MAPK4 Accelerates Progression of Cervical Squamous Cell Carcinoma by Positively Regulating SLC3A2 Expression
-
摘要:目的
探索MAPK4在宫颈鳞状细胞癌(CSCC)中的作用以鉴定候选的预后预测生物标志物和分子治疗靶点。
方法在TCGA队列中进行Kaplan-Meier生存分析并利用临床样本开展免疫组织化学实验探索MAPK4与患者预后的相关性;基于MAPK4 mRNA水平构建列线图。Western Blot、CCK-8、克隆形成和Transwell细胞功能实验明确MAPK4在宫颈鳞状细胞癌中的异常表达和作用;DIA蛋白组测序鉴定MAPK4调节的效应分子;敲降MAPK4并过表达效应分子,联合上述细胞功能实验揭示MAPK4调节效应分子介导肿瘤进程的作用。
结果MAPK4 mRNA水平升高与蛋白高表达的CSCC患者预后更差;基于MAPK4构建的列线图可准确预测患者1、3、5年生存率。相比于正常宫颈组织,MAPK4蛋白在肿瘤中表达上调,敲降MAPK4可显著抑制宫颈鳞状细胞癌细胞ME180和SiHa增殖、克隆形成和迁移侵袭能力。SLC3A2是MAPK4的下游效应分子,敲降MAPK4后过表达SLC3A2可减弱敲降MAPK4对细胞增殖、克隆形成和迁移侵袭的抑制作用。
结论MAPK4是宫颈鳞状细胞癌患者候选的预后预测生物标志物。MAPK4正向调节SLC3A2蛋白表达加速肿瘤进程,是宫颈鳞状细胞癌患者潜在的分子治疗靶点。
Abstract:ObjectiveTo explore the role of MAPK4 in cervical squamous cell carcinoma (CSCC) for the identification of candidate prognostic prediction biomarkers and molecular therapeutic targets.
MethodsThe TCGA cohort was subjected to Kaplan-Meier survival analysis. Immunohistochemistry experiments were conducted on clinical samples to explore the correlation between MAPK4 and patient prognosis. A nomogram was constructed based on MAPK4 mRNA levels. Western blot, CCK-8, colony formation, and Transwell cell function experiments were performed to clarify the abnormal expression and role of MAPK4 in CSCC. DIA proteome sequencing was used to identify effector molecules regulated by MAPK4. Combined with the above cell function experiments, the knockdown of MAPK4 and the overexpression of effector molecules revealed that MAPK4 regulated effector molecules to mediate tumor progression.
ResultsCSCC patients with elevated MAPK4 mRNA levels and high protein expression have a worse prognosis. The constructed nomogram based on MAPK4 can accurately predict the 1-, 3-, and 5-year survival rates of patients. Compared with that in normal cervical tissues, MAPK4 protein expression was up-regulated in tumors. MAPK4 knockdown substantially inhibited the proliferation, colony formation, migration, and invasion of CSCC ME180 and SiHa cells. SLC3A2 is a downstream effector molecule of MAPK4. Overexpression SLC3A2 can weaken the inhibitory effect of MAPK4 knockdown on cell proliferation, colony formation, migration, and invasion.
ConclusionMAPK4 is a candidate prognostic biomarker for patients with CSCC. MAPK4 positively regulates SLC3A2 protein expression and accelerates tumor progression, making it a potential molecular therapeutic target for patients with CSCC.
-
Key words:
- Cervical squamous cell carcinoma /
- MAPK4 /
- SLC3A2 /
- Tumor progression /
- Biomarkers /
- Therapeutic targets
-
0 引言
宫颈癌(cervical cancer, CC)是常见的生殖系统肿瘤,其发病率和死亡率均位居女性肿瘤第四位[1]。2020年全球宫颈癌新发病例约60.4万例,新增死亡约34.1万例[2]。既往研究表明宫颈癌的发生、上皮内瘤变和肿瘤后期进展均与高危型人乳头瘤状病毒(Human papilloma virus, HPV)持续感染密切相关[3-5]。尽管HPV疫苗在一定程度上降低了宫颈癌的发病率和死亡率,但在低收入国家和地区,宫颈癌发病人数逐年升高且呈年轻化趋势[6]。宫颈鳞状细胞癌(Cervical squamous cell carcinoma, CSCC)是宫颈癌中最主要的病理亚型,占全部宫颈癌的75%~90%[7]。经手术治疗的早期CSCC患者预后良好,而经同步放化疗标准治疗的局部晚期患者预后不佳。目前CSCC恶性进展的机制尚未阐明,临床中亦缺乏有效的预后生物标志物和靶向治疗策略。探索CSCC进展的分子机制和生物标志物,筛选鉴定疗效显著的分子治疗靶点和靶向药物是提高患者生存率的关键。
丝裂原活化蛋白激酶(Mitogen-activated protein kinases,MAPKs)是一类进化保守的丝氨酸-苏氨酸激酶,其通过三级激酶级联的形式(MAPKKK/MAPKK/MAPK)转导细胞信号[8-9],调节肿瘤细胞增殖、迁移侵袭和代谢等过程[10-12]。在哺乳动物细胞中已发现4种最典型的MAPKs,分别为ERK1/2,c-JNK1/2/3,p38和ERK5[13]。鉴于MAPKs在肿瘤中的关键调控作用,已有多种以该家族分子作为药物靶点的抑制剂被开发应用于临床试验,例如ERK1/2抑制剂优立替尼[14]和GDC-0994等[15]。与典型的MAPKs不同,MAPK4是一种非典型的MAPK家族成员,其缺乏执行磷酸化激活功能的经典Thr-X-Tyr激活基序[16-17]。MAPK4在肿瘤细胞中的功能可能与经典的MAPKs蛋白家族成员不同,目前其在CSCC中的作用及其调控机制尚未阐明。本研究旨在探索MAPK4在CSCC细胞中的作用与调控机制,为揭示CSCC恶性进程的分子机制提供关键实验证据,同时为CSCC的预后预测及靶向治疗提供候选的生物标志物和分子治疗靶点。
1 材料与方法
1.1 生物信息学分析
1.1.1 数据下载
从UCSC数据库(https://xenabrowser.net/datapages/)下载239例具有完整生存信息的TCGA-CSCC队列转录组数据(FPKM定量数据)和临床病理参数信息。将上述转录组数据经Log2(FPKM+1)转换后用于后续分析。
1.1.2 预后生存分析
结合患者转录组和生存数据,利用“survival”和“survminer”R包进行Kaplan-Meier生存分析,根据最佳Cut-off值将患者分成MAPK4 mRNA高水平组和低水平组。利用在线数据网站Kaplan-Meier plotter(https://kmplot.com/analysis/)分析MAPK4转录水平对其他肿瘤患者预后的影响。
1.1.3 单因素/多因素Cox回归分析和列线图构建
基于患者生存数据,纳入MAPK4转录水平、年龄、肿瘤分期和肿瘤分级,利用R包“survival”进行单因素和多因素Cox回归分析。基于患者MAPK4 mRNA水平和肿瘤分期,利用“rms”R包构建列线图以预测患者1年、3年和5年生存率并绘制校准曲线以评估预测值与实际观测值的一致性。
1.2 临床组织样本收集与检测
1.2.1 组织样本收集
用于免疫组织化学检测的68例CSCC石蜡切片样本和用于Western Blot的7对正常宫颈/CSCC样本均来源于2019年7月-2021年7月在中国医学科学院肿瘤医院临床诊断后的剩余样本。上述患者均签署了知情同意书,本研究的设计和实施得到中国医学科学院肿瘤医院伦理委员会批准(24/308-4588)。
1.2.2 免疫组织化学检测
制备4 μm厚度的石蜡切片,使用通用二步法检测试剂盒(中杉金桥,北京,PV-9000)进行免疫组织化学检测,根据试剂盒说明书操作。主要流程如下:切片脱蜡后水化,使用3%H2O2阻断内源性过氧化物酶活性,微波抗原修复,MAPK4(三鹰生物,武汉,26102-1-AP)抗体孵育后在4℃过夜,DAB显色,苏木精对比染色,温水返蓝,酒精浸泡,二甲苯脱水,中性树脂封片后烘干,扫描后拍照,利用Image J软件进行统计分析。
1.3 细胞实验
1.3.1 细胞培养
人胚肾细胞293T、CSCC细胞ME180和SiHa均由中国医学科学院肿瘤医院分子肿瘤学国家重点实验室常规保存,293T使用含10%血清的DMEM(普诺赛,武汉,PM150210A)培养基培养,ME180使用含10%血清的McCoy's 5A(普诺赛,武汉,PM150710A)培养基培养,SiHa使用含10%血清的MEMα(普诺赛,武汉,PM150421A)培养基培养,上述细胞均置于恒温37℃且含有5% CO2的细胞培养箱中培养。
1.3.2 siRNA和质粒转染
敲降MAPK4的siRNA序列为siMAPK4-1:5′-ACUACACCAAAGCCAUCGACATT-3′,siMAPK4-2:5′-GAAGGUCGCUGUGAAGAAGAUTT-3′。敲降SLC3A2的siRNA序列为siSLC3A2-1:5′-GCCUGGACUCUUCUCCUAUAUTT-3′,siSLC3A2-2:5′-GCUGGGUCCAAUUCACAAGAATT-3′。对照组siRNA序列为5′-UUCUCCGAACGUGUCACGUTT-3′。上述siRNA均购自苏州吉玛基因股份有限公司。用于瞬时转染的对照质粒pLVX-puro、pLVX-MAPK4-Puro和pLVX-SLC3A2-Puro由本实验室构建保存。按照产品说明书,使用Opti-MEM培养基(Thermo Fisher Scientific,美国,31985070)和Lipofectamine 2000(Thermo Fisher Scientific,美国,11668019)转染试剂瞬时转染 siRNA或质粒至细胞。
1.3.3 Western blot检测
利用细胞裂解液裂解实验组和对照组细胞并提取蛋白,用BCA蛋白定量法检测蛋白浓度,根据说明书进行SDS-PAGE凝胶电泳并将蛋白转移至PVDF膜,与MAPK4和SLC3A2(Santa Cruz,美国,sc-390154)抗体进行杂交并使用ECL发光液检测试剂显示杂交信号,以Vinculin(Abcam,英国,ab129002)作为内参对照。
1.3.4 细胞增殖能力的检测
利用CCK-8试剂盒(GlpBio,美国,GK10001)检测细胞增殖能力的改变。将实验组和对照组细胞用胰蛋白酶消化并计数,按照2×103个/孔将细胞接种至96孔板,每组设计3个平行孔。每隔24 h检测450 nm(OD450)处的吸光度值,连续检测4天,绘制细胞生长曲线。
1.3.5 克隆形成实验
将实验组和对照组细胞用胰酶消化并计数,在6孔板的每个孔中接种500个细胞,每组设置3个复孔。连续培养10~12天后,1×PBS润洗,甲醇固定30 min。弃固定液,在6孔板中加入0.5%结晶紫染色20 min,流水冲洗,扫描并计数。
1.3.6 迁移侵袭实验
胰酶消化细胞并计数,Transwell小室下方加入700 μl含有20%血清的培养基,在小室上方加入100 μl含1×104个细胞的细胞悬液(无血清),培养箱内静置培养。1×PBS润洗小室,固定液(甲醇∶丙酮1∶1)固定30 min。弃固定液,0.5%结晶紫染色20 min,流水冲洗,轻轻擦去小室上层细胞。裁剪小室底膜,中性树脂和盖玻片封片,扫描并计数。
1.3.7 细胞周期检测
利用细胞周期检测试剂盒(凯基生物,南京,KGA512)进行细胞周期检测。实验组和对照组细胞(各设置3个复孔)转染siRNA 24 h后胰酶消化,1100 r/min离心5 min,收集细胞沉淀。加入预冷的70%乙醇,4℃固定10~12 h。1100 r/min离心5 min,弃乙醇, 1×PBS润洗,1100 r/min离心5 min。加入RNase A溶液,在37℃水浴锅中孵育15 min,PI染色30 min,流式细胞仪检测。
1.4 质谱检测
1.4.1 DIA定量蛋白组测序
在人胚肾细胞293T中瞬时转染pLVX-puro(n=3)和pLVX-MAPK4-puro(n=3)48 h后收集细胞,使用数据非依赖性采集模式(Data independent acquisition,DIA)定量蛋白组技术对6个样品分别进行细胞裂解、蛋白酶解、色谱分离和质谱检测。Q ExactiveHF(Thermo Fisher Scientific)进行蛋白质鉴定分析。使用DIA-NN 1.8.1软件[18]对质谱仪产生的原始文件进行数据库检索。质谱检测和数据采集由中国科学院上海药物研究所公共技术服务中心完成。
1.4.2 差异蛋白鉴定与通路富集分析
利用“limma”R包分析MAPK4过表达组和对照组差异蛋白,以P adjust<0.05,Fold Change>1.2(上调蛋白)或<0.83(下调蛋白)作为差异蛋白筛选标准。R包“clusterprofiler”进行KEGG和GO富集分析以明确差异蛋白所介导的信号通路和细胞组分。
1.5 统计学方法
利用SPSS 22.0软件进行统计分析,两组之间的比较用Student-t检验;三组或三组以上的比较用方差分析,P<0.05为差异具有统计学意义。
2 结果
2.1 MAPK4高表达CSCC患者相比低表达患者具有更差的预后
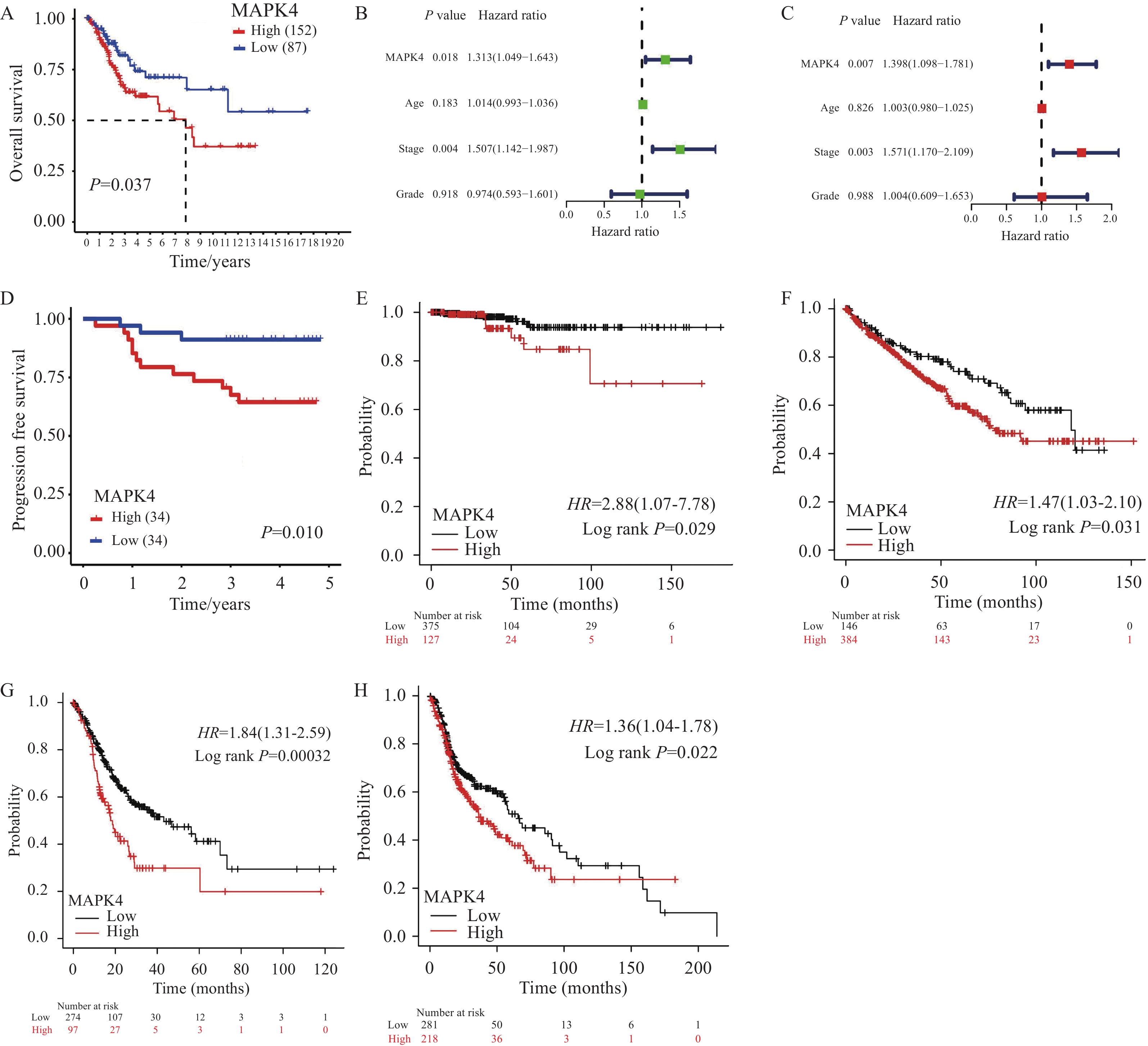
为了明确MAPK4对CSCC患者预后的影响,我们首先对TCGA-CSCC队列进行Kaplan-Meier生存分析,根据最佳cut-off值将患者分为MAPK4 mRNA高水平组(n=152)和低水平组(n=87),发现高水平组患者相比低水平组具有更短的生存时间(P=0.037),见图1A。随后基于MAPK4 mRNA和生存数据,纳入患者年龄、肿瘤分期和肿瘤分级分别进行单因素和多因素Cox回归分析,以探究CSCC预后的独立风险因素,见图1B~C。多因素Cox回归分析结果表明,MAPK4 mRNA水平(Hazard ratio=1.398,P=0.007)和肿瘤分期(Hazard ratio=1.571,P=0.003)均为患者预后的独立风险因素,见图1C。进一步在68例CSCC临床组织样本中进行免疫组织化学检测MAPK4蛋白表达,发现MAPK4蛋白高表达组患者相比于低表达组具有更短的无进展生存时间(P=0.01),见图1D。此外,在Kaplan Meier plotter分析网站探索MAPK4对其他肿瘤预后的影响,发现在甲状腺癌(P=0.029)、肾透明细胞癌(P=0.031)、胃腺癌(P=0.00032)和头颈鳞癌(P=0.022)中,MAPK4 mRNA高水平组患者相比低水平组同样是具有更短的生存时间,见图1E~H。上述分析结果表明,MAPK4高表达与包括CSCC在内的多种肿瘤恶性进程相关,MAPK4可作为候选的CSCC患者预后生物标志物。
![]() 图 1 MAPK4高表达CSCC患者相比低表达患者具有更差的预后Figure 1 CSCC patients with high MAPK4 expression have a worse prognosis than those with low MAPK4 expressionA: Kaplan-Meier survival analysis showed that the overall survival time of patients in the high-level MAPK4 mRNA group (n=152) was shorter than that in the low-level group (n=87) (Log rank test); B-C: univariate (B) and multivariate (C) Cox regression analyses based on MAPK4 mRNA level, age, tumor stage, and tumor grade; D: patients with high MAPK4 protein expression had a shorter progression-free survival time (n=68, Log rank test); E-H: Kaplan-Meier plotter online analysis showed that elevated MAPK4 mRNA levels in thyroid carcinoma (E) (n=502, Log rank test), kidney renal clear cell carcinoma (F) (n=530, Log rank test), stomach adenocarcinoma (G) (n=371, Log rank test), and head and neck squamous cell carcinoma (H) (n=499, Log rank test) had shorter overall survival time.
图 1 MAPK4高表达CSCC患者相比低表达患者具有更差的预后Figure 1 CSCC patients with high MAPK4 expression have a worse prognosis than those with low MAPK4 expressionA: Kaplan-Meier survival analysis showed that the overall survival time of patients in the high-level MAPK4 mRNA group (n=152) was shorter than that in the low-level group (n=87) (Log rank test); B-C: univariate (B) and multivariate (C) Cox regression analyses based on MAPK4 mRNA level, age, tumor stage, and tumor grade; D: patients with high MAPK4 protein expression had a shorter progression-free survival time (n=68, Log rank test); E-H: Kaplan-Meier plotter online analysis showed that elevated MAPK4 mRNA levels in thyroid carcinoma (E) (n=502, Log rank test), kidney renal clear cell carcinoma (F) (n=530, Log rank test), stomach adenocarcinoma (G) (n=371, Log rank test), and head and neck squamous cell carcinoma (H) (n=499, Log rank test) had shorter overall survival time.2.2 基于MAPK4构建列线图以预测CSCC患者预后
由于MAPK4 mRNA水平和肿瘤分期均为患者预后的独立风险因素,基于MAPK4 mRNA水平和肿瘤分期,利用233例具有完整肿瘤分期信息的TCGA-CSCC患者构建列线图模型以预测患者预后。列线图显示,不同的MAPK4 mRNA水平和肿瘤分期均对应不同的患者评分,患者总评分越高,其1年、3年和5年的生存率更低,见图2A。校准曲线表明列线图所预测的1年、3年和5年生存率与实际观察值具有良好的符合程度,见图2B~D。基于MAPK4构建的可视化列线图是CSCC患者预后预测的候选分类工具。
![]() 图 2 基于MAPK4构建列线图以预测CSCC患者预后Figure 2 Construction of a nomogram based on MAPK4 to predict the prognosis of CSCC patientsA: Construction of a nomogram based on the patient’s MAPK4 mRNA level and tumor stage to predict the patient’s prognosis (n=233); B-D: calibration curves showing that the nomogram accurately predicts patient survival at 1 year (B), 3 years (C), and 5 years (D).
图 2 基于MAPK4构建列线图以预测CSCC患者预后Figure 2 Construction of a nomogram based on MAPK4 to predict the prognosis of CSCC patientsA: Construction of a nomogram based on the patient’s MAPK4 mRNA level and tumor stage to predict the patient’s prognosis (n=233); B-D: calibration curves showing that the nomogram accurately predicts patient survival at 1 year (B), 3 years (C), and 5 years (D).2.3 MAPK4蛋白表达促进CSCC细胞增殖和迁移侵袭
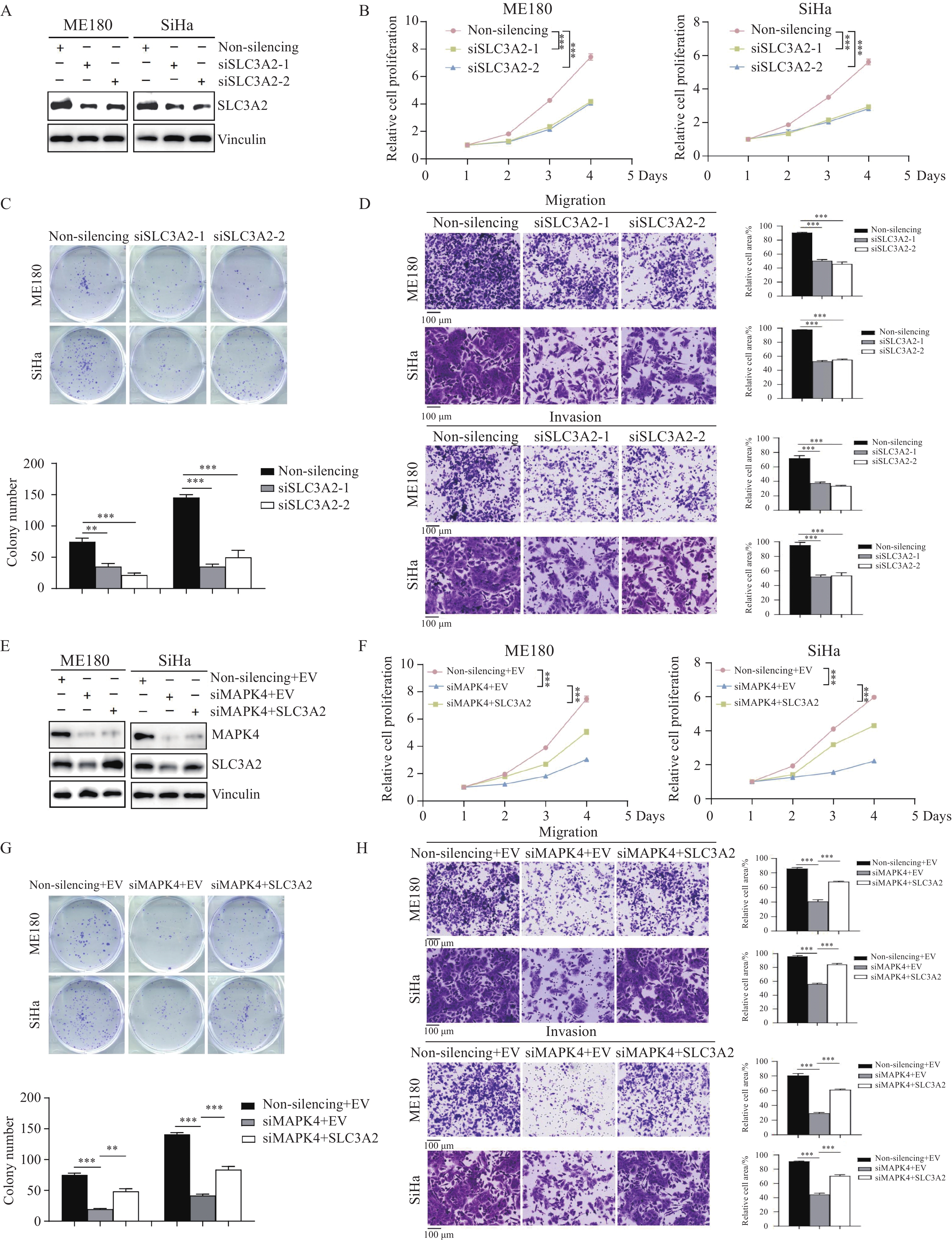
MAPK4高表达CSCC患者相比低表达患者的预后更差,提示MAPK4是驱动CSCC恶性进程的关键基因,目前MAPK4在CSCC中的具体作用和机制尚不明确。通过Western blot检测MAPK4在7对配对的正常宫颈和CSCC组织中的表达,发现MAPK4在CSCC组织中蛋白表达高于正常宫颈组织,见图3A。CCK-8和克隆形成实验表明敲降MAPK4可显著抑制CSCC细胞ME180和SiHa的增殖和克隆形成能力,见图3B~D。此外,敲降MAPK4可显著抑制CSCC细胞迁移和侵袭能力,见图3E。综上所述,MAPK4可促进细胞增殖、迁移和侵袭从而加速CSCC进程,是MAPK4高表达患者预后更差的驱动因素。
![]() 图 3 MAPK4蛋白表达促进CSCC细胞增殖和迁移侵袭Figure 3 MAPK4 protein expression promotes proliferation, migration, and invasion of CSCC cellsA: Western blot results showed that MAPK4 protein expression was higher in CSCC tissues than in normal cervical tissues; B: Western blot detected the protein inhibition efficiency of siRNA knockdown MAPK4; C-E: MAPK4 knockdown significantly inhibited cell proliferation (C) (mean±SEM, n=3, one-way ANOVA), colony formation (D) (mean±SEM, n=3, one-way ANOVA), migration, and invasion (E) (mean±SEM, n=4, one-way ANOVA). **: P<0.01, ***: P<0.001.
图 3 MAPK4蛋白表达促进CSCC细胞增殖和迁移侵袭Figure 3 MAPK4 protein expression promotes proliferation, migration, and invasion of CSCC cellsA: Western blot results showed that MAPK4 protein expression was higher in CSCC tissues than in normal cervical tissues; B: Western blot detected the protein inhibition efficiency of siRNA knockdown MAPK4; C-E: MAPK4 knockdown significantly inhibited cell proliferation (C) (mean±SEM, n=3, one-way ANOVA), colony formation (D) (mean±SEM, n=3, one-way ANOVA), migration, and invasion (E) (mean±SEM, n=4, one-way ANOVA). **: P<0.01, ***: P<0.001.2.4 DIA蛋白组测序鉴定MAPK4的下游效应分子
为了从蛋白组层面解析MAPK4促进肿瘤进程所调控的核心效应分子和生物学通路,我们在模式细胞293T中利用DIA蛋白组定量测序鉴定MAPK4过表达组(n=3)与对照组(n=3)之间的差异蛋白。6个样本共定量到蛋白
6742 个,一半以上样本中具有定量信息的蛋白6678 个,所有样本中均具有定量信息的蛋白6295 个。按照P adjust<0.05,Fold Change>1.2(上调蛋白)或<0.83(下调蛋白)作为筛选标准,共得到97个上调蛋白和84个下调蛋白。火山图展示了差异蛋白的分布情况,以P adjust进行排序,排名前5的上调蛋白包括SLC3A2、VTN、TMEM102、ECD和LETMD1,排名前5的下调蛋白包括PGM5、GSE1、LGALS1、JUN和RAD9A,见图4A。为了揭示差异蛋白所影响的生物学通路和细胞组分,对差异蛋白进行KEGG通路富集分析,发现其主要富集在铁死亡等生物学通路,见图4B;对差异蛋白进行GO富集分析,发现其参与调节线粒体基质、线粒体内膜和线粒体蛋白复合体等细胞组分,见图4C。2.5 MAPK4通过调节SLC3A2表达加速CSCC细胞增殖、迁移和侵袭
根据DIA蛋白组定量分析数据,按照P adjust进行排序,发现SLC3A2是MAPK4过表达后上调最为显著的蛋白。结合前面的通路富集分析结果,SLC3A2可能是MAPK4加速CSCC恶性进展的下游效应分子。随后在CSCC细胞中敲降SLC3A2,发现敲降SLC3A2可同样抑制ME180和SiHa细胞的增殖及克隆形成能力,见图5A~C。Transwell实验表明敲降SLC3A2可抑制细胞的迁移和侵袭能力,见图5D。进一步,Western blot实验结果表明敲降MAPK4可下调SLC3A2蛋白表达,见图5E;而在敲降MAPK4的细胞中瞬转SLC3A2质粒,发现过表达SLC3A2可抑制敲降MAPK4导致的细胞增殖、克隆形成、迁移和侵袭能力的降低过程,见图5F~H。上述结果表明SLC3A2是MAPK4的下游效应分子,MAPK4可通过正向调控SLC3A2蛋白表达促进CSCC细胞增殖、迁移和侵袭,最终加速肿瘤进程。
![]() 图 5 MAPK4通过调节SLC3A2表达加速CSCC细胞增殖、迁移和侵袭Figure 5 MAPK4 accelerates CSCC cell proliferation, migration, and invasion by regulating SLC3A2 expressionA: Western blot detected the protein inhibition efficiency of siRNA knockdown SLC3A2; B-D: MAPK4 knockdown significantly inhibited cell proliferation (B) (mean±SEM, n=3, one-way ANOVA), colony formation (C) (mean±SEM, n=3, one-way ANOVA), migration, and invasion (D) (mean±SEM, n=4, one-way ANOVA); E: overexpression SLC3A2 in MAPK4-knockdown CSCC cells; F-H: overexpression SLC3A2 in MAPK4 knockdown cells can restore the proliferation (F) (mean±SEM, n=3, one-way ANOVA), colony formation (G) (mean±SEM, n=3, one-way ANOVA), migration, and invasion (H) (mean±SEM, n=4, one-way ANOVA) of CSCC cells. EV(Empty vector): pLVX-puro, SLC3A2: pLVX-SLC3A2-puro. ***: P<0.001, **: P<0.01.
图 5 MAPK4通过调节SLC3A2表达加速CSCC细胞增殖、迁移和侵袭Figure 5 MAPK4 accelerates CSCC cell proliferation, migration, and invasion by regulating SLC3A2 expressionA: Western blot detected the protein inhibition efficiency of siRNA knockdown SLC3A2; B-D: MAPK4 knockdown significantly inhibited cell proliferation (B) (mean±SEM, n=3, one-way ANOVA), colony formation (C) (mean±SEM, n=3, one-way ANOVA), migration, and invasion (D) (mean±SEM, n=4, one-way ANOVA); E: overexpression SLC3A2 in MAPK4-knockdown CSCC cells; F-H: overexpression SLC3A2 in MAPK4 knockdown cells can restore the proliferation (F) (mean±SEM, n=3, one-way ANOVA), colony formation (G) (mean±SEM, n=3, one-way ANOVA), migration, and invasion (H) (mean±SEM, n=4, one-way ANOVA) of CSCC cells. EV(Empty vector): pLVX-puro, SLC3A2: pLVX-SLC3A2-puro. ***: P<0.001, **: P<0.01.3 讨论
高危型HPV持续性感染是宫颈癌的明确致病因素[19]。在西方发达国家,尽管HPV疫苗的推广已显著降低了女性罹患宫颈癌的风险[20],但在发展中国家,由于疫苗供应不足和经济收入限制,宫颈癌仍是威胁女性健康的重要社会问题[21]。约50%的患者初诊时即为局部晚期宫颈癌[22],经同步放化疗标准方案治疗的局部晚期患者预后不佳,开发临床诊断治疗新策略和新方案,推动患者个体化治疗是领域内的重要科学问题。
本研究通过整理239例TCGA-CSCC样本的转录组数据和生存信息,利用生存分析和Cox回归分析,发现MAPK4 mRNA和肿瘤分期均为患者生存预后的独立风险因素。在临床中肿瘤分期是CSCC预后的高危因素,表明研究队列选择和生物信息学分析的合理性。进一步,我们开展免疫组织化学实验从蛋白水平证明了MAPK4高表达CSCC患者具有更差的预后,mRNA和蛋白水平的结果一致。本研究还以MAPK4 mRNA和肿瘤分期为基础构建了列线图以预测患者的生存率,校准曲线证明该可视化的列线图具有良好的预测效应。综上可知,本研究数据分析合理,临床组织样本与公共数据库结果可互相验证。此外,MAPK4及其相关的列线图可作为候选的分类工具预测CSCC患者的预后,研究结果具备一定的转化应用价值。
既往研究已报道MAPK4在其他肿瘤中的促癌作用及调节机制。前列腺癌中MAPK4通过抑制GATA2蛋白泛素化降解激活雄激素受体信号从而加速肿瘤细胞增殖[23]。三阴性乳腺癌中MAPK4激活AKT通路促进肿瘤细胞增殖和移植瘤生长[24],此外MAPK4还通过上调PDK1蛋白表达介导肿瘤恶性进程[25]。Shen等利用免疫共沉淀和体外激酶实验发现MAPK4直接结合AKT进而磷酸化AKT T308位点,MAPK4还通过激活mTORC2通路上调AKT S473位点磷酸化水平[26]。非小细胞肺癌中MAPK4通过促进血管生成从而加速裸鼠移植瘤生长[27]。本研究发现,在CSCC细胞中敲降MAPK4表达可抑制肿瘤细胞增殖、克隆形成、迁移和侵袭能力,与MAPK4在其他肿瘤中通常发挥促癌作用一致。为了进一步明确MAPK4促进CSCC肿瘤进程的作用机制,我们通过DIA蛋白组测序鉴定MAPK4调控的下游效应分子。一方面,根据P adjust进行排序,发现SLC3A2是MAPK4过表达后上调最为显著的蛋白;另一方面,通过KEGG通路富集分析发现差异蛋白富集最显著的通路为铁死亡生物学通路。既往文献已报道,SLC3A2是SLC3家族分子中一种Ⅱ型跨膜糖蛋白,其胞膜外的半胱氨酸位点可通过二硫键与6种L型氨基酸转运体(LAT1、LAT2、xCT、y+LAT1、y+LAT2和asc1)共价结合[28-30]。SLC3A2/xCT可转运半胱氨酸供细胞合成抗氧化剂谷胱甘肽,维持细胞内活性氧的稳态并限制细胞毒性脂质过氧化作用从而抑制细胞铁死亡[31]。在头颈鳞癌中SLC3A2高表达与患者预后不良正相关,抑制SLC3A2表达可降低肿瘤细胞增殖活力并增强其放疗敏感性[32]。Wu等研究发现在膀胱癌细胞中抑制SLC3A2表达可上调活性氧和Fe2+水平促进肿瘤细胞铁死亡[33]。SLC3A2在喉癌中表达上调且与患者预后不良正相关,抑制SLC3A2可通过降低mTOR磷酸化水平进而促进肿瘤细胞铁死亡[34]。目前SLC3A2在CSCC中的作用及其分子机制尚无文献报道。本研究通过CCK-8、克隆形成、迁移和侵袭实验证明SLC3A2在CSCC中同样发挥促癌作用。我们进一步利用回复实验明确SLC3A2是MAPK4的下游调节分子,MAPK4通过上调SLC3A2表达促进CSCC恶性进程。然而,MAPK4正向调节SLC2A2表达的具体分子机制仍不清楚,MAPK4/SLC3A2是否通过影响铁死亡促进CSCC肿瘤进程亦需深入探索。
本研究首次揭示了MAPK4/SLC3A2的调控关系,并发现MAPK4和MAPK4相关的列线图可能用于CSCC的预后预测,MAPK4是CSCC一个候选的分子治疗靶点。研究结果为阐明CSCC的恶性进程机制提供了实验证据,为患者个体化治疗提供了候选分子靶点。目前本研究仍存在一定局限性:一方面,缺乏多中心的大样本队列验证MAPK4和列线图对CSCC预后预测的价值;另一方面,MAPK4上调SLC3A2蛋白表达促进CSCC进程的详细分子机制仍未阐明。后续我们拟纳入多中心CSCC样本检测MAPK4的mRNA和蛋白表达水平,进一步验证MAPK4及其相关的列线图在患者预后预测中的临床转化应用价值。此外,我们还将从公共数据库、临床组织样本、体外细胞和体内动物四个层面开展实验充分揭示MAPK4/SLC3A2在CSCC中的具体调控机制。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:余 竟:实验设计、数据分析、实验实施及文章撰写邓 露:文献调研及部分实验实施赵雨亭:数据分析袁振龙:统计学分析、文章校对吴令英:基金支持、思路设计、文章审阅 -
-
[1] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[2] Singh D, Vignat J, Lorenzoni V, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative[J]. Lancet Glob Health, 2023, 11(2): e197-e206. doi: 10.1016/S2214-109X(22)00501-0
[3] Kalliala I, Athanasiou A, Veroniki AA, et al. Incidence and mortality from cervical cancer and other malignancies after treatment of cervical intraepithelial neoplasia: a systematic review and meta-analysis of the literature[J]. Ann Oncol, 2020, 31(2): 213-227. doi: 10.1016/j.annonc.2019.11.004
[4] Guo C, Qu X, Tang X, et al. Spatiotemporally deciphering the mysterious mechanism of persistent HPV-induced malignant transition and immune remodelling from HPV-infected normal cervix, precancer to cervical cancer: Integrating single-cell RNA-sequencing and spatial transcriptome[J]. Clin Transl Med, 2023, 13(3): e1219. doi: 10.1002/ctm2.1219
[5] Sivars L, Jylhä C, Crona Guterstam Y, et al. Cell-free human papillomavirus (HPV) DNA is a sensitive biomarker for prognosis and for early detection of relapse in locally advanced cervical cancer[J]. Clin Cancer Res, 2024, 30(13): 2764-2771. doi: 10.1158/1078-0432.CCR-23-3941
[6] Yang M, Du J, Lu H, et al. Global trends and age-specific incidence and mortality of cervical cancer from 1990 to 2019: an international comparative study based on the Global Burden of Disease[J]. BMJ Open, 2022, 12(7): e055470. doi: 10.1136/bmjopen-2021-055470
[7] Lin S, Sun Y, Cao C, et al. Single-nucleus RNA sequencing reveals heterogenous microenvironments and specific drug response between cervical squamous cell carcinoma and adenocarcinoma[J]. EBioMedicine, 2023, 97: 104846. doi: 10.1016/j.ebiom.2023.104846
[8] Bahar ME, Kim HJ, Kim DR. Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies[J]. Signal Transduct Target Ther, 2023, 8(1): 455. doi: 10.1038/s41392-023-01705-z
[9] Iroegbu JD, Ijomone OK, Femi-Akinlosotu OM, et al. ERK/MAPK signalling in the developing brain: Perturbations and consequences[J]. Neurosci Biobehav Rev, 2021, 131: 792-805. doi: 10.1016/j.neubiorev.2021.10.009
[10] Jin H, Huang X, Pan Q, et al. The EIF3H-HAX1 axis increases RAF-MEK-ERK signaling activity to promote colorectal cancer progression[J]. Nat Commun, 2024, 15(1): 2551. doi: 10.1038/s41467-024-46521-3
[11] Lin X, Ye R, Li Z, et al. KIAA1429 promotes tumorigenesis and gefitinib resistance in lung adenocarcinoma by activating the JNK/ MAPK pathway in an m(6)A-dependent manner[J]. Drug Resist Updat, 2023, 66: 100908. doi: 10.1016/j.drup.2022.100908
[12] Dimri M, Humphries A, Laknaur A, et al. NAD(P)H Quinone Dehydrogenase 1 Ablation Inhibits Activation of the Phosphoinositide 3-Kinase/Akt Serine/Threonine Kinase and Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Pathways and Blocks Metabolic Adaptation in Hepatocellular Carcinoma[J]. Hepatology, 2020, 71(2): 549-568. doi: 10.1002/hep.30818
[13] Park HB, Baek KH. E3 ligases and deubiquitinating enzymes regulating the MAPK signaling pathway in cancers[J]. Biochim Biophys Acta Rev Cancer, 2022, 1877(3): 188736. doi: 10.1016/j.bbcan.2022.188736
[14] Sullivan RJ, Infante JR, Janku F, et al. First-in-Class ERK1/2 Inhibitor Ulixertinib (BVD-523) in Patients with MAPK Mutant Advanced Solid Tumors: Results of a Phase I Dose-Escalation and Expansion Study[J]. Cancer Discov, 2018, 8(2): 184-195. doi: 10.1158/2159-8290.CD-17-1119
[15] Weekes C, Lockhart A, Lorusso P, et al. A Phase Ib Study to Evaluate the MEK Inhibitor Cobimetinib in Combination with the ERK1/2 Inhibitor GDC-0994 in Patients with Advanced Solid Tumors[J]. Oncologist, 2020, 25(10): 833-e1438. doi: 10.1634/theoncologist.2020-0292
[16] Aberg E, Perander M, Johansen B, et al. Regulation of MAPK-activated protein kinase 5 activity and subcellular localization by the atypical MAPK ERK4/MAPK4[J]. J Biol Chem, 2006, 281(46): 35499-35510. doi: 10.1074/jbc.M606225200
[17] Kant S, Schumacher S, Singh MK, et al. Characterization of the atypical MAPK ERK4 and its activation of the MAPK-activated protein kinase MK5[J]. J Biol Chem, 2006, 281(46): 35511-35519. doi: 10.1074/jbc.M606693200
[18] Demichev V, Messner CB, Vernardis SI, et al. DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput[J]. Nat Methods, 2020, 17(1): 41-44. doi: 10.1038/s41592-019-0638-x
[19] Malagon T, Franco EL, Tejada R, et al. Epidemiology of HPV-associated cancers past, present and future: towards prevention and elimination[J]. Nat Rev Clin Oncol, 2024, 21(7): 522-538. doi: 10.1038/s41571-024-00904-z
[20] Brisson M, Kim JJ, Canfell K, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries[J]. Lancet, 2020, 395(10224): 575-590. doi: 10.1016/S0140-6736(20)30068-4
[21] Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis[J]. Lancet Glob Health, 2020, 8(2): e191-e203. doi: 10.1016/S2214-109X(19)30482-6
[22] Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer[J]. N Engl J Med, 1999, 340(15): 1144-1153. doi: 10.1056/NEJM199904153401502
[23] Shen T, Wang W, Zhou W, et al. MAPK4 promotes prostate cancer by concerted activation of androgen receptor and AKT[J]. J Clin Invest, 2021, 131(4): 135465. doi: 10.1172/JCI135465
[24] Wang W, Han D, Cai Q, et al. MAPK4 promotes triple negative breast cancer growth and reduces tumor sensitivity to PI3K blockade[J]. Nat Commun, 2022, 13(1): 245. doi: 10.1038/s41467-021-27921-1
[25] Han D, Wang W, Jeon JH, et al. Cooperative activation of PDK1 and AKT by MAPK4 enhances cancer growth and resistance to therapy[J]. PLoS Biol, 2023, 21(8): e3002227. doi: 10.1371/journal.pbio.3002227
[26] Wang W, Shen T, Dong B, et al. MAPK4 overexpression promotes tumor progression via noncanonical activation of AKT/mTOR signaling[J]. J Clin Invest, 2019, 129(3): 1015-1029. doi: 10.1172/JCI97712
[27] Chen J, Yang J, Liu Y, et al. MAPK4 facilitates angiogenesis by inhibiting the ERK pathway in non-small cell lung cancer[J]. Cancer Innov, 2024, 3(3): e117. doi: 10.1002/cai2.117
[28] Fort J, de la Ballina LR, Burghardt HE, et al. The structure of human 4F2hc ectodomain provides a model for homodimerization and electrostatic interaction with plasma membrane[J]. J Biol Chem, 2007, 282(43): 31444-31452. doi: 10.1074/jbc.M704524200
[29] Verrey F, Closs EI, Wagner CA, et al. CATs and HATs: the SLC7 family of amino acid transporters[J]. Pflugers Arch, 2004, 447(5): 532-542. doi: 10.1007/s00424-003-1086-z
[30] Rodriguez CF, Escudero-Bravo P, Diaz L, et al. Structural basis for substrate specificity of heteromeric transporters of neutral amino acids[J]. Proc Natl Acad Sci U S A, 2021, 118(49): e2113573118. doi: 10.1073/pnas.2113573118
[31] Bridges RJ, Natale NR, Patel SA. System xc(-) cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS[J]. Br J Pharmacol, 2012, 165(1): 20-34. doi: 10.1111/j.1476-5381.2011.01480.x
[32] Digomann D, Kurth I, Tyutyunnykova A, et al. The CD98 Heavy Chain Is a Marker and Regulator of Head and Neck Squamous Cell Carcinoma Radiosensitivity[J]. Clin Cancer Res, 2019, 25(10): 3152-3163. doi: 10.1158/1078-0432.CCR-18-2951
[33] Wu P, Zhao L, Kong G, et al. Study on the Role and Mechanism of SLC3A2 in Tumor-Associated Macrophage Polarization and Bladder Cancer Cells Growth[J]. Technol Cancer Res Treat, 2024, 23: 15330338241246649.
[34] Wu F, Xiong G, Chen Z, et al. SLC3A2 inhibits ferroptosis in laryngeal carcinoma via mTOR pathway[J]. Hereditas, 2022, 159(1): 6. doi: 10.1186/s41065-022-00225-0



 吴令英: 博士,主任医师,博士生导师,北京协和医学院长聘教授和“教学名师”。中国临床肿瘤学会副理事长,中国妇幼保健协会常务理事,中国临床肿瘤学会妇科肿瘤专家委员会主任委员,中国妇幼保健协会妇幼微创专业委员会主任委员,中国抗癌协会妇科肿瘤专业委员会副主任委员,中国临床肿瘤学会临床研究专家委员会副主任委员,中国老年学及老年医学学会妇科分会副会长和中华医学会妇科肿瘤专业委员会常委等。Cancer Biology & Medicine、Oncology and Translational Medicine、《中华妇产科杂志》和《中华放射肿瘤学杂志》等杂志编委。长期从事妇科肿瘤的临床治疗与转化医学研究,作为负责人主持近30余项国际和国内多中心临床研究,主持国家重点研发计划项目和国家自然科学基金等20余项科研课题。作为通讯作者将近5年的研究成果发表于Journal of Clinical Oncology、JAMA Oncology和Clinical Cancer Research等杂志,牵头制定“中国临床肿瘤学会卵巢癌诊疗指南”等多项指南
。
吴令英: 博士,主任医师,博士生导师,北京协和医学院长聘教授和“教学名师”。中国临床肿瘤学会副理事长,中国妇幼保健协会常务理事,中国临床肿瘤学会妇科肿瘤专家委员会主任委员,中国妇幼保健协会妇幼微创专业委员会主任委员,中国抗癌协会妇科肿瘤专业委员会副主任委员,中国临床肿瘤学会临床研究专家委员会副主任委员,中国老年学及老年医学学会妇科分会副会长和中华医学会妇科肿瘤专业委员会常委等。Cancer Biology & Medicine、Oncology and Translational Medicine、《中华妇产科杂志》和《中华放射肿瘤学杂志》等杂志编委。长期从事妇科肿瘤的临床治疗与转化医学研究,作为负责人主持近30余项国际和国内多中心临床研究,主持国家重点研发计划项目和国家自然科学基金等20余项科研课题。作为通讯作者将近5年的研究成果发表于Journal of Clinical Oncology、JAMA Oncology和Clinical Cancer Research等杂志,牵头制定“中国临床肿瘤学会卵巢癌诊疗指南”等多项指南
。
 下载:
下载: