Value of Inflammatory Load in Predicting Prognosis of Elderly Patients with Epithelial Ovarian Cancer
-
摘要:目的
探讨血液炎症负荷在预测老年上皮性卵巢癌患者预后中的价值。
方法回顾性分析诊断为上皮性卵巢癌的老年(初诊年龄≥65岁)患者的临床资料和相关外周血参数。应用单变量和多变量Cox回归构建炎症相关的血液评分系统,使用Kaplan-Meier法进行生存分析,采用Cox风险比例回归分析确定独立预后影响因素,基于独立预后影响因素构建列线图模型并使用ROC曲线、C指数及校准曲线评价列线图模型。
结果血液中炎症负荷高的患者预后更差(P=0.002)。与炎症负荷低组相比,炎症负荷高的上皮性卵巢癌患者临床分期更晚、腹水量更多(P<0.05)。Cox风险比例回归分析显示ACCI、CA125值、残留病灶和血液中炎症负荷是影响总生存期的独立预后因素(P<0.05)。
结论血液中的炎症负荷是老年上皮性卵巢癌患者预后的生物标志物,通过对血液中炎症负荷评分可为卵巢癌患者的疗效监测和治疗干预提供帮助。
Abstract:ObjectiveTo explore the value of blood inflammatory load in predicting overall survival of elderly patients with epithelial ovarian cancer (EOC).
MethodsElderly patients with EOC were selected, and their clinical data and peripheral blood parameters were collected. We constructed an inflammation-related blood scoring system using univariate and multivariate Cox regression analysis. The Kaplan-Meier method was used for survival analysis. We used Cox proportional hazards analysis to identify the independent prognostic factors. A nomogram model was constructed based on independent prognostic factors, and the receiver operating characteristic curve, C-index, and calibration curve were used to evaluate the model.
ResultsPatients with high blood inflammatory load had worse prognosis (P=0.002). Compared with the low inflammatory load group, patients with high inflammatory load had later clinical stages and larger ascites volume (P<0.05). Cox regression analysis showed that ACCI, CA125, residual lesions, and blood score were independent factors affecting overall survival (P<0.05).
ConclusionThe blood inflammatory load is the biomarker for the prognosis of elderly patients with EOC. Scoring the inflammatory load in the blood can assist in efficacy monitoring and treatment intervention of ovarian cancer patients.
-
Key words:
- Inflammation /
- Ovarian cancer /
- Aged /
- Prognosis
-
0 引言
上皮性卵巢恶性肿瘤(epithelial ovarian cancer, EOC)是常见的妇科恶性肿瘤之一,据全球恶性肿瘤统计,EOC新发病例占所有女性癌症的3.4%,死亡例数占所有女性因癌症死亡病例的4.7%[1],约70%患者诊断时为疾病的中晚期[2]。尽管外科手术的进步及新系统疗法的引入,患者的总生存率仍未得到提高。老年患者在EOC患者中占一半以上[1],随着患者年龄和并发症的增加,与治疗相关不良反应的风险也将增加,这导致了老年患者接受标准治疗方案的人数减少[3-5]。因此,如何早期发现并积极干预对提高老年患者治疗效果和改善预后是亟待解决的课题。
近年来,随着肿瘤临床和基础研究的不断深入,研究者们发现患者自身的炎症状态与肿瘤的发生发展密切相关[6],故其可能是有潜力的肿瘤诊断和预后标志物。炎症相关的标志物如中性粒细胞与淋巴细胞比值(neutrophil-to-lymphocyte ratio, NLR)、淋巴细胞与单核细胞比值(lymphocyte to monocyte ratio, LMR)、血小板与淋巴细胞比值(platelet to lymphocyte ratio, PLR)、白蛋白与球蛋白比值(albumin-to-globulin ratio, AGR)、纤维蛋白原与淋巴细胞比值(fibrinogen-to-lymphocyte ratio, FLR)已被证明在多种癌症中具有临床意义[7-9]。
本研究我们通过外周血相关指标评估老年性EOC的全身炎症状态,探索炎症负荷对老年性EOC的预后预测和诊疗价值。
1 资料与方法
1.1 一般资料
回顾性收集2013年12月至2022年4月在武汉大学中南医院就诊的老年(初诊年龄≥65岁)上皮性卵巢癌(epithelial ovarian cancer, EOC)患者的临床资料和相关外周血参数。纳入标准:(1)初诊且初诊年龄均≥65岁;(2)经病理确诊为上皮性卵巢癌;(3)可获得完整的临床病理资料。排除标准:(1)近期曾患感染性疾病且未治愈者;(2)患有血液系统疾病者;(3)曾使用对血液学有影响的药物。本研究最终纳入138例患者。患者资料收集和研究已获得伦理委员会的批准(伦理号:临研伦[2021087])。
1.2 数据收集
收集患者的临床信息,主要包括年龄、体重指数(body mass index, BMI)、并发症、血液学指标和肿瘤专科信息。血液学指标为患者治疗前一周内的血液学检查结果,包括CA125、血液常规检查(中性粒细胞绝对值计数、淋巴细胞绝对值计数、单核细胞绝对值计数、血小板计数)、血清白蛋白、球蛋白以及纤维蛋白原水平。肿瘤专科信息包括:是否选择接受姑息治疗、国际妇产科联合会(Federation International of Gynecology and Obstetrics, FIGO)分期、病理类型、残留病灶(R0定义为手术后无肉眼可见的残留病灶,R1即为残留病灶大小≤1 cm, R2为残留病灶大小>1 cm)、术后并发症、是否接受腹腔热灌注治疗(hyperthermic intraperitoneal chemotherapy, HIPEC)。采取电话、门诊随诊、复查资料的方式对患者进行随访(初始治疗后2年内每2~4月随访一次,3年后每3~6月随访一次,5年后每12月随访一次),记录患者的死亡时间。患者总生存时间(overall survival, OS)为组织学或细胞学诊断与任何原因导致的死亡之间的时间间隔。年龄调整查尔森合并症指数(age-adjusted Charlson comorbidity index, ACCI)将患者的年龄作为校正变量纳入最终查尔森合并症指数以评估患者合并症情况,是预测各种癌症预后的有用工具[10]。
1.3 统计学方法
所有数据使用R 4.3.0(http://www.R-project ct.org/)软件进行统计分析。血清白蛋白、单核细胞计数、血小板计数的临界值根据R软件survminer包的操作系统计算得出:单核细胞计数临界值为0.29,血小板计数临界值为249.00,白蛋白临界值为36.50。根据获得的临界值将患者分为两组(高组和低组)。采用单因素和多因素Cox回归分析根据炎症相关参数构建炎症相关的血液评分。使用Kaplan-Meier曲线描述组间的生存差异。利用时间依赖ROC曲线评价评分系统的预测效果。使用Kruskal-Wallis检验及费舍尔精确检验检测各个临床变量与本研究构建的血液评分之间的相关性。采用单因素和多因素Cox比例风险回归分析OS的独立预后因素。使用C指数、ROC曲线、校准曲线评估列线图的预测准确性。P<0.05为差异有统计学意义。
2 结果
2.1 老年上皮性卵巢癌患者的临床特征
本研究共纳入138例老年上皮性卵巢癌患者,患者年龄范围65~84岁,中位年龄68岁,平均年龄69.86±4.68岁。其中有88例高级别浆液性卵巢癌,其他病理类型上皮性卵巢癌有50例。根据FIGO分期标准(第8版),Ⅰ~Ⅱ期23例,Ⅲ~Ⅳ期115例。
2.2 外周血指数的生存分析
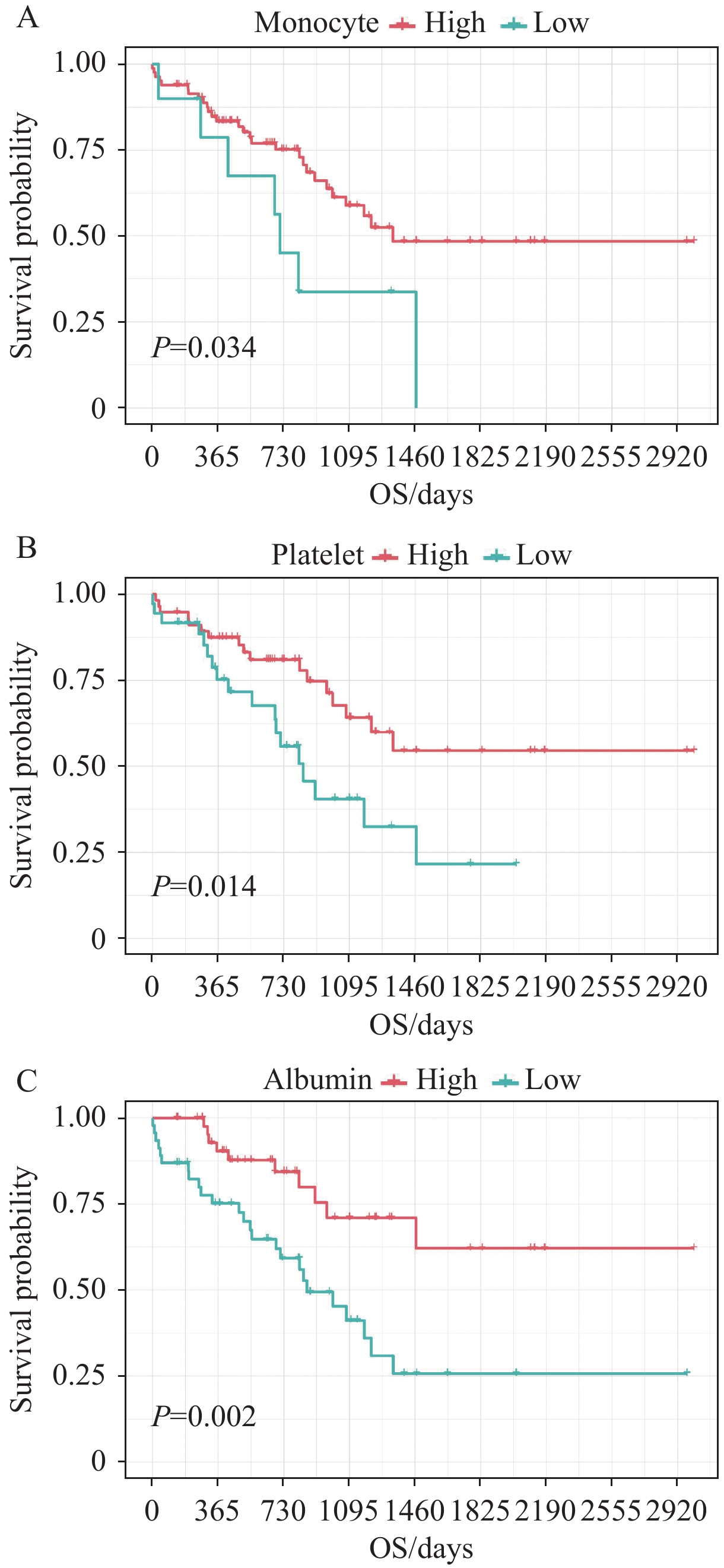
单因素Cox回归分析结果显示:血清白蛋白、单核细胞计数、血小板计数与OS显著相关(P<0.05),见表1。分析发现:高单核细胞计数(≥0.29)、高血小板计数(≥249.00)、高白蛋白值(≥36.50)是预后保护因素(均P<0.05),见图1。
表 1 单因素Cox回归分析外周血参数中OS的影响因素Table 1 Cox univariate analysis of the peripheral blood factors affecting OSCharacteristics HR 95%CI P NLR 0.467 0.143-1.525 0.207 LMR 1.691 0.850-3.361 0.134 PLR 2.304 0.812-6.536 0.117 AGR 2.034 0.922-4.483 0.078 FLR(g/109) 2.323 0.962-5.610 0.061 Neutrophil(109/L) 1.636 0.828-3.233 0.157 Lymphocyte(109/L) 3.110 0.743-13.020 0.120 Monocyte(109/L) 2.388 1.040-5.483 0.040 Platelet(109/L) 2.261 1.160-4.407 0.017 Albumin(g/L) 3.013 1.444-6.285 0.003 Globulin(g/L) 0.575 0.250-1.319 0.192 Fibrinogen(g/L) 1.623 0.827-3.184 0.159 Notes: NLR: neutrophil-to-lymphocyte ratio; LMR: lymphocyte to monocyte ratio; PLR: platelet to lymphocyte ratio; AGR: albumin-to-globulin ratio; FLR: fibrinogen-to-lymphocyte ratio. 2.3 构建老年上皮性卵巢癌炎症血液评分
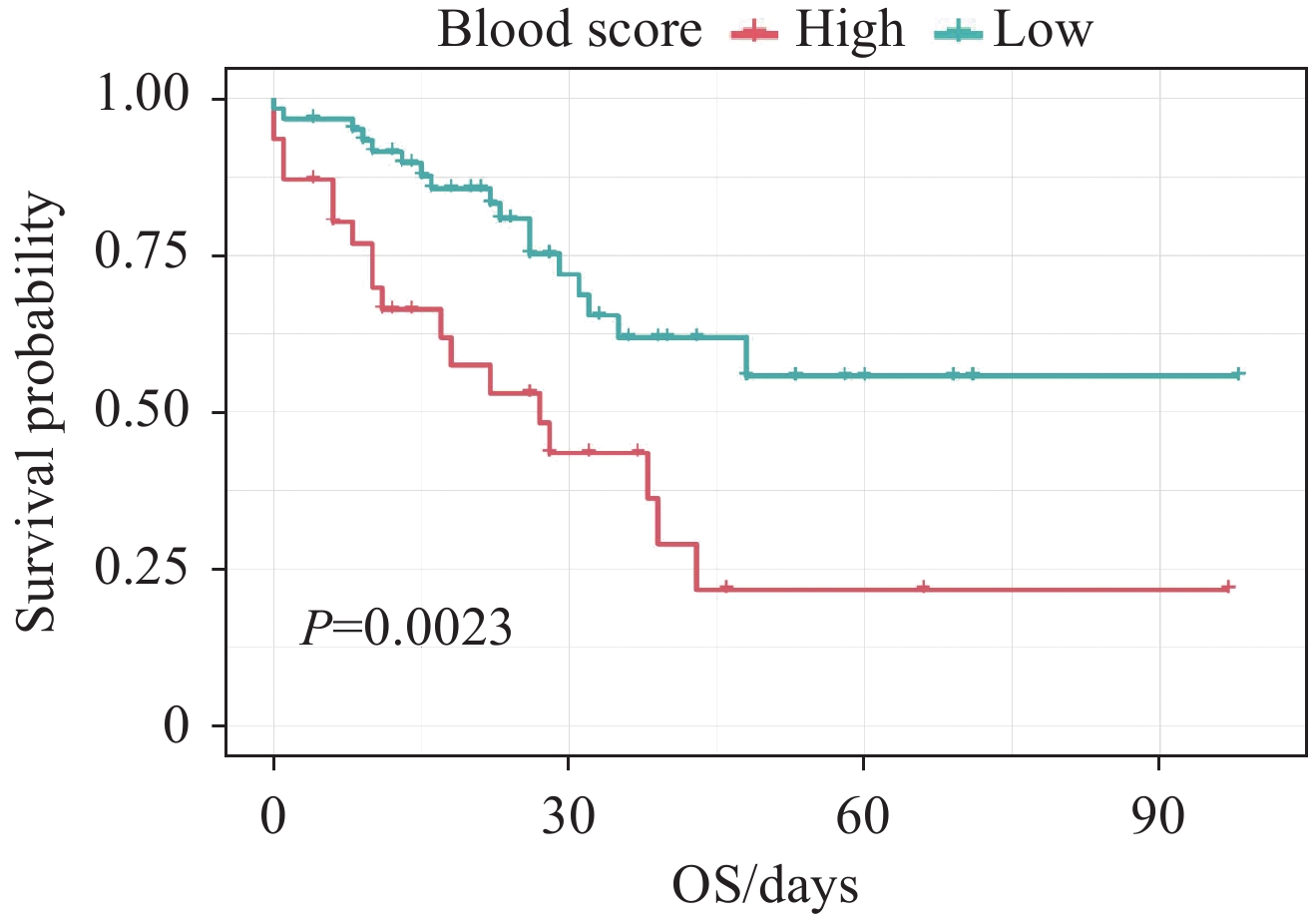
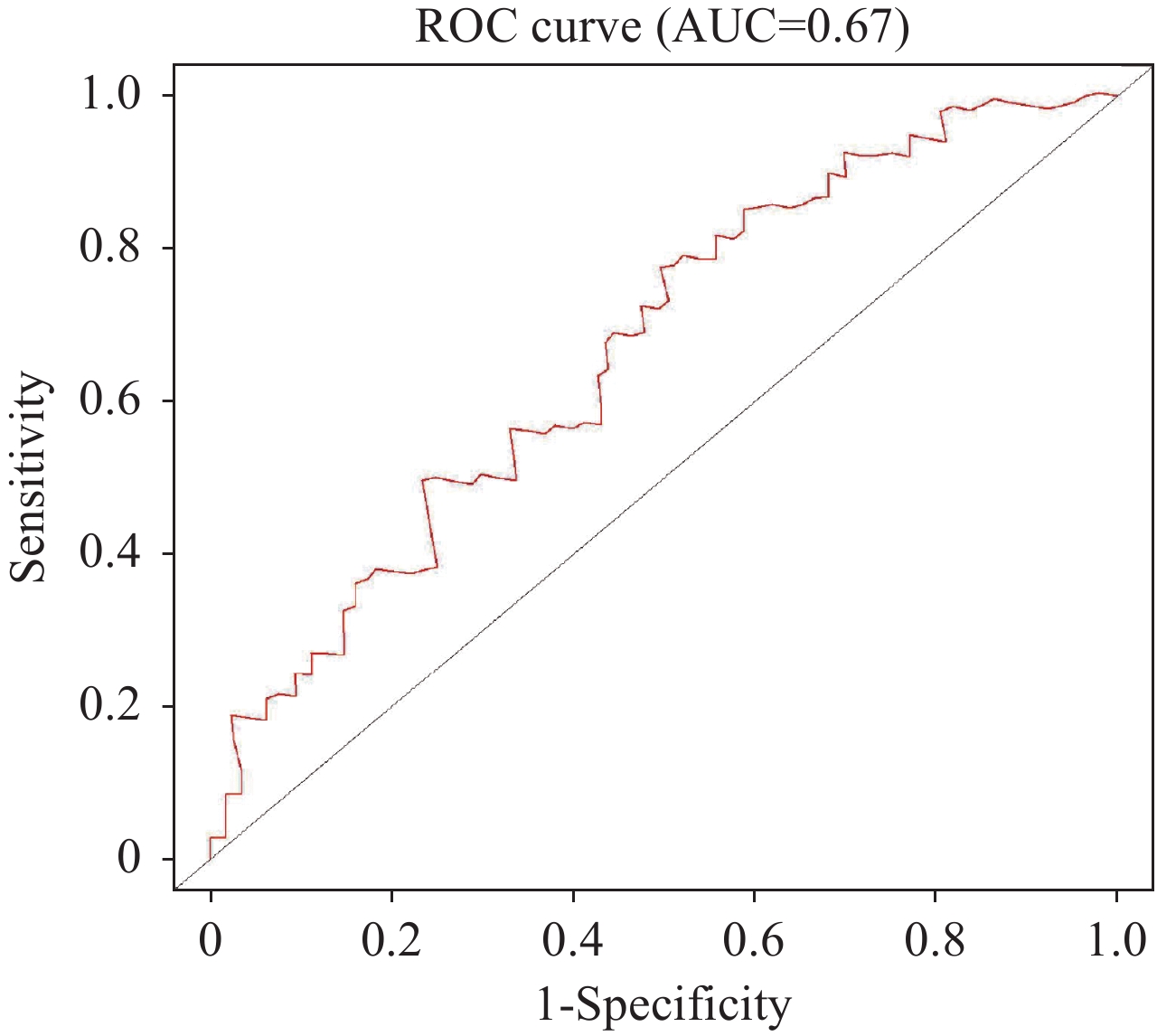
通过多因素Cox回归分析,并根据赤池信息量准则纳入血清白蛋白、单核细胞计数、血小板计数构建炎症血液评分,见表2。具体计算公式如下:炎症血液评分=1.189×单核细胞计数(109/L)−0.005×血小板计数(109/L)−0.074×白蛋白(g/L)。Kaplan-Meier分析结果显示血液中炎症负荷高的患者预后更差(P=0.002),见图2。ROC曲线下面积显示炎症血液评分能较好地预测老年EOC患者的预后(AUC=0.67),见图3。
表 2 多因素Cox回归分析外周血指数中OS的影响因素Table 2 Cox multivariate analysis of the peripheral blood factors affecting OSCharacteristics β HR 95%CI P Monocyte(109/L) 1.189 3.282 0.647-16.663 0.152 Platelet(109/L) −0.005 0.995 0.992-0.999 0.013 Albumin(g/L) −0.074 0.928 0.869-0.992 0.029 根据血液评分的中值将老年EOC患者分成高评分组和低评分组。与低评分组相比,血液评分高的上皮性卵巢癌患者临床分期更晚,腹水量更多,接受HIPEC治疗更少(均P<0.05),见表3。
表 3 血液评分与老年EOC患者临床特征相关性分析Table 3 Correlation between blood score and clinical characteristics of elderly patients with epithelial ovarian cancerCharacteristics All Low blood
scoreHigh blood
scoreP Age (years, n) 0.630 65-75 117 74 43 ≥76 21 12 9 BMI (n) 0.409 ≥25 35 20 15 <25 92 61 31 Histology (n) 0.374 High-grade
serous carcinoma88 57 31 Low-grade serous
carcinoma3 3 0 Mucinous carcinoma 9 5 4 Clear cell carcinoma 6 3 3 Endometrioid carcinoma 4 3 1 Carcinosarcoma 3 0 3 Hepatoid
adenocarcinoma1 1 0 Mixed carcinoma 4 3 1 Insufficient pathological
information20 11 9 ACCI (median, IQR) 3(2-4) 3(2-4) 3(2-4) 0.144 CA125 (U/ml, n) 0.354 < 1000 69 44 25 ≥ 1000 53 29 24 FIGO stage (n) 0.009 Ⅰ-Ⅱ 23 20 3 Ⅲ-Ⅳ 115 66 49 Palliative care (n) 0.051 Yes 16 6 10 No 122 80 42 Residual disease (n) 0.148 R0 57 44 13 R1 26 16 10 R2 29 17 12 Postoperative complication (n) 0.522 No complication & I 73 52 21 Ⅱ-Ⅳ 39 25 14 HIPEC (n) 0.031 Yes 12 11 1 No 126 75 51 Ascites (ml, n) 0.024 < 1000 68 52 16 ≥ 1000 45 25 20 Notes: Elderly patients with EOC who visited from December 2013 to April 2022 were selected. Some patients did't conduct routine test of BMI and CA125 before their initial surgery. Additionally, CA125 was not a routine test for patients 10 years ago. Therefore, BMI and CA125 data were incomplete. The clinical characteristics of patients who had undergone surgery were residual disease, postoperative complication, and ascites volume. Sixteen patients who received palliative treatment and 10 patients who had not yet undergone surgery did not have these data content. And there was one patient's initial diagnosis report assessed a large amount of ascites(>1000 ml). 2.4 接受抗癌治疗的老年EOC患者的预后影响因素分析
排除16例接受姑息治疗的患者,对122例患者进行单因素及多因素Cox回归分析,评估影响接受抗癌治疗的老年EOC患者预后的因素,见表4。单因素Cox回归分析结果表明:ACCI、病理类型、CA125、残留病灶和血液评分是患者OS的影响因素(均P<0.05)。多因素Cox回归分析结果显示:ACCI、CA125、残留病灶和血液评分是患者OS的独立影响因素(均P<0.05)。
表 4 单变量和多变量回归分析OS的影响因素Table 4 Univariate and multivariate regression analysis of influencing factors for OSCharacteristics Univariate analysis Multivariate analysis HR 95%CI P HR 95%CI P Age (years) 65-75 1 - - - ≥76 1.043 0.303-3.589 0.946 - - - BMI <25 1 - - - ≥25 0.734 0.275-1.959 0.536 - - - Histology High-grade serous carcinoma 1 1 Low-grade serous carcinoma 8.374×108 9.295×107-7.545×109 <0.001 1.266×109 0-Inf 0.997 Mucinous carcinoma 9.669×107 2.220×107-4.210×108 <0.001 1.339×108 0-Inf 0.998 Clear cell carcinoma 1 1-1 - - - - Endometrioid carcinoma 1.280×107 4.647×106-3.523×107 <0.001 1.816×107 0-Inf 0.998 Carcinosarcoma 9.903×106 1.302×106-7.534×107 <0.001 1.287×107 0-Inf 0.998 Hepatoid adenocarcinoma 1 1-1 - 7.235×106 0-Inf 0.998 Mixed carcinoma 6.905×106 9.023×105-5.284×107 <0.001 7.353×106 0-Inf 0.998 Insufficient pathological information 0.512 0-Inf 1.000 0.831 0-Inf 1.000 ACCI 1.846 1.251-2.724 0.002 1.779 1.066-2.968 0.027 CA125 (U/ml) < 1000 1 1 ≥ 1000 0.146 0.061-0.350 <0.001 0.222 0.067-0.742 0.015 FIGO stage Ⅰ-Ⅱ 1 - - - Ⅲ-Ⅳ 1.136 0.149-8.677 0.902 - - - Residual disease R0 1 1 R1 3.800 1.603-9.008 0.002 4.108 1.094-15.419 0.036 R2 5.396 2.217-13.140 <0.001 6.318 1.746-22.869 0.005 Postoperative complication No complication & I 1 - - - Ⅱ-Ⅳ 2.148 0.892-5.174 0.088 - - - HIPEC No 1 - - - Yes 1.467 0.338-6.370 0.609 - - - Ascites (ml) < 1000 1 - - - ≥ 1000 0.574 0.217-1.521 0.264 - - - Blood score High 1 1 Low 0.224 0.095-0.530 0.001 0.253 0.087-0.734 0.011 Note: -: not applicable. 2.5 构建并评价列线图模型
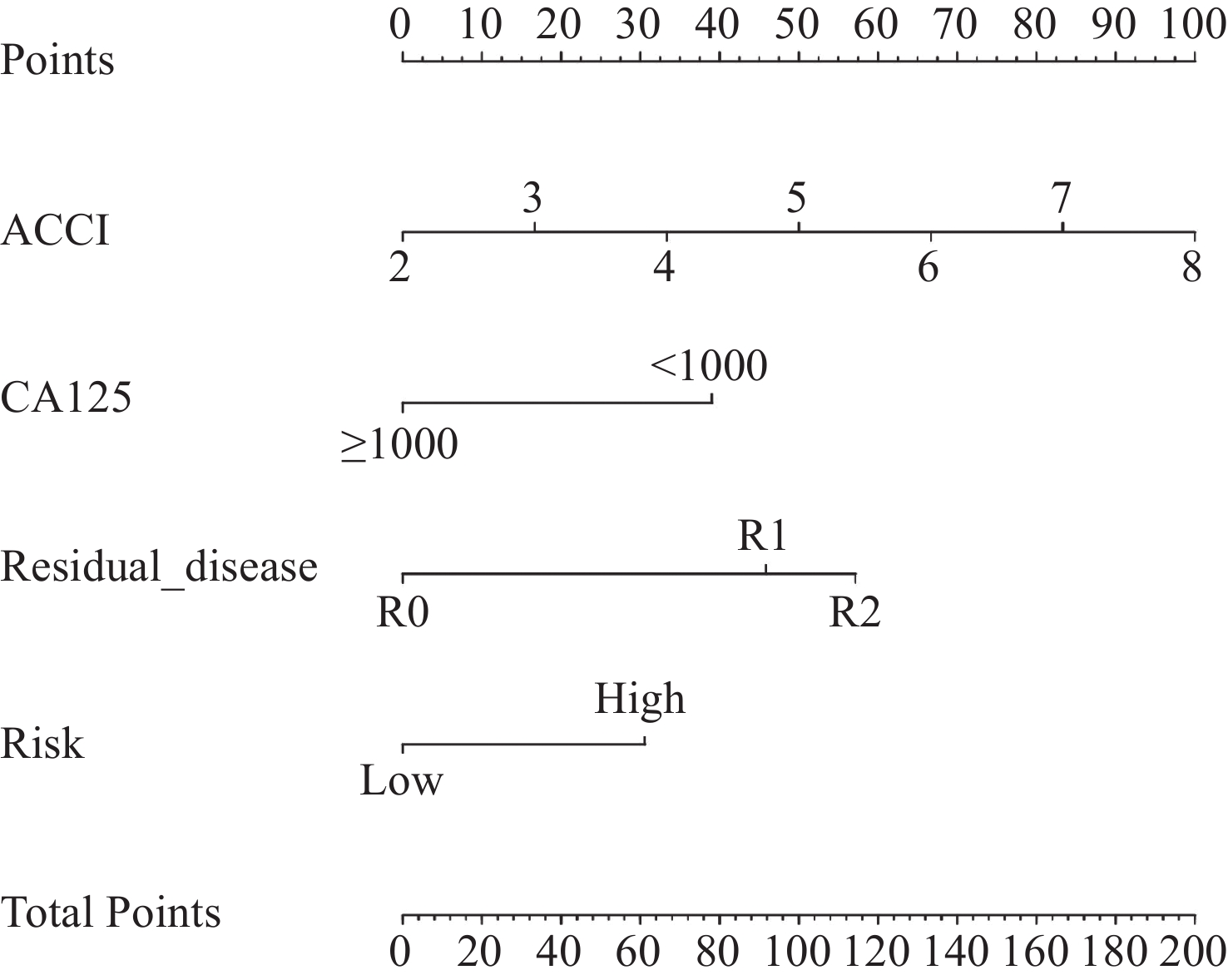
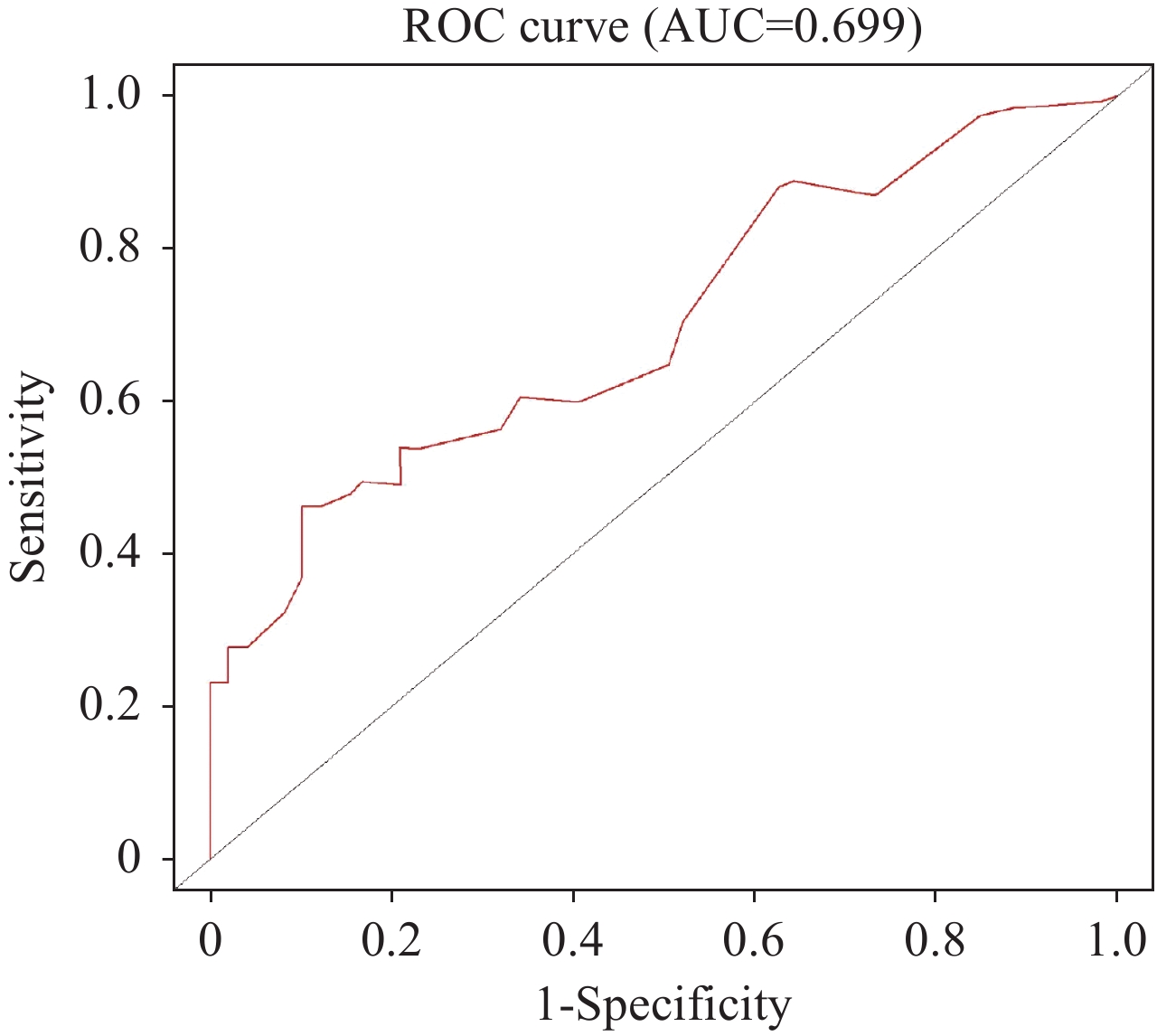
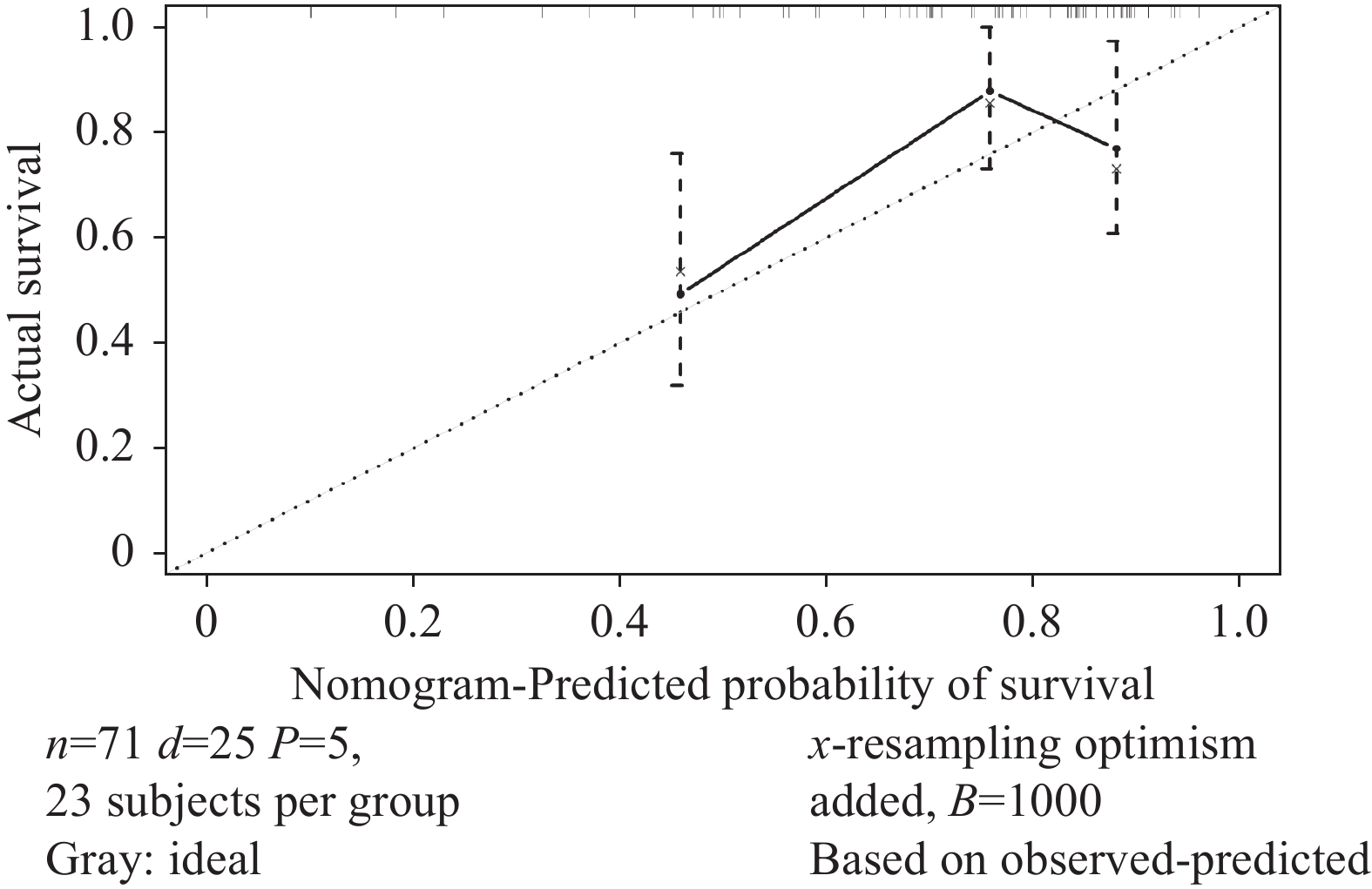
根据确定的独立预后影响因素,构建老年EOC患者的列线图以预测其OS(图4)。计算其C指数为0.705(95%CI: 0.589~0.820),时间依赖性ROC曲线AUC=0.699(图5),校准曲线显示列线图模型对OS的预测与观察结果间一致性较好(图6),列线图模型能较好预测预后。
3 讨论
全身系统性炎症是肿瘤患者中最具代表性的宿主肿瘤相互作用,炎症负荷与恶性肿瘤患者的预后密切相关。炎症负荷每增加一个标准差,癌症患者预后不良的风险就增加10.3%[11]。近年来,随着肿瘤临床研究的不断深入,研究者们发现具有高炎症负荷的癌症,如胰腺癌、肺癌和卵巢癌[12-14],提示持续监测炎症负荷尤为重要。
研究显示可以使用常见血液检查中常规测量的各种生化或血液学标志物或根据这些测量得出的比值来评估全身炎症状态[8]。中性粒细胞与血小板均可以产生细胞因子和趋化因子促进癌症相关的炎症,并促进肿瘤的发生发展[15-16]。单核细胞可以分化为肿瘤相关巨噬细胞促进肿瘤的浸润和转移[17]。淋巴细胞可以促进对癌症的细胞毒性免疫反应[18]。除了血液常规检查以外,血液生化中的白蛋白和球蛋白值也可以反应炎症和营养水平并与癌症患者的预后相关联[7]。纤维蛋白原通过调节肿瘤细胞的增殖、侵袭和迁移,或调节肿瘤微环境中免疫细胞的功能,在癌症中发挥关键作用[19]。NLR、PLR、AGR在肺癌患者队列中被证明为独立预后指标[20]。在一项多中心的回顾性研究中发现LMR与接受手术治疗的小细胞肺癌患者预后显著相关[21]。
老年EOC患者随着年龄的增加,生存率降低,只有33%的80~84岁的患者在诊断后存活1年,其预后较年轻患者有明显差距[4]。本研究在老年EOC患者中验证了中性粒细胞计数、血小板计数、单核细胞计数、淋巴细胞计数、白蛋白值、球蛋白值、纤维蛋白原值,以及NLR、LMR、PLR、AGR、FLR与预后的相关性。结果表明单核细胞计数是老年EOC患者预后的危险因素,但血小板计数和白蛋白值出现了不一致的结果,这可能是由于在单因素分析中没有考虑到其他因素的影响,导致出现了辛普森悖论的情况。因此在多因素分析中,通过调整控制其他因素的影响,揭示血小板计数和白蛋白值是预后的保护性因素。此外,我们通过统计学方法建立预测老年EOC患者预后的炎症血液评分系统,对比其他外周血炎症指标ROC曲线的曲线下面积(NLR:0.472,LMR:0.462,PLR:0.441,AGR:0.450,FLR:0.499),血液评分的AUC值为0.67,其预测准确性优于其他炎症指标。因此,本研究的评分系统能更好地预测老年EOC患者的预后。血液评分较高的患者通常更有可能出现FIGO分期晚、术前大量腹水。这也提示我们在对老年性EOC患者做出临床处理时要把血液评分高的患者纳入高危人群,从而更好提高此类患者的生活质量和围手术期患者安全。
目前已有研究发现多种与EOC患者预后有关的因素,如年龄、BMI、并发症、病理类型、FIGO分期等[22]。本研究使用ACCI评分评估老年EOC患者的并发症状态,ACCI指数纳入患者年龄作为校正变量能较好预测癌症患者预后[23]。在单因素和多因素Cox回归分析中,我们发现了并发症、CA125、残留病灶和炎症血液评分与OS的相关性有统计学意义。基于这些结果,我们建立了用于预测老年EOC患者预后的列线图。列线图模型的C指数为0.705,AUC值为0.699,具有较好的预测性能。然而,本研究回顾性队列病例数不足以分出验证集对此评分系统及模型进行外部验证,因此在未来进一步研究中,需要更大的样本量、良好的同质性和外部验证,以进一步验证此结论。
总之,本研究验证了炎症负荷与老年上皮性卵巢癌患者预后间的相关性,并构建了与炎症相关的血液评分系统和列线图模型,结果表明,该模型具有较强的临床实用性和较好的预后预测能力,并且通过血液中炎症负荷的评分可对卵巢癌患者的疗效监测和治疗干预提供帮助。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:杨丹妮:研究设计、资料收集、统计分析、论文撰写赵梦娜:研究设计、资料收集冯晓叶:文献查阅、资料收集童继玉:研究设计、文献查阅、统计分析王 华:研究设计、文献查阅蔡红兵:研究指导、论文审阅、论文修改 -
表 1 单因素Cox回归分析外周血参数中OS的影响因素
Table 1 Cox univariate analysis of the peripheral blood factors affecting OS
Characteristics HR 95%CI P NLR 0.467 0.143-1.525 0.207 LMR 1.691 0.850-3.361 0.134 PLR 2.304 0.812-6.536 0.117 AGR 2.034 0.922-4.483 0.078 FLR(g/109) 2.323 0.962-5.610 0.061 Neutrophil(109/L) 1.636 0.828-3.233 0.157 Lymphocyte(109/L) 3.110 0.743-13.020 0.120 Monocyte(109/L) 2.388 1.040-5.483 0.040 Platelet(109/L) 2.261 1.160-4.407 0.017 Albumin(g/L) 3.013 1.444-6.285 0.003 Globulin(g/L) 0.575 0.250-1.319 0.192 Fibrinogen(g/L) 1.623 0.827-3.184 0.159 Notes: NLR: neutrophil-to-lymphocyte ratio; LMR: lymphocyte to monocyte ratio; PLR: platelet to lymphocyte ratio; AGR: albumin-to-globulin ratio; FLR: fibrinogen-to-lymphocyte ratio. 表 2 多因素Cox回归分析外周血指数中OS的影响因素
Table 2 Cox multivariate analysis of the peripheral blood factors affecting OS
Characteristics β HR 95%CI P Monocyte(109/L) 1.189 3.282 0.647-16.663 0.152 Platelet(109/L) −0.005 0.995 0.992-0.999 0.013 Albumin(g/L) −0.074 0.928 0.869-0.992 0.029 表 3 血液评分与老年EOC患者临床特征相关性分析
Table 3 Correlation between blood score and clinical characteristics of elderly patients with epithelial ovarian cancer
Characteristics All Low blood
scoreHigh blood
scoreP Age (years, n) 0.630 65-75 117 74 43 ≥76 21 12 9 BMI (n) 0.409 ≥25 35 20 15 <25 92 61 31 Histology (n) 0.374 High-grade
serous carcinoma88 57 31 Low-grade serous
carcinoma3 3 0 Mucinous carcinoma 9 5 4 Clear cell carcinoma 6 3 3 Endometrioid carcinoma 4 3 1 Carcinosarcoma 3 0 3 Hepatoid
adenocarcinoma1 1 0 Mixed carcinoma 4 3 1 Insufficient pathological
information20 11 9 ACCI (median, IQR) 3(2-4) 3(2-4) 3(2-4) 0.144 CA125 (U/ml, n) 0.354 < 1000 69 44 25 ≥ 1000 53 29 24 FIGO stage (n) 0.009 Ⅰ-Ⅱ 23 20 3 Ⅲ-Ⅳ 115 66 49 Palliative care (n) 0.051 Yes 16 6 10 No 122 80 42 Residual disease (n) 0.148 R0 57 44 13 R1 26 16 10 R2 29 17 12 Postoperative complication (n) 0.522 No complication & I 73 52 21 Ⅱ-Ⅳ 39 25 14 HIPEC (n) 0.031 Yes 12 11 1 No 126 75 51 Ascites (ml, n) 0.024 < 1000 68 52 16 ≥ 1000 45 25 20 Notes: Elderly patients with EOC who visited from December 2013 to April 2022 were selected. Some patients did't conduct routine test of BMI and CA125 before their initial surgery. Additionally, CA125 was not a routine test for patients 10 years ago. Therefore, BMI and CA125 data were incomplete. The clinical characteristics of patients who had undergone surgery were residual disease, postoperative complication, and ascites volume. Sixteen patients who received palliative treatment and 10 patients who had not yet undergone surgery did not have these data content. And there was one patient's initial diagnosis report assessed a large amount of ascites(>1000 ml). 表 4 单变量和多变量回归分析OS的影响因素
Table 4 Univariate and multivariate regression analysis of influencing factors for OS
Characteristics Univariate analysis Multivariate analysis HR 95%CI P HR 95%CI P Age (years) 65-75 1 - - - ≥76 1.043 0.303-3.589 0.946 - - - BMI <25 1 - - - ≥25 0.734 0.275-1.959 0.536 - - - Histology High-grade serous carcinoma 1 1 Low-grade serous carcinoma 8.374×108 9.295×107-7.545×109 <0.001 1.266×109 0-Inf 0.997 Mucinous carcinoma 9.669×107 2.220×107-4.210×108 <0.001 1.339×108 0-Inf 0.998 Clear cell carcinoma 1 1-1 - - - - Endometrioid carcinoma 1.280×107 4.647×106-3.523×107 <0.001 1.816×107 0-Inf 0.998 Carcinosarcoma 9.903×106 1.302×106-7.534×107 <0.001 1.287×107 0-Inf 0.998 Hepatoid adenocarcinoma 1 1-1 - 7.235×106 0-Inf 0.998 Mixed carcinoma 6.905×106 9.023×105-5.284×107 <0.001 7.353×106 0-Inf 0.998 Insufficient pathological information 0.512 0-Inf 1.000 0.831 0-Inf 1.000 ACCI 1.846 1.251-2.724 0.002 1.779 1.066-2.968 0.027 CA125 (U/ml) < 1000 1 1 ≥ 1000 0.146 0.061-0.350 <0.001 0.222 0.067-0.742 0.015 FIGO stage Ⅰ-Ⅱ 1 - - - Ⅲ-Ⅳ 1.136 0.149-8.677 0.902 - - - Residual disease R0 1 1 R1 3.800 1.603-9.008 0.002 4.108 1.094-15.419 0.036 R2 5.396 2.217-13.140 <0.001 6.318 1.746-22.869 0.005 Postoperative complication No complication & I 1 - - - Ⅱ-Ⅳ 2.148 0.892-5.174 0.088 - - - HIPEC No 1 - - - Yes 1.467 0.338-6.370 0.609 - - - Ascites (ml) < 1000 1 - - - ≥ 1000 0.574 0.217-1.521 0.264 - - - Blood score High 1 1 Low 0.224 0.095-0.530 0.001 0.253 0.087-0.734 0.011 Note: -: not applicable. -
[1] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[2] Schoutrop E, Moyano-Galceran L, Lheureux S, et al. Molecular, cellular and systemic aspects of epithelial ovarian cancer and its tumor microenvironment[J]. Semin Cancer Biol, 2022, 86(Pt 3): 207-223.
[3] Wethington SL, Armstrong DK, Gaillard SL. Vulnerable Older Adults With Ovarian Cancer-Time to Stop Undertreating[J]. JAMA Oncol, 2021, 7(6): 831-833. doi: 10.1001/jamaoncol.2021.0468
[4] Falandry C, Rousseau F, Mouret-Reynier MA, et al. Efficacy and Safety of First-line Single-Agent Carboplatin vs. Carboplatin Plus Paclitaxel for Vulnerable Older Adult Women With Ovarian Cancer: A GINECO/GCIG Randomized Clinical Trial[J]. JAMA Oncol, 2021, 7(6): 853-861. doi: 10.1001/jamaoncol.2021.0696
[5] Vergote I, van Nieuwenhuysen E, de Waele S, et al. Prospective non-interventional BELOVA/BGOG-ov16 study on safety of frontline bevacizumab in elderly patients with FIGO stage Ⅳ ovarian cancer: a study of the Belgian and Luxembourg Gynaecological Oncology Group[J]. Int J Gynecol Cancer, 2022, 32(6): 753-760. doi: 10.1136/ijgc-2021-003190
[6] Denk D, Greten FR. Inflammation: the incubator of the tumor microenvironment[J]. Trends Cancer, 2022, 8(11): 901-914. doi: 10.1016/j.trecan.2022.07.002
[7] Yamamoto T, Kawada K, Obama K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients[J]. Int J Mol Sci, 2021, 22(15): 8002. doi: 10.3390/ijms22158002
[8] Nøst TH, Alcala K, Urbarova I, et al. Systemic inflammation markers and cancer incidence in the UK Biobank[J]. Eur J Epidemiol, 2021, 36(8): 841-848. doi: 10.1007/s10654-021-00752-6
[9] Bai G, Zhou Y, Rong Q, et al. Development of Nomogram Models Based on Peripheral Blood Score and Clinicopathological Parameters to Predict Preoperative Advanced Stage and Prognosis for Epithelial Ovarian Cancer Patients[J]. J Inflamm Res, 2023, 16: 1227-1241. doi: 10.2147/JIR.S401451
[10] Fink CA, Weykamp F, Adeberg S, et al. Comorbidity in limited disease small-cell lung cancer: Age-adjusted Charlson comorbidity index and its association with overall survival following chemoradiotherapy[J]. Clin Transl Radiat Oncol, 2023, 42: 100665.
[11] Xie H, Ruan G, Ge Y, et al. Inflammatory burden as a prognostic biomarker for cancer[J]. Clin Nutr, 2022, 41(6): 1236-1243. doi: 10.1016/j.clnu.2022.04.019
[12] Plaja A, Teruel I, Ochoa-de-Olza M, et al. Prognostic Role of Neutrophil, Monocyte and Platelet to Lymphocyte Ratios in Advanced Ovarian Cancer According to the Time of Debulking Surgery[J]. Int J Mol Sci, 2023, 24(14): 11420. doi: 10.3390/ijms241411420
[13] Ohara Y, Valenzuela P, Hussain SP. The interactive role of inflammatory mediators and metabolic reprogramming in pancreatic cancer[J]. Trends Cancer, 2022, 8(7): 556-569. doi: 10.1016/j.trecan.2022.03.004
[14] Terlizzi M, Colarusso C, Falanga A, et al. Induction of Inflammation Disrupts the Negative Interplay between STING and S1P Axis That Is Observed during Physiological Conditions in the Lung[J]. Int J Mol Sci, 2023, 24(9): 8303. doi: 10.3390/ijms24098303
[15] Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis[J]. J Hematol Oncol, 2021, 14(1): 173. doi: 10.1186/s13045-021-01187-y
[16] Schlesinger M. Role of platelets and platelet receptors in cancer metastasis[J]. J Hematol Oncol, 2018, 11(1): 125. doi: 10.1186/s13045-018-0669-2
[17] Ugel S, Canè S, De Sanctis F, et al. Monocytes in the Tumor Microenvironment[J]. Annu Rev Pathol, 2021, 16: 93-122. doi: 10.1146/annurev-pathmechdis-012418-013058
[18] Melssen MM, Sheybani ND, Leick KM, et al. Barriers to immune cell infiltration in tumors[J]. J Immunother Cancer, 2023, 11(4): e006401. doi: 10.1136/jitc-2022-006401
[19] Yu J, Li J, Shen J, et al. The role of Fibrinogen-like proteins in Cancer[J]. Int J Biol Sci, 2021, 17(4): 1079-1087. doi: 10.7150/ijbs.56748
[20] Song M, Zhang Q, Song C, et al. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer[J]. J Cachexia Sarcopenia Muscle, 2022, 13(5): 2504-2514. doi: 10.1002/jcsm.13032
[21] Lang C, Egger F, Alireza Hoda M, et al. Lymphocyte-to-monocyte ratio is an independent prognostic factor in surgically treated small cell lung cancer: An international multicenter analysis[J]. Lung Cancer, 2022, 169: 40-46. doi: 10.1016/j.lungcan.2022.05.010
[22] Cheng H, Xu JH, Kang XH, et al. Nomograms for predicting overall survival and cancer-specific survival in elderly patients with epithelial ovarian cancer[J]. J Ovarian Res, 2023, 16(1): 75. doi: 10.1186/s13048-023-01144-y
[23] Koseki Y, Hikage M, Fujiya K, et al. Utility of a modified age-adjusted Charlson Comorbidity Index in predicting cause-specific survival among patients with gastric cancer[J]. Eur J Surg Oncol, 2021, 47(8): 2010-2015. doi: 10.1016/j.ejso.2021.01.026
-
期刊类型引用(0)
其他类型引用(1)



 下载:
下载: