 84.43±12.24 43.22±9.83 35.69±7.57 (n=44) TNF-α 117.64±13.78 247.16±16.34 188.75±15.38 140.56±14.53 134.24±11.41 (n=44) IL-6 between groups t/P 0.7198 /0.4733 9.0804 /<0.0001 13.9725 /<0.0001 8.4714 /<0.0001 6.2228 /<0.0001 TNF-α between groups t/P 1.0682 /0.2880 6.8237 /<0.0001 8.9533 /<0.0001 5.4886 /<0.0001 5.3852 /<0.0001 表 8 联合组和对照组食管癌患者放疗前后促纤维化细胞因子水平比较 (ng/ml, ${{\bar x}} \pm {{s}} $)
Table 8 Comparison of profibrotic cytokine levels before and after radiotherapy in combined and control groups of esophageal cancer patients (ng/ml, ${{\bar x}} \pm {{s}} $)
Groups Projects Pre-IMRT End of IMRT Time after IMRT Three months Six months One year Combined group (N=53) TGF-β1 23.16±3.35 59.53±5.87 50.25±5.24 31.27±4.43 25.15±3.17 (n=50) VEGF 83.44±14.86 156.57±23.38 127.91±17.65 96.93±15.54 87.13±11.63 (n=50) Control group (N=50) TGF-β1 22.09±3.71 76.46±8.14 62.52±6.55 42.11±5.08 34.24±4.16 (n=44) VEGF 82.16±13.54 178.44±25.71 144.73±18.39 116.87±16.63 98.26±18.35 (n=44) TGF-β1 between groups t/P 1.5378 /0.1272 12.1583 /<0.0001 10.5274 /<0.0001 11.5598 /<0.0001 11.9950 /<0.0001 VEGF between groups t/P 0.4561 /0.6493 4.5208 /<0.0001 4.7364 /<0.0001 6.2907 /<0.0001 3.5549 /0.0003 表 9 联合组和对照组食管癌患者不良反应比较 (n)
Table 9 Comparison of adverse reactions between combined and control groups of esophageal cancer patients (n)
Adverse events Combined group (n=53) Control group (n=50) χ2 P Ⅰ Ⅱ Ⅲ Ⅳ Incidence (%) Ⅰ Ⅱ Ⅲ Ⅳ Incidence (%) Nausea and vomiting 13 9 2 0 45.3 14 4 3 0 42.0 0.113 0.737 Diminished appetite 17 12 6 0 66.0 17 8 5 0 60.0 0.403 0.526 Bloating 8 3 0 0 20.8 3 4 1 0 16.0 0.378 0.534 Constipation 7 2 0 0 17.0 6 4 0 0 20.0 0.156 0.693 Esophagitis 17 9 2 0 52.8 20 7 3 0 60.0 0.538 0.463 Fatigue 22 5 2 0 54.7 21 6 1 0 56.0 0.017 0.896 Myelosuppression 12 8 11 9 75.5 8 10 12 7 74.0 0.030 0.864 Rash 7 3 0 0 18.9 1 0 0 0 2.0 7.674 0.006 Peripheral neuritis 4 1 0 0 9.4 2 1 0 0 6.0 0.080 0.778 Hepatic impairment 3 1 0 0 7.6 3 2 0 0 10.0 0.008 0.927 -
[1] Chen YY, Wang M, Zuo CY, et al. Nrf-2 as a novel target in radiation induced lung injury[J]. Heliyon, 2024, 10(8): e29492. doi: 10.1016/j.heliyon.2024.e29492
[2] Dasgupta Q, Jiang A, Wen AM, et al. A human lung alveolus-on-a-chip model of acute radiation-induced lung injury[J]. Nat Commun, 2023, 14(1): 6506. doi: 10.1038/s41467-023-42171-z
[3] Farh ME, Kim HJ, Kim SY, et al. Transcriptional changes in radiation-induced lung injury: a comparative analysis of two radiation doses for preclinical research[J]. Int J Mol Sci, 2024, 25(7): 3766. doi: 10.3390/ijms25073766
[4] Amirkhosravi A, Mirtajaddini Goki M, Heidari MR, et al. Combination of losartan with pirfenidone: a protective anti-fibrotic against pulmonary fibrosis induced by bleomycin in rats[J]. Sci Rep, 2024, 14(1): 8729. doi: 10.1038/s41598-024-59395-8
[5] Türkkan G, Willems Y, Hendriks LEL, et al. Idiopathic pulmonary fibrosis: Current knowledge, future perspectives and its importance in radiation oncology[J]. Radiother Oncol, 2021, 155: 269-277. doi: 10.1016/j.radonc.2020.11.020
[6] Chen C, Zeng BW, Xue D, et al. Pirfenidone for the prevention of radiation-induced lung injury in patients with locally advanced oesophageal squamous cell carcinoma: Aprotocol for a randomised controlled trial[J]. BMJ Open, 2022, 12(10): e060619. doi: 10.1136/bmjopen-2021-060619
[7] Freites-Martinez A, Santana N, Arias-Santiago S, et al. Using the common terminology criteria for adverse events(CTCAE-Versión 5.0) to Evaluate the severity of events of anticancer therapies[J]. Actas Dermosifiliogr(Engl Ed), 2021, 112(1): 90-92. doi: 10.1016/j.ad.2019.05.009
[8] Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma[J]. Semin Liver Dis, 2010, 30(1): 52-60. doi: 10.1055/s-0030-1247132
[9] Inoo H, Sakanaka K, Fujii K, et al. Association of volumetric-modulated arc therapy with radiation pneumonitis in thoracic esophageal cancer[J]. J Radiat Res, 2022, 63(4): 646-656. doi: 10.1093/jrr/rrac021
[10] Beach TA, Finkelstein JN, Chang CY. Epithelial responses in radiation-induced lung injury (RILI) allow chronic inflammation and fibrogenesis[J]. Radiat Res, 2023, 199(5): 439-451.
[11] Shi XY, Zhu YQ, Ting CJ, et al. Single-cell transcriptomic analysis of radiation-induced lung injury in rat[J]. Biomol Biomed, 2024, 24(5): 1331-1349. doi: 10.17305/bb.2024.10357
[12] Jahnke L, Perrenoud V, Zandi S, et al. Modulation of extracellular matrix composition and chronic inflammation with pirfenidone promotes scar reduction in retinal wound repair[J]. Cells, 2024, 13(2): 164. doi: 10.3390/cells13020164
[13] AI-Kuraishy H M, Batiha GES, Faidah H, et al. Pirfenidone and post-Covid-19 pulmonary fibrosis: invoked again for realistic goals[J]. Inflammopharmacology, 2022, 30(6): 2017-2026. doi: 10.1007/s10787-022-01027-6
[14] Amirkhosravi A, Heidari MR, Karami-Mohajeri S, et al. Losartan enhances the suppressive effect of pirfenidone on the bleomycin-induced epithelial-mesenchymal transition and oxidative stress in A549 cell line[J]. Iran J Basic Med Sci, 2023, 26(8): 972-978.
[15] Gutiérrez J, López D, Sandoval A S, et al. P-3 Pirfenidone prevents obesity-associated nonalcoholic steatohepatitis and cardiac fibrosis through hormonal reprogramming[J]. Ann Hepatol, 2024, 29(1 suppl): 101190.
[16] Munoz-Espinosa LE, Torre A, Cisneros L, et al. Prolonged-release pirfenidone in patients with compensated cirrhosis. Final results of the multicenter study ODISEA, controlled against placebo, plus standardized care 2023[J]. Ann Hepatol, 2024, 29(2 suppl): 101472.
[17] Mohamed HE, Abdelhady MA, Elmaghraby AM, et al. Empagliflozin and pirfenidone confer renoprotection through suppression of glycogen synthase kinase-3β and promotion of tubular regeneration in rats with induced metabolic syndrome[J]. Toxicol Appl Pharm, 2024, 485: 116892. doi: 10.1016/j.taap.2024.116892
[18] Schreiber J, Schütte W, Koerber W, et al. Clinical course of mild-to-moderate idiopathic pulmonary fibrosis during therapy with pirfenidone: results of the non-interventional study AERplus[J]. Pneumologie, 2024, 78(4): 236-243. doi: 10.1055/a-2267-2074
[19] Ying HJ, Fang M, Hang QQ, et al. Pirfenidone modulates macrophage polarization and ameliorates radiation-induced lung fibrosis by inhibiting the TGF-β1/Smad3 pathway[J]. J Cell Mol Med, 2021, 25(18): 8662-8675. doi: 10.1111/jcmm.16821
[20] Moustafa M, Lipson KE, Akbarpour M, et al. Late intervention with radiation-induced lung fibrosis -comparing pamrevlumab anti-CTGF therapy vs. pirfenidone vs. nintedanib as mono-, dual- and triple-therapy combinations[J]. Int J Radiat Oncol, 2020, 108(3S): S76.
[21] Mummudi N, David S, Tandon S, et al. PROphylactic pirfenidone for prevention of radiation induced pneumonitis in patients with lung cancer (PROPeR study)[J]. J Thorac Oncol, 2023, 18 (11 suppl): S299.
[22] Manie MF, Fawzy HM, El-Sayed El-SM. Hydroxytyrosol alleviates methotrexate-induced pulmonary fibrosis in rats: involvement of TGF-β1, tissue factor, and VEGF[J]. Biol Pharm Bull, 2024, 47(1): 303-310. doi: 10.1248/bpb.b23-00477
[23] Monroy HC, Arceo S, Cabral AG, et al. P-21 fibrosis development and malignancies are delayed by pirfenidone while increasing SIRT1 nuclear translocation and histone 3 deacetylations in a hepatocarcinoma model[J]. Ann Hepatol, 2024, 29(1 suppl): 101208.
[24] Igbo BT, Jentsch C, Linge A, et al. Correlation of microscopic tumor extension with tumor microenvironment in esophageal cancer patients[J]. Strahlenther Onkol, 2024, 200(7): 595-604. doi: 10.1007/s00066-024-02234-6
[25] Florescu DN, Boldeanu MV, Șerban RE, et al. Correlation of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, inflammatory markers, and tumor markers with the diagnosis and prognosis of colorectal cancer[J]. Life(Basel), 2023, 13(12): 2261.
[26] Fernandes S, Oliver-De La Cruz, Morazzo S, et al. TGF-β induces matrisome pathological alterations and EMT in patient-derived prostate cancer tumoroids[J]. Matrix Biol, 2024, 125: 12-30. doi: 10.1016/j.matbio.2023.11.001
[27] Ilson DH. Emerging evidence for VEGF and immune checkpoint inhibition in oesophagogastric cancer[J]. Lancet Gastroenterol Hepatol, 2022, 7(3): 200-201. doi: 10.1016/S2468-1253(21)00428-3
[28] Wang G, Zhou XW, Guo ZL, et al. The Anti-fibrosis drug Pirfenidone modifies the immunosuppressive tumor microenvironment and prevents the progression of renal cell carcinoma by inhibiting tumor autocrine TGF-β[J]. Cancer Biol Ther, 2022, 23(1): 150-162. doi: 10.1080/15384047.2022.2035629
[29] Miranda-Roblero HO, Monroy-Ramírez HC, Galicia-Moreno M, et al. P-43 Pirfenidone prevents neoplastic lesions development by oxidative, fibrogenic, antiproliferative and epigenetic mechanisms regulation in a model of chemical hepatocarcinogenesis [J]. Ann Hepatol, 2023, 28(1 suppl): 100945.
[30] Luo DQ, Zeng XL, Zhang SL, et al. Pirfenidone suppressed triple-negative breast cancer metastasis by inhibiting the activity of the TGF-β/SMAD pathway[J]. J Cell Mol Med, 2023, 27(3): 456-469. doi: 10.1111/jcmm.17673
[31] Zhang S, Wang Y, Luo D, et al. Pirfenidone inhibits TGF-β1-induced metabolic reprogramming during epithelial-mesenchymal transition in non-small cell lung cancer[J]. J Cell Mol Med, 2024, 28(3): e18059. doi: 10.1111/jcmm.18059



 下载:
下载: