Risk Factors and Prognosis of Patients with Para-Aortic Lymph Node Metastasis of Advanced Esophagogastric Junction Malignancy
-
摘要:目的
通过对比进展期食管胃结合部恶性肿瘤患者一般临床病理特征及区域淋巴结转移状态,探讨患者主动脉旁淋巴结转移阳性的危险因素及其预后。
方法收集224例手术根治性切除食管胃结合部恶性肿瘤患者临床病理资料,对影响第16组淋巴结转移的危险因素进行单因素χ2检验和多因素Logistic回归分析。生存分析采用Kaplan-Meier法,生存率比较采用Log rank检验。
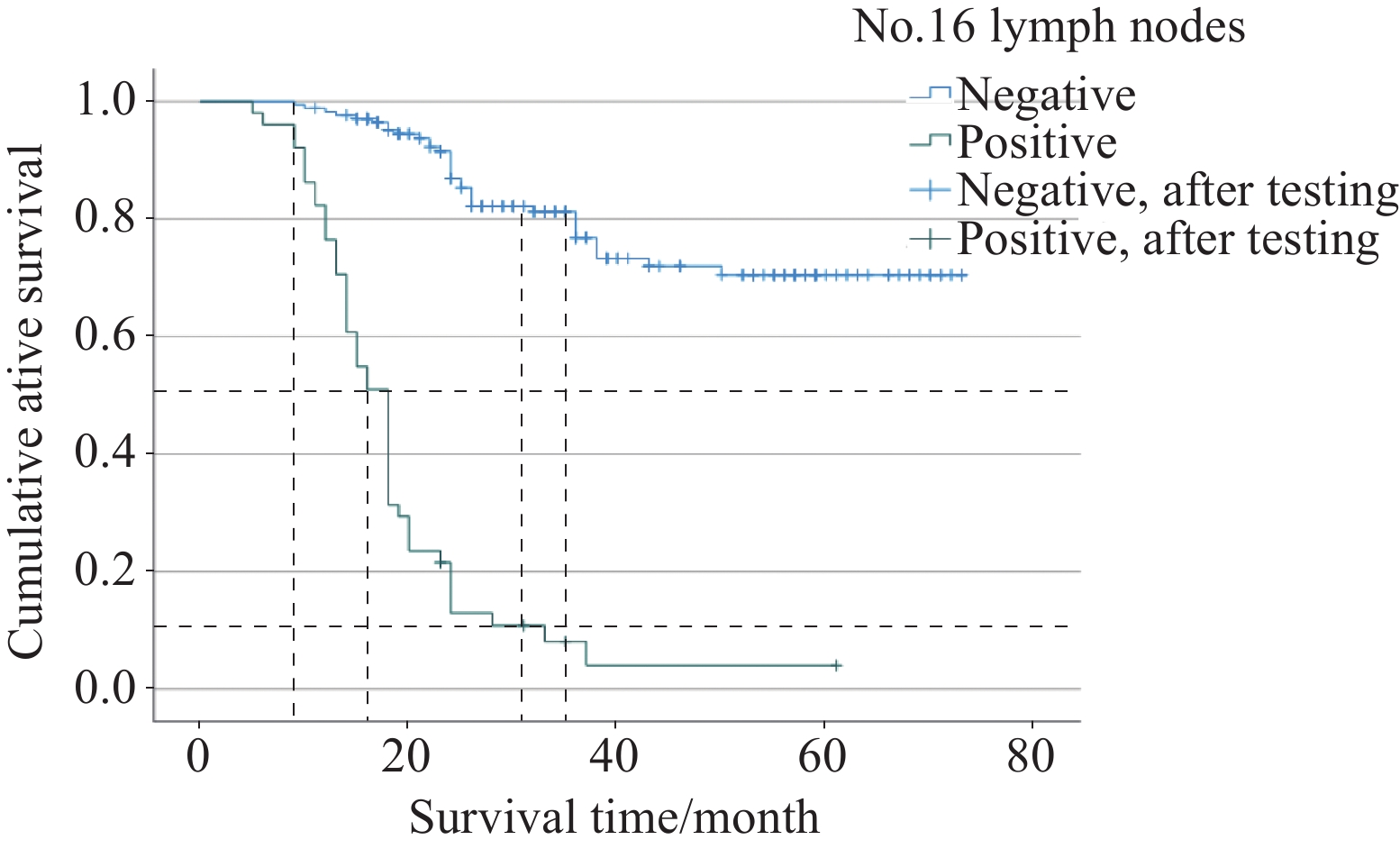
结果(1)单因素分析见Siewert分型、肿瘤大小、病理分期、T分期、N分期与主动脉旁淋巴结转移阳性相关(均P<0.05)。多因素分析发现Siewert分型、肿瘤大小均是其转移阳性的独立危险因素(均P<0.001)。(2)纳入的17组区域淋巴结与主动脉旁淋巴结转移相关的单因素分析中,除No.5、No.6、No.111、No.112组淋巴结与主动脉旁淋巴结转移阳性不相关(均P>0.05),剩余13组区域淋巴结均与其相关。多因素分析发现,No.7、No.11p、No.110组淋巴结均是其转移阳性的独立危险因素(均P<0.05)。进一步分析发现,当至少其中一组区域淋巴结转移时,阳性转移率高达47.4%(3组均阴性时仅4.7%)。(3)主动脉旁淋巴结转移阳性患者术后1年、3年累积生存率分别为76.5%、8.1%,阴性患者分别为98.3%、76.8%。Log rank检验表明两组总生存率存在明显差异(P<0.001)。
结论(1)进展期食管胃结合部恶性肿瘤患者术前检查应明确患者的肿瘤分型及大小,建议SiewertⅡ、Ⅲ型和肿瘤直径>6 cm的患者行主动脉旁淋巴结清扫术。(2)术中行No.7、No.11p、No.110淋巴结冰冻活检病理,发现转移阳性后行主动脉旁淋巴结清扫术,有助于术中对清扫主动脉旁淋巴结的判断。
-
关键词:
- 食管胃结合部恶性肿瘤 /
- 区域淋巴结 /
- 主动脉旁淋巴结 /
- 影响因素 /
- 预后
Abstract:ObjectiveTo determine the risk factors and prognostic survival of patients with para-aortic lymph node metastasis of advanced esophagogastric junction malignancy by comparing their general clinicopathological characteristics and regional lymph node metastasis status with those of patients with negative para-aortic lymph node metastasis.
MethodsThis single-center retrospective case study collected the clinical and pathological data of 224 patients with esophagogastric junction malignant tumors undergoing radical resection. Single factor affecting lymph node metastasis in group 16 was analyzed by chi square test, and multiple factors were examined using logistic regression. Kaplan-Meier method was used for survival analysis, and Log rank test was used for survival rate comparison.
ResultsAmong the 224 patients with advanced esophagogastric junction malignant tumors, (1) Univariate analysis showed that Siewert classification, tumor diameter, pathological stage, T stage, and N stage were associated with positive para-aortic lymph node metastasis (P<0.05). Meanwhile, multivariate logistic analysis showed that Siewert type and tumor diameter were independent risk factors for positive metastasis (P<0.05); (2) Among the 17 groups of regional lymph nodes with para-aortic lymph node metastasis, univariate analysis revealed that No.5, No.6, No.111, and No.112 lymph nodes were not correlated with positive para-aortic lymph node metastasis (P>0.05). The remaining 13 groups of regional lymph nodes were all associated with para-aortic lymph node metastasis. Meanwhile, multivariate logistic analysis revealed that No.7, No.11p, and No.110 lymph nodes were independent risk factors for metastasis (P<0.05). When the regional lymph node metastasis in these three groups was negative, the positive rate of para-aortic lymph node metastasis was only 4.7%. When at least one of these groups had regional lymph node metastasis, the positive metastasis rate was up to 47.4%; (3) The 1- and 3-year cumulative survival rates of the patients with positive para-aortic lymph node metastasis after surgery were 76.5% and 8.1%, respectively, and those in negative patients were 98.3% and 76.8%, respectively. Log rank test showed a significant difference in overall survival rate between the patients with positive and negative para-aortic lymph node metastasis (P<0.001).
Conclusion(1) Preoperative examination of patients with advanced esophagogastric junction malignant tumors should clarify the tumor type and maximum diameter. Patients with Siewert type Ⅱ and Ⅲ and maximum tumor diameter of >6 cm are recommended to undergo para-aortic lymph node dissection. (2) Frozen biopsy of lymph nodes of No.7 and No.11p is performed during the operation, and the para-aortic lymph nodes should be dissected if the metastasis is found to be positive, which is helpful for the clinical surgeon to judge the dissection of the para-aortic lymph nodes.
-
0 引言
目前,全球癌症的发病和死亡人数仍在持续增长,2020年新发肿瘤1 929.3万例、死亡995.8万例,癌症在许多国家的死因顺位上已经超越心血管疾病等高死亡率慢性疾病[1]。沈阳市城区居民全死因中,恶性肿瘤也仅次于心脏病[2],严重危害沈阳人民健康。为了解沈阳市城区居民恶性肿瘤发病及生存情况,并提出防治措施,现对2011—2018年沈阳市城区居民恶性肿瘤发病及生存趋势进行分析。
1 资料与方法
1.1 资料来源
2011—2018年沈阳市城区恶性肿瘤发病和生存资料来源于中国肿瘤登记中心肿瘤随访与登记报告系统。报告范围为具有沈阳市城市国家级监测点(和平区、沈河区、大东区、皇姑区和铁西区)户籍居民发病的全部恶性肿瘤(ICD10编码C00-C97)。人口资料来源于沈阳市公安局提供的每年份性别、年龄别的平均人口数。
1.2 质量评价
根据《中国肿瘤登记工作指导手册》[3]和国际癌症研究中心(IARC)/国际癌症登记协会(IACR)[4-5]对登记质量的有关要求,对数据的可靠性、完整性、有效性进行评估。沈阳市城区肿瘤发病数据从2008年起连续五年被国际五大洲癌症协会收录,连续十年被中国肿瘤登记年报收录。沈阳市城区2011—2018年上报新发肿瘤数据质量评价为病理诊断率(MV%)为66.63%、只有死亡医学证明书比例(DCO%)为2.43%、死亡发病比(M/I)为0.65,均符合质量要求。
1.3 统计学方法
提取2011—2018年沈阳城区恶性肿瘤发病数据,以国际疾病分类法ICD-10进行分类,并用IARCcrgTools软件进行数据审核。利用Excel2007计算粗发病率、标化率(中标率、世标率)、年龄别发病率、累积发病率(0~74岁)、截缩发病率(35~64岁)、前十位肿瘤发病顺位和生存率等指标;利用SPSS23.0统计软件对恶性肿瘤男女发病率及生存率进行χ2检验,检验水准α=0.05;采用寿命表法计算观察生存率,病例随访时间截止至2020-12-31;采用EdererⅡ方法(Ederer and Heise, 1959)计算期望生存率(expected survival rate, ESR)和相对生存率(relative survival rate, RSR);利用美国癌症中心研究所开发的Joinpoint 3.5.3软件计算发病率和生存率年度变化百分比(APC%),检验水准α=0.05。中标率采用2000年全国人口普查,世标率采用Segi's世界标准人口结构进行计算。
2 结果
2.1 总体发病分布
2011—2018年沈阳市上报新发恶性肿瘤109 873例,发病率为364.70/10万,中标率190.00/10万,世标率185.63/10万,0~74岁累积率为21.17%,35~64岁截缩率为311.66/10万。2011—2014年男、女、合计恶性肿瘤发病率及标化率均呈大幅上升趋势(P < 0.01);2015—2018年男、女、合计恶性肿瘤发病率缓慢上升但标化率缓慢下降(P≥0.05),见表 1。
表 1 2011—2018年沈阳市城区居民恶性肿瘤发病率(1/105)Table 1 Malignant tumor incidence of urban residents in Shenyang from 2011 to 2018 (1/105)
2.2 性别分布
2011—2018年沈阳市城区恶性肿瘤男性发病率为380.62/10万,女性发病率为349.42/10万,男、女发病率之比为1.09:1,8年间男性恶性肿瘤发病率高于女性,差异有统计学意义(χ2=201.63, P < 0.05),见表 1。
2.3 年龄分布
多数肿瘤在0~30岁组开始发病,30~40岁组缓慢上升,在40岁以后开始大幅度上升,在80~岁组达到发病高峰,85+岁组发病率略有下降,这可能与85+岁组人口急剧减少有关。男女以50~55岁组为界,发病率呈现X形状,见图 1。
2.4 发病顺位
2011—2018年沈阳市城区男性恶性肿瘤发病前10位的依次是肺癌、结直肠癌、肝癌、胃癌、膀胱癌、食管癌、胰腺癌、前列腺癌、肾癌、甲状腺癌;其中前5位肺癌(28.77%)、结直肠癌(16.40%)、肝癌(9.18%)、胃癌(9.15%)、膀胱癌(4.36%)占男性恶性肿瘤的67.86%。8年间肺癌、结直肠癌、膀胱癌、胰腺癌、前列腺癌、肾癌、甲状腺癌发病率均呈上升趋势(P < 0.05);而肝癌(P=0.00, P=0.05)、胃癌(P=0.02, P=0.07)、食管癌(P=0.08, P=0.24)发病率则先上升后下降,见表 2。
表 2 2011—2018年沈阳市城区男性恶性肿瘤发病顺位(1/105)Table 2 Incidence rank of malignant tumors in male residents in urban areas of Shenyang from 2011 to 2018 (1/105)
女性恶性肿瘤发病率前10位的依次是乳腺癌、肺癌、结直肠癌、宫颈癌、甲状腺癌、胃癌、肝癌、卵巢癌、胰腺癌、子宫体癌;其中前5位乳腺癌(22.63%)、肺癌(18.72%)、结直肠癌(12.84%)、宫颈癌(5.73%)、甲状腺癌(5.71%)占女性恶性肿瘤65.63%;8年间除宫颈癌、胃癌、肝癌、卵巢癌、子宫体癌外,乳腺癌、肺癌、结直肠癌、甲状腺癌、胰腺癌发病率均呈上升趋势(P < 0.05),见表 3。
表 3 2011—2018年沈阳市城区女性恶性肿瘤发病顺位(1/105)Table 3 Incidence rank of malignant tumors in female residents in urban areas of Shenyang from 2011 to 2018 (1/105)
2.5 恶性肿瘤5年生存率
2011—2015年沈阳市城区居民恶性肿瘤5年生存率为40.49%,相对生存率为47.84%。5年间合计观察生存率呈上升趋势,差异有统计学意义(P=0.04),其中男性为31.82%,女性为49.58%,男女观察生存率均呈上升趋势(P=0.04, P=0.03),且女性5年生存率高于男性(χ2=187.62, P < 0.05),见表 4。
表 4 2011—2015年沈阳市城区居民恶性肿瘤5年生存率(%)Table 4 Five-year survival rate of malignant tumors in urban residents in Shenyang from 2011 to 2015 (%)
2.6 发病前十位恶性肿瘤5年生存率顺位
2011—2015年沈阳市城区男性发病前十位的恶性肿瘤5年生存率顺位依次是甲状腺癌(86.25%)、肾癌(64.19%)、膀胱癌(59.43%)、结直肠癌(48.41%)、前列腺癌(47.55%)、胃癌(31.63%)、食管癌(20.56%)、肝癌(17.20%)、肺癌(16.79%)、胰腺癌(8.67%),见表 5。女性依次是甲状腺癌(91.81%)、乳腺癌(76.50%)、子宫体癌(73.17%)、子宫颈癌(65.18%)、结直肠癌(49.04%)、卵巢癌(43.34%)、胃癌(32.47%)、肺癌(21.20%)、肝癌(14.41%)、胰腺癌(10.01%),见表 6。
表 5 2011—2015年沈阳市城区男性发病前十位恶性肿瘤5年生存率(%)Table 5 Five-year survival rate of top ten malignant tumors among males in urban areas of Shenyang from 2011 to 2015 (%) 表 6 2011—2015年沈阳市城区女性发病前十位恶性肿瘤5年生存率(%)Table 6 Five-year survival rate of top ten malignant tumors among females in urban areas of Shenyang from 2011 to 2015 (%)
表 6 2011—2015年沈阳市城区女性发病前十位恶性肿瘤5年生存率(%)Table 6 Five-year survival rate of top ten malignant tumors among females in urban areas of Shenyang from 2011 to 2015 (%)
男女5年生存率最高均为甲状腺癌,最低均为胰腺癌。相同癌种中,肺癌、甲状腺癌5年生存率女性高于男性(χ2=48.29, χ2=9.85, P < 0.01),差异有统计学意义;肝癌男性高于女性(χ2=5.32, P < 0.05),差异有统计学意义;结直肠癌(χ2=0.37, P≥0.05)、胃癌(χ2=0.33, P≥0.05)、胰腺癌(χ2=0.99, P≥0.05)男女5年生存率差异无统计学意义。
2.7 发病前十位恶性肿瘤5年生存率变化趋势
2011—2015年沈阳市男性发病前十位的恶性肿瘤除结直肠癌、前列腺癌、肝癌外,其余七位中,甲状腺癌(APC%=12.97, P=0.03)、肾癌(APC%=7.86, P=0.01)、膀胱癌(APC%=10.04, P=0.00)、胃癌(APC%=6.57, P=0.05)、食管癌(APC%=6.05, P=0.03)、肺癌(APC%=11.81, P=0.04)、胰腺癌(APC%=25.57, P=0.02)5年生存率均呈上升趋势,见表 5。
女性发病前十位的恶性肿瘤除子宫体癌、结直肠癌、胃癌、肺癌外,其余六位的甲状腺癌(APC%=7.93, P=0.01)、乳腺癌(APC%=3.87, P=0.05)、宫颈癌(APC%=4.96, P=0.00)、卵巢癌(APC%=10.75, P=0.03)、肝癌(APC%=20.09, P=0.01)、胰腺癌(APC%=49.75, P=0.01)5年生存率均呈上升趋势,见表 6。
3 讨论
从发病情况看,沈阳市2011—2018年城区居民恶性肿瘤发病率持续上升(P=0.00, P=0.67),发病中标率(190.00/10万)低于2015年中国城市恶性肿瘤发病中标率(193.93/10万)[6]和中国东部恶性肿瘤发病中标率(194.36/10万)[7],高于中部恶性肿瘤发病中标率(183.36/10万)[8],低于2006—2015年辽宁省五城市恶性肿瘤标化发病率(199.15/10万)[9],高于2016年安徽(179.70/10万)[10]和江苏(182.61/10万)[11]、2017年黑龙江(174.27/10万)[12],目前发病率处于全国中等水平。
2011—2018年沈阳市恶性肿瘤发病率男性高于女性(χ2=201.63, P < 0.05),与中国分布相一致[13],且随着年龄的增长呈明显上升趋势,而沈阳市老龄人口亦呈逐年上升趋势,这既反映了人口老龄化进程的加快,也反映了癌症相关危险因素暴露时间的增加[14]是沈阳市癌症高发的重要原因。因此要针对病因和危险因素,致力于通过精准、适度和有效的干预,降低癌症发生风险。
2011—2018年男女恶性肿瘤发病顺位前三位与国家《2018肿瘤登记年报》发布中国城市数据[8]一致。8年间,男性发病前十位除肝癌、胃癌、食管癌发病率先上升后下降,其他癌种均呈上升趋势;女性除了妇科肿瘤和肝癌、胃癌外,其他发病前十的恶性肿瘤发病率均呈上升趋势。研究表明与感染或贫困相关的癌症正逐渐被经济发达国家的常见癌症所取代[15]。而沈阳市由于经济的发展和生活水平的提高,不良生活方式的加剧,不仅原有主要高发癌症尚未有明显下降趋势,西方国家高发的大肠癌、前列腺癌和女性乳腺癌等癌症发病又迅速增加。
从生存情况看,2011—2015年沈阳市城区恶性肿瘤5年观察生存率为40.49%、相对生存率为47.84%,接近于2018年全国公布的全部癌症的5年生存率(40.5%)[16],2019年辽宁省公布的城市癌症5年标化生存率(41.5%)[17],2012—2016年上海市青浦区5年相对生存率为47.41%[18],高于姑苏区2008—2013年癌症患者的5年相对生存率(42.2%)[19]。女性的生存率总体高于男性(χ2=187.62, P < 0.05)。
随着医疗技术水平的提升,沈阳市癌症5年生存率也大幅上升,但仍有部分癌种5年生存率无上升趋势,这与癌症种类构成不同和筛查手段落后造成生存率差异有重要关系。2011—2015年沈阳市城区恶性肿瘤5年生存率最高的癌种为甲状腺癌,其次是女性的乳腺癌(76.50%)、子宫体癌(73.17%)、宫颈癌(65.18%);男女生存率排在后三位是肝癌(17.20%、14.41%)、肺癌(16.79%、21.20%)、胰腺癌(8.67%、10.01%);生存率最低的均为胰腺癌。而这些生存率较高的癌症可以通过早期筛查项目,寻找出高危人群或早期患者,进行早发现、早诊断和早治疗的“二级预防”,是有效提升癌症生存的关键手段;对于生存率较低的癌症我们要依托于生物医学各学科不断发展的各种新技术、新手段,不断探索癌症相关标志物,优化筛查策略,早期发现、规范治疗,以提升患者生存率。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:牛 权:数据收集、整理、统计分析,文章撰写毕小刚:文章审阅及修改 -
表 1 患者一般临床病理资料
Table 1 General clinicopathological data of patients
Variable n=224(%) Gender Male 190(84.8) Female 34(15.2) Age(years) 64.45(40-89) Siewert type Ⅰ 8(3.6) Ⅱ 151(67.4) Ⅲ 65(29.0) Neoadjuvant chemotherapy Yes 16(7.1) No 208(92.9) Maximum tumor diameter(cm) Mean 4.64(1-13) Degree of differentiation(adenocarcinoma) G1 7(3.1) G2 66(29.5) G3 52(23.2) G4 99(44.2) Pathologic stage Ⅱa 41(18.3) Ⅱb 47(21.0) Ⅲa 44(19.6) Ⅲb 33(14.7) Ⅲc 7(3.1) Ⅳ 52(23.2) T stage T2 18(8.0) T3 115(51.3) T4a 87(38.8) T4b 4(1.8) N stage N0 51(22.8) N1 42(18.8) N2 54(24.1) N3 77(34.4) Surgical modality Proximal gastrectomy 72(32.1) Total gastrectomy 152(67.9) 表 2 一般临床病理资料与第16组淋巴结转移相关的单因素分析
Table 2 Univariate analysis of general clinicopathological data related to No.16 lymph node metastasis
Variable n (%) No.16 positive
metastasis rate/
% (n)χ2 P Gender 0.598 0.439 Male 190(84.8) 23.7(45) Female 34(15.2) 17.6(6) Age (years) 0.734 0.392 ≥70 64(28.6) 26.6(17) <70 160(71.4) 21.3(34) Siewert type 15.514 <0.001 Ⅰ 8(3.6) 12.5(1) Ⅱ 151(67.4) 15.9(24) Ⅲ 65(29.0) 40.0(26) Neoadjuvant
chemotherapy0.049 0.764 Yes 16(7.1) 25.0(4) No 208(92.9) 22.6(47) Maximum tumor
diameter(cm)15.750 <0.001 >6 38(17.0) 47.4(18) ≤6 186(83.0) 17.7(33) Degree of
differentiation0.794 0.373 G1-2 73(32.6) 19.2(14) G3-4 151(67.4) 24.5(37) Pathologic stage 218.422 <0.001 Ⅱ-Ⅲ 172(76.8) 0.0(0) Ⅳ 52(23.2) 98.1(51) T stage 5.770 0.015 T2 18(8.0) 0.0(0) T3-T4 206(92.0) 24.8(51) N stage 46.880 <0.001 N0-N1 93(41.5) 0.0(0) N2-N3 131(58.5) 38.9(51) 表 3 患者一般临床病理资料与第16组淋巴结转移相关的多因素Logistic分析
Table 3 Multivariate Logistic analysis of general clinicopathological data of patients related to No.16 lymph node metastasis
Variable Regression coefficient Standard error Wald Degree of freedom P OR (95%CI) Siewert type 1.174 0.320 13.472 1 <0.001 3.235 (1.728-6.055) Maximum tumor diameter 1.429 0.377 14.332 1 <0.001 4.173 (1.992-8.742) 表 4 各区域淋巴结与第16组淋巴结转移相关的单因素分析
Table 4 Univariate analysis of lymph nodes in each region associated with No.16 lymph node metastasis
Station Positive rate in
each group
/%(n)No.16 positive
metastasis rate
/%(n)χ2 P No.1 53.1(119) 37.0(44) 29.140 <0.001 No.2 15.6(35) 40.0(14) 7.005 0.008 No.3 63.8(143) 32.9(47) 22.938 <0.001 No.4 20.1(45) 51.1(23) 25.727 <0.001 No.5 1.3(3) 66.7(2) 3.332 0.131 No.6 1.3(3) 66.7(2) 3.332 0.131 No.7 36.2(81) 49.4(40) 51.112 <0.001 No.8a 12.5(28) 67.9(19) 36.998 <0.001 No.8p 4.5(10) 90.0(9) 26.907 <0.001 No.9 9.4(21) 81.0(17) 44.613 <0.001 No.10 6.3(14) 78.6(11) 26.446 <0.001 No.11p 16.5(37) 67.6(25) 50.587 <0.001 No.11d 3.6(8) 62.5(5) 7.448 0.016 No.12 3.1(7) 85.7(6) 24.511 <0.001 No.110 12.9(29) 75.9(22) 53.405 <0.001 No.111 0.4(1) 100(1) 3.407 0.228 No.112 2.2(5) 60.0(3) 4.032 0.079 Notes: No.1: right lymph node of cardia; No.2: left lymph node of cardia; No.3: lymph nodes on the lesser curvature of the stomach; No.4: lymph nodes on the greater curvature of the stomach; No.5: suprapyloric lymph nodes; No.6: subpyloric lymph nodes; No.7: lymph nodes of left gastric artery; No.8a: anterior superior lymph nodes of common hepatic artery; No.8p: posterior lymph nodes of common hepatic artery; No.9: celiac trunk lymph nodes; No.10: splenic hilum lymph nodes; No.11p: proximal splenic artery lymph node; No.11d: distal splenic artery lymph nodes; No.12: lymph nodes within the hepatoduodenal ligament; No.110: periesophageal lymph nodes in the lower thoracic region; No.111: supradiaphragmatic lymph nodes; No.112: posterior mediastinal lymph nodes. 表 5 各组淋巴结与第16组淋巴结转移相关的多因素Logistic分析
Table 5 Multivariate Logistic analysis of lymph nodes in each group associated with No.16 lymph node metastasis
Station Regression coefficient Standard error Wald Degree of freedom P OR (95%CI) No.1 1.264 0.656 3.718 1 0.054 3.541(0.979-12.800) No.2 −0.644 0.733 0.771 1 0.380 0.525(0.125-2.211) No.3 1.064 0.753 1.999 1 0.157 2.899(0.663-12.676) No.4 0.410 0.635 0.418 1 0.518 1.508(0.435-5.229) No.7 1.108 0.563 3.877 1 0.049 3.029(1.005-9.126) No.8a 1.098 0.715 2.358 1 0.125 2.998(0.738-12.175) No.8p 0.745 1.510 0.243 1 0.622 2.106(0.109-40.655) No.9 1.153 0.832 1.919 1 0.166 3.168(0.620-16.191) No.10 1.099 0.996 1.217 1 0.270 3.002(0.426-21.162) No.11p 1.651 0.648 6.496 1 0.011 5.214(1.464-18.563) No.11d 1.648 1.074 2.353 1 0.125 5.195(0.633-42.641) No.12 1.789 1.243 2.356 1 0.131 5.196(0.725-40.256) No.110 2.407 0.646 13.881 1 <0.001 11.101(3.129-39.383) 表 6 No.7、No.11p、No.110与第16组淋巴结转移率的关系
Table 6 Relationship between the rate of lymph node metastasis between No.7, No.11p, No.110 and No.16
Lymph node metastasis status in No.7, No.11p, No.110 No.16 lymph node metastasis status No.16 positive metastasis rate (%) Metastasis (–) Metastasis(+) All negative results 123 6 4.7 Positive results for at least a regional of lymph nodes 50 45 47.4 表 7 第16组淋巴结转移患者生存分析时间的平均值
Table 7 Mean value of the survival analysis time of patients with No.16 lymph node metastasis
No.16 lymph node
metastasis statusEstimate Standard
error95%CI Negative 59.780 1.926 56.005-63.555 Positive 18.998 1.644 15.776-22.220 Population 50.000 1.952 46.173-53.826 -
[1] Rodriguez GM, DePuy D, Aljehani M, et al. Trends in epidemiology of esophageal cancer in the US, 1975-2018[J]. JAMA Netw Open, 2023, 6(8): e2329497. doi: 10.1001/jamanetworkopen.2023.29497
[2] 中国医师协会内镜医师分会腹腔镜外科专业组, 国际食管疾病学会中国分会, 中国食管胃结合部腺癌研究协作组, 等. 食管胃结合部腺癌外科治疗中国专家共识(2024年版)[J]. 中华胃肠外科杂志, 2024, 27(2): 109-126. [Laparoscopic Surgery Group of the Endoscopist Branch in the Chinese Medical Doctor Association (CMDA), Chinese Society for Diseases of the Esophagus (CSDE), China Esophageal Gastric Junction Adenocarcinoma Research Collaboration Group, et al. Chinese expert consensus on the surgical treatment for adenocarcinoma of esophagogastric junction (Edition 2024)[J]. Zhonghua Wei Chang Wai Ke Za Zhi, 2024, 27(2): 109-126.] doi: 10.3760/cma.j.cn441530-20231212-00213 Laparoscopic Surgery Group of the Endoscopist Branch in the Chinese Medical Doctor Association (CMDA), Chinese Society for Diseases of the Esophagus (CSDE), China Esophageal Gastric Junction Adenocarcinoma Research Collaboration Group, et al. Chinese expert consensus on the surgical treatment for adenocarcinoma of esophagogastric junction (Edition 2024)[J]. Zhonghua Wei Chang Wai Ke Za Zhi, 2024, 27(2): 109-126. doi: 10.3760/cma.j.cn441530-20231212-00213
[3] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[4] Manabe N, Matsueda K, Haruma K. Epidemiological Review of Gastroesophageal Junction Adenocarcinoma in Asian Countries[J]. Digestion, 2022, 103(1): 29-36. doi: 10.1159/000519602
[5] Hosokawa Y, Kinoshita T, Konishi M, et al. Clinicopathological features and prognostic factors of adenocarcinoma of the esophagogastric junction according to Siewert classification: experiences at a single institution in Japan[J]. Ann Surg Oncol, 2012, 19(2): 677-683. doi: 10.1245/s10434-011-1983-x
[6] Hasegawa S, Yoshikawa T. Adenocarcinoma of the esophagogastric junction: incidence, characteristics, and treatment strategies[J]. Gastric Cancer, 2010, 13(2): 63-73. doi: 10.1007/s10120-010-0555-2
[7] Nomura E, Sasako M, Yamamoto S, et al. Gastric Cancer Surgical Study Group of the Japan Clinical Oncology Group. Risk factors for para-aortic lymph node metastasis of gastric cancer from a randomized controlled trial of JCOG9501[J]. Jpn J Clin Oncol, 2007, 37(6): 429-433. doi: 10.1093/jjco/hym067
[8] Kurokawa Y, Takeuchi H, Doki Y, et al. Mapping of Lymph Node Metastasis From Esophagogastric Junction Tumors: A Prospective Nationwide Multicenter Study[J]. Ann Surg, 2021, 274(1): 120-127. doi: 10.1097/SLA.0000000000003499
[9] de Manzoni G, Di Leo A, Roviello F, et al. Tumor site and perigastric nodal status are the most important predictors of para-aortic nodal involvement in advanced gastric cancer[J]. Ann Surg Oncol, 2011, 18(8): 2273-2280. doi: 10.1245/s10434-010-1547-5
[10] Liang H, Deng J. Evaluation of rational extent lymphadenectomy for local advanced gastric cancer[J]. Chin J Cancer Res, 2016, 28(4): 397-403. doi: 10.21147/j.issn.1000-9604.2016.04.02
[11] Fujitani K, Miyashiro I, Mikata S, et al. Pattern of abdominal nodal spread and optimal abdominal lymphadenectomy for advanced Siewert type Ⅱ adenocarcinoma of the cardia: results of a multicenter study[J]. Gastric Cancer, 2013, 16(3): 301-308. doi: 10.1007/s10120-012-0183-0
[12] Goto H, Tokunaga M, Miki Y, et al. The optimal extent of lymph node dissection for adenocarcinoma of the esophagogastric junction differs between Siewert type Ⅱ and Siewert type Ⅲ patients[J]. Gastric Cancer, 2014, 18(2): 375-381.
[13] Yoshikawa T, Takeuchi H, Hasegawa S, et al. Theoretical therapeutic impact of lymph node dissection on adenocarcinoma and squamous cell carcinoma of the esophagogastric junction[J]. Gastric Cancer, 2016, 19(1): 143-149. doi: 10.1007/s10120-014-0439-y
[14] Wang JB, Lin MQ, Li P, et al. The prognostic relevance of parapyloric lymph node metastasis in Siewert type Ⅱ/Ⅲ adenocarcinoma of the esophagogastric junction[J]. Eur J Surg Oncol, 2017, 43(12): 2333-2340. doi: 10.1016/j.ejso.2017.08.017
[15] Cai MZ, Lv CB, Cai LS, et al. Priority of lymph node dissection for advanced esophagogastric junction adenocarcinoma with the tumor center located below the esophagogastric junction[J]. Medicine (Baltimore), 2019, 98(51): e18451. doi: 10.1097/MD.0000000000018451
[16] Chen XD, Chen QC, Xu R, et al. Therapeutic value of lymph node dissection for Siewert type Ⅱ and Ⅲ adenocarcinoma: meta-analysis[J]. BJS Open, 2024, 8(1): zrad138. doi: 10.1093/bjsopen/zrad138
[17] Junfeng Z, Yingxue H, Peiwu Y. Systematic review of risk factors for metastasis to para-aortic lymph nodes in gastric cancer[J]. Surg Oncol, 2013, 22(4): 210-216. doi: 10.1016/j.suronc.2013.10.003
[18] Dong YP, Deng JY. Advances in para-aortic nodal dissection in gastric cancer surgery: A review of research progress over the last decade[J]. World J Clin Cases, 2020, 8(13): 2703-2716. doi: 10.12998/wjcc.v8.i13.2703
[19] Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer[J]. N Engl J Med, 2008, 359(5): 453-462. doi: 10.1056/NEJMoa0707035
[20] Liu K, Yang K, Zhang W, et al. Changes of Esophagogastric Junctional Adenocarcinoma and Gastroesophageal Reflux Disease Among Surgical Patients During 1988-2012: A Single-institution, High-volume Experience in China[J]. Ann Surg, 2016, 263(1): 88-95. doi: 10.1097/SLA.0000000000001148
[21] Yabusaki H, Nashimoto A, Matsuki A, et al. Comparison of the surgical treatment strategies for Siewert type Ⅱ squamous cell carcinoma in the same area as esophagogastric junction carcinoma: data from a single Japanese high-volume cancer center[J]. Surg Today, 2014, 44(8): 1522-1528. doi: 10.1007/s00595-013-0773-4
[22] Hasegawa S, Yoshikawa T, Rino Y, et al. Priority of lymph node dissection for Siewert type Ⅱ/Ⅲ adenocarcinoma of the esophagogastric junction[J]. Ann Surg Oncol, 2013, 20(13): 4252-4259. doi: 10.1245/s10434-013-3036-0
[23] Zhang C, He Y, Schwarz RE, et al. Evaluation of para-aortic nodal dissection for locoregionally advanced gastric cancer with 1-3 involved para-aortic nodes[J]. Chin Med J (Engl), 2014, 127(3): 435-441. doi: 10.3760/cma.j.issn.0366-6999.20130664
-
期刊类型引用(3)
1. 贺明,汤成,梁艳,黄义娟,陶然. 2014-2016年重庆市九龙坡区新发恶性肿瘤生存分析. 社区医学杂志. 2023(03): 115-118+123 .  百度学术
百度学术
2. 李红,徐幽琼,郑婉辉,陆璐. 福州市2018年恶性肿瘤发病与死亡分析. 现代肿瘤医学. 2023(18): 3481-3485 .  百度学术
百度学术
3. 亓琳,张欢欢,沈自芳,杨蕊. 彩色多普勒超声特征对乳腺无症状炎性改变和浸润性导管癌的鉴别诊断价值. 中国医药导报. 2023(28): 164-167 .  百度学术
百度学术
其他类型引用(0)



 下载:
下载: