-
摘要:目的
探讨SMARCA4(BRG1)缺失性癌患者的临床病理特征、免疫表型及诊治要点。
方法回顾性分析11例SMARCA4(BRG1)缺失性癌患者的临床资料,总结其HE染色后的形态及免疫组织化学特征,并结合文献进行分析。
结果11例患者中男性8例,女性3例;中位年龄60岁。8例行根治性手术切除,3例行传统化疗联合靶向及免疫治疗。镜下肿瘤细胞胞质丰富、红染,呈上皮样、横纹肌样或梭形,具明显的嗜酸性核仁,核分裂象易见(>5/10 HPF),肿瘤组织内见多灶状坏死,脉管内见大量癌栓,可伴间质黏液样变性。11例肿瘤细胞SMARCA4(BRG1)表达缺失,同时表达上皮源性CK和间叶源性Vim标记,SMARCB1(INI1)表达保留,p53突变型,肿瘤细胞呈高增殖活性(Ki-67>60%),突触素Syn呈中等强度阳性。3例为错配修复蛋白缺陷,分别表现为MLH1/PMS2、PMS2、MSH6表达缺失,其中1例经Sanger测序法检测证实为高度微卫星不稳定型。
结论SMARCA4(BRG1)缺失性癌发病率低,易与其他肿瘤混淆,术前诊断困难,需依靠免疫组织化学明确诊断。
-
关键词:
- SMARCA4(BRG1) /
- SWI/SNF复合体 /
- 免疫表型
Abstract:ObjectiveTo investigate the clinicopathological features, immunophenotype, diagnosis and treatment of SMARCA4 (BRG1)-deficient carcinoma.
MethodsClinical data of 11 patients with SMARCA4 (BRG1)-deficient cancer were collected. The morphologic and immunohistochemical features of this tumour were summarized, and the relevant literature was reviewed.
ResultsAmong the 11 cases of SMARCA4 (BRG1)-deficient carcinoma, eight were male and three were female, with median age of 60. Seven patients underwent radical resection, and four underwent traditional joint targeted chemotherapy and immunotherapy. Microscopically, the tumor cells were epithelioid, rhabdoid or spindle-shaped, with prominent eosinophilic nucleoli and frequent mitoses (>5/10 HPF). Multiple foci of necrosis were found in the tumor tissue, a large number of tumor emboli in the blood vessels and myxoid stromal degeneration. Among these cases, 11 cases showed loss of SMARCA4 (BRG1) expression, whereas the CK and Vim markers were expressed, SMARCB1 (INI1) expression was retained, and p53 mutation was detected. The tumor cells showed high proliferation activity (Ki-67>60%), and synaptophsin was moderately positive. Three cases were mismatch repair deficient and respectively showed the loss of MLH1/PMS2, PMS2 and MSH6 expression.
ConclusionThe incidence of SMARCA4 (BRG1) -dificient carcinoma is low. It can be easily confused with other tumors and is difficult to be diagnosed before operation, which requires confirmation by immunohistochemistry.
-
Key words:
- SMARCA4(BRG1) /
- SWI/SNF complex /
- Immunophenotype
-
0 引言
酵母交配型转换/蔗糖不发酵复合物(switch/sucrose non-fermentable complex, SWI/SNF复合物)的突变广泛分布于人类各种肿瘤中,其中SMARCA4(BRG1)是继ARID1A之后恶性肿瘤中突变第二频繁的SWI/SNF基因,具代表性的肿瘤为卵巢高血钙型小细胞癌[1],新近文献也陆续报道了一些高侵袭性的SMARCA4(BRG1)缺失性未分化肿瘤,可发生于头颈部、胸腔、消化道和女性生殖道等部位,但均十分少见[2-4]。因肿瘤细胞分化原始,除SMARCA4(BRG1)等SWI/SNF复合物相关标志物外,缺乏特异性分化,诊治过程中易被误诊为各种类型的低分化或未分化肿瘤。本文回顾性分析11例SMARCA4(BRG1)缺失性癌临床病理学特征,以提升对这类疾病的认识,减少漏诊和误诊。
1 资料与方法
1.1 病例资料
收集南昌大学第一附属医院病理科2018年3月至2023年6月诊断的SMARCA4(BRG1)缺失性癌11例,所有病例均有完整的临床病史、影像资料、病理切片和免疫组织化学结果,并经两名高年资医师复阅切片。
1.2 方法
所有标本均经4.0%中性甲醛固定,常规脱水,石蜡包埋,4 μm厚切片,HE染色,光学显微镜观察。免疫组织化学染色采用EnVision法,采用的抗体包括广谱细胞角蛋白(CKpan,克隆号AE1/AE3)、Vim、S-100、CK7、CK20、CD34、CDX2、CD117、Glypican-3、p53、CD56、Syn、LCA、Ki-67均购自福州迈新生物技术开发有限公司;SMARCA4(BRG1)、SMARCB1(INI1)、SALL4均购自北京中杉金桥生物技术有限公司;SMARCA2(BRM)购自美国Abcam公司。错配修复蛋白MLH1、MSH2、MSH6、PMS2均购自丹麦DAKO公司。 一抗抗体均为工作液,具体操作步骤严格按试剂盒说明书进行。
2 结果
2.1 临床特征
11例SMARCA4(BRG1)缺失性癌中男性3例,女性8例;年龄45~73岁(中位年龄60岁)。发病部位位于子宫3例、胃2例、小肠1例、右半结肠1例、直肠2例、肝脏2例。3例(例1~3)发生于子宫者表现为异常阴道流血,8例(例4~11)发生于消化系统者表现为腹痛、腹胀,或伴恶心、呕吐。8例行根治性手术切除辅以化疗,3例行普通化疗联合靶向及免疫治疗,具体见表1。
表 1 11例SMARCA4(BRG1)缺失性癌患者临床特征Table 1 Clinical characteristics of 11 cases of SMARCA4(BRG1)-deficient carcinomaPatients Age (year)
/GenderPosition Diameter (cm) Pathological diagnosis Therapeutic method 1 53/F Uterus 2.0 Undifferentiated endometrial carcinoma Surgery+chemotherapy 2 52/F Uterus 1.2 Endometrioid adenocarcinoma Ⅰ+
Undifferentiated carcinomaSurgery+chemotherapy+
radiotherapy3 60/F Uterus 2.1 Endometrioid adenocarcinoma Ⅱ+
Undifferentiated carcinomaSurgery+chemotherapy 4 71/M Distal stomach 9.0 Predisposed to undifferentiated carcinoma Surgery+chemotherapy 5 73/M Stomach - Malignant tumor Surgery+chemotherapy+
Targeted therapy6 62/F Small intestine 9.0 Undifferentiated carcinoma Surgery+chemotherapy 7 66/F Rectum 11.0 Mixed carcinoma Surgery+chemotherapy 8 48/M Right semicolon 8.5 60% adenocarcinoma
+40% undifferentiated carcinomaSurgery+chemotherapy 9 58/F Rectum - Undifferentiated carcinoma Chemotherapy+
Targeted therapy10 66/F Liver - Malignant tumor Targeted therapy+
immunotherapy11 45/F Liver - Undifferentiated carcinoma Chemotherapy Notes: M: male; F: female; -: not available. 2.2 辅助检查
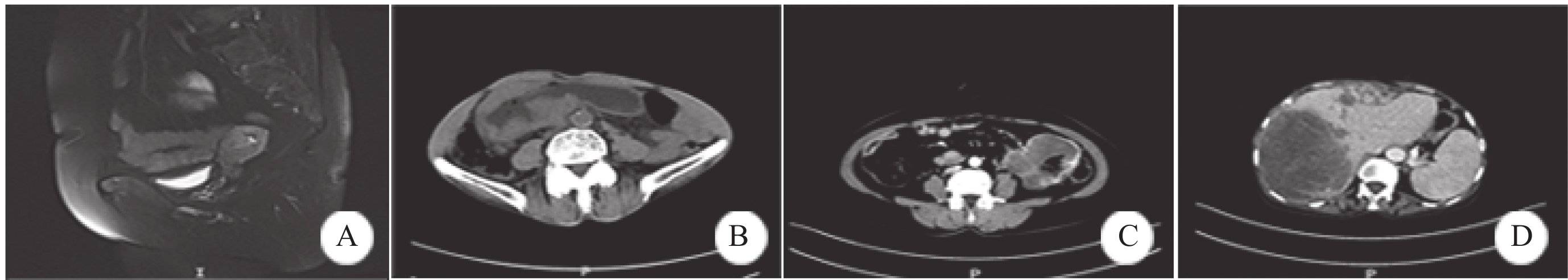
3例(例1~3)位于子宫者均行磁共振成像(MRI)检查,T2WI显示宫腔内软组织信号影,破坏子宫内膜与子宫肌界面,低信号带发生中断,侵入子宫肌层,增强扫描肿块强化程度低于子宫肌,见图1A。例4行腹部CT检查,显示胃窦部明显不均匀增厚,最厚约4.5 cm,管腔狭窄,增强扫描呈不均匀强化,见图1B。例5行胃镜检查,显示胃体大弯见菜花样新生物,中央凹陷,质脆,弹性消失。例6~9位于肠道者均行腹部CT检查,显示肠壁不规则明显增厚,并形成肿块,肿块直径约7~11 cm,见图1C;其中2例行肠镜检查,显示升结肠、直肠巨大新生物,质硬,弹性消失。例10~11位于肝脏者行腹部CT检查,显示肝轮廓饱满,肝内见多发类圆形低密度影,直径达12 cm,见图1D。
![]() 图 1 SMARCA4(BRG1)缺失性癌患者影像学检查Figure 1 Imaging examination of patients with SMARCA4(BRG1) deficient carcinomaA: case 3, MRI showed a uterine mass with a size of 2.5×2.0 cm; B: case 4, CT showed gastric wall thickening and stenosis; C: case 6, CT showed a mass of 8.4×6.6 cm in the left abdominal cavity. It adhered to the intestinal canal; D: case 10, CT showed a mass of 12×9.6 cm in the right lobe of the liver.
图 1 SMARCA4(BRG1)缺失性癌患者影像学检查Figure 1 Imaging examination of patients with SMARCA4(BRG1) deficient carcinomaA: case 3, MRI showed a uterine mass with a size of 2.5×2.0 cm; B: case 4, CT showed gastric wall thickening and stenosis; C: case 6, CT showed a mass of 8.4×6.6 cm in the left abdominal cavity. It adhered to the intestinal canal; D: case 10, CT showed a mass of 12×9.6 cm in the right lobe of the liver.2.3 病理特征
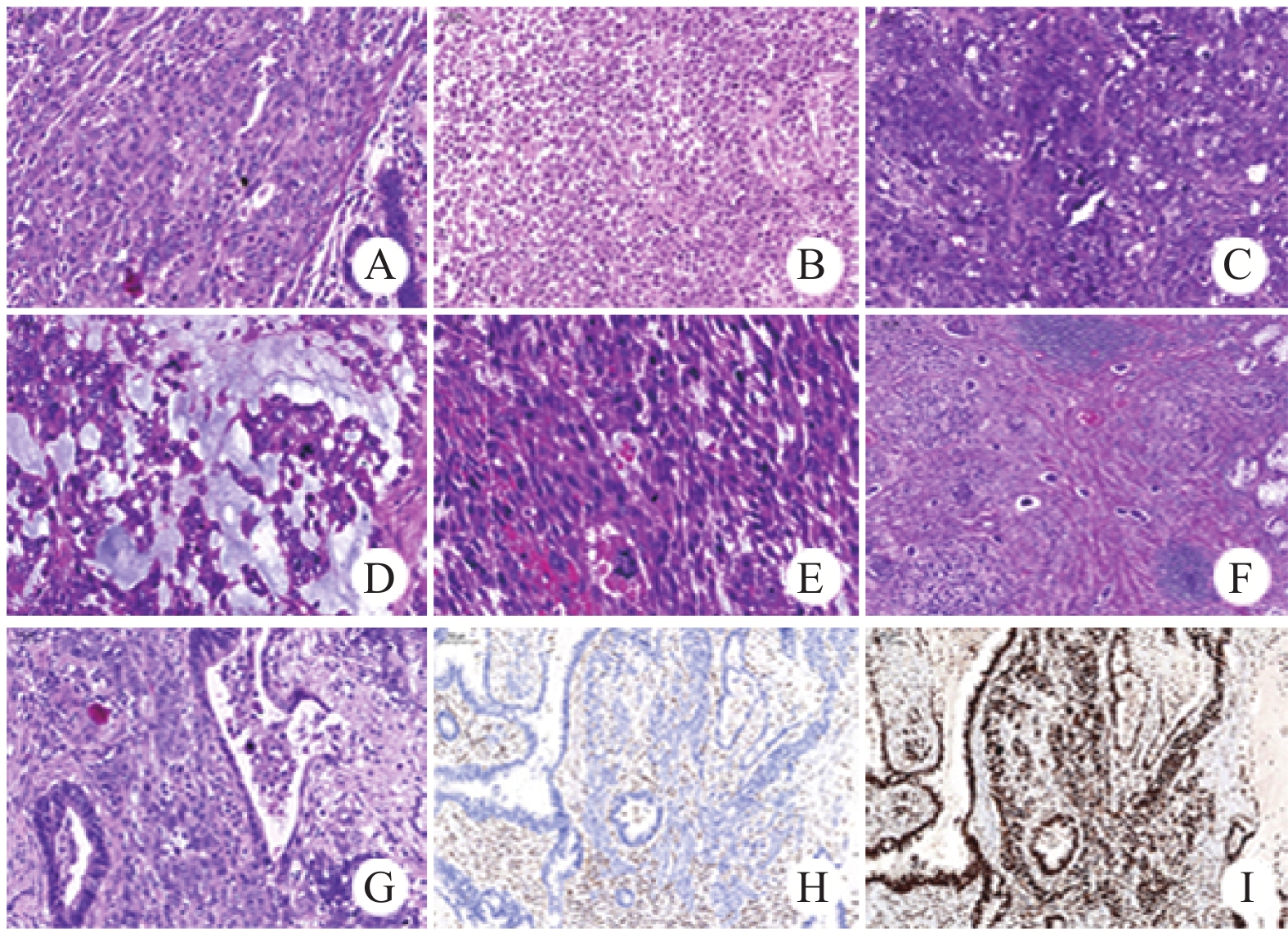
大体检查:例1~3显示子宫内膜增厚,内膜粗糙,局灶增厚并形成软组织肿块,平均最大径1.8 cm,范围1.2~2.1 cm;例4、6~8显示胃壁、肠腔内巨大肿块,切面灰白、鱼肉样,质硬,平均最大径9.1 cm,范围8~11 cm(肿瘤大体图请扫描OSID码)。例5、9为胃体、直肠活检标本;例10~11为肝脏穿刺标本。镜下观察:肿瘤细胞体积中等大小,胞质丰富、红染,呈上皮样形态,见图2A;局灶区域呈横纹肌样形态,并显示失黏附性,见图2B;细胞核染色质细腻或呈空泡状,可见明显的嗜酸性核仁,见图2C;例6部分区肿瘤间质黏液样变性,见图2D,肿瘤细胞具多形性,并见梭形细胞成分,见图2E。肿瘤细胞核分裂象易见(>5/10 HPF),肿瘤组织内见多灶状坏死,脉管内见大量癌栓,见图2F。此外,7例根治性切除标本中2例出现淋巴结转移(例4和8),1例患者腹腔内见多发播散病灶,并同时转移至右侧卵巢,转移灶内肿瘤细胞部分呈腺样分化(例6),见图2G、H、I。
![]() 图 2 SMARCA4(BRG1)缺失性癌病理形态学表现Figure 2 Pathological features of SMARCA4(BRG1) deficient carcinomaA: epithelioid morphology (HE ×200); B: rhabdoid morphology (HE ×200); C: evident eosinophilic nucleoli (HE ×200); D: interstitial mucoid degeneration (HE ×400); E: spindle cell morphology (HE ×400); F: numerous intravascular tumor emboli; G: adenoid differentiation, H: SMARCA4(BRG1) negative expression (Envision×200); I: SMARCB1(INI1) positive expression (Envision ×400).
图 2 SMARCA4(BRG1)缺失性癌病理形态学表现Figure 2 Pathological features of SMARCA4(BRG1) deficient carcinomaA: epithelioid morphology (HE ×200); B: rhabdoid morphology (HE ×200); C: evident eosinophilic nucleoli (HE ×200); D: interstitial mucoid degeneration (HE ×400); E: spindle cell morphology (HE ×400); F: numerous intravascular tumor emboli; G: adenoid differentiation, H: SMARCA4(BRG1) negative expression (Envision×200); I: SMARCB1(INI1) positive expression (Envision ×400).2.4 免疫组织化学特征
11例肿瘤细胞SMARCA4(BRG1)表达缺失,同时表达上皮源性CK和间叶源性Vim标记,SMARCB1(INI1)表达保留,SMARCA2(BRM)1例表达缺失,p53突变型,肿瘤细胞呈高增殖活性(Ki-67>60%),突触素Syn呈中等强度阳性。9例局灶性表达原始细胞标记SALL4或CD34(其中7例表达SALL4,2例表达CD34),见图3。3例发生于子宫内膜,肿瘤细胞ER、PR、Pax-8、WT-1均呈阴性。6例发生于胃肠道,肿瘤细胞CK7、CK20、CDX2均呈阴性。2例发生于肝脏,其中1例Glypican-3呈强阳性。
![]() 图 3 SMARCA4(BRG1)缺失性癌免疫组织化学表型Figure 3 Immunohistochemical phenotype of SMARCA4(BRG1) deficient carcinomaA: CK exhibited focal positive expression(EnVision ×200); B: Vim positive expression (EnVision ×200); C: SMARCA4(BRG1) negative expression (EnVision ×200); D: SMARCB1(INI1) positive expression (EnVision ×200); E: p53 was mutant (EnVision ×400); F: tumor cells showed high proliferation activity (EnVision ×200); G: Syn positive expression (EnVision ×200); H: SALL4 partially positive expression (EnVision ×200); I: CD34 focally positive expression (EnVision ×200).
图 3 SMARCA4(BRG1)缺失性癌免疫组织化学表型Figure 3 Immunohistochemical phenotype of SMARCA4(BRG1) deficient carcinomaA: CK exhibited focal positive expression(EnVision ×200); B: Vim positive expression (EnVision ×200); C: SMARCA4(BRG1) negative expression (EnVision ×200); D: SMARCB1(INI1) positive expression (EnVision ×200); E: p53 was mutant (EnVision ×400); F: tumor cells showed high proliferation activity (EnVision ×200); G: Syn positive expression (EnVision ×200); H: SALL4 partially positive expression (EnVision ×200); I: CD34 focally positive expression (EnVision ×200).2.5 DNA错配修复蛋白检测
3例出现错配修复蛋白缺失,均发生于子宫,表现为1个或2个蛋白表达缺失,其中1例经Sanger测序法检测证实为高度微卫星不稳定型,见表2。
表 2 11例SMARCA4(BRG1)缺失性癌免疫组织化学表型Table 2 Immunohistochemical phenotype of 11 cases of SMARCA4(BRG1)-deficient carcinomaNo Position CK Vim BRG1 INI1 Syn SALL4 CD34 Ki-67 MMR Molecular detection MLH1 MSH2 MSH6 PMS2 1 Uterus - + - + + + - 70% - + + - undetected 2 Uterus focal + + - + + - + 70% + + + - MSI-H 3 Uterus - focal + - + + + - 80% + + - + undetected 4 Stomach + + - + + - - 90% + + + + undetected 5 Stomach + + - + + - - 70% + + + + undetected 6 Small intestine focal + + - + + - + 95% + + + + undetected 7 Rectum focal + + - + + + - 60% + + + + undetected 8 Right semicolon + + - + + + - 70% + + + + undetected 9 Rectum focal + + - + + + - 80% + + + + undetected 10 Liver + + - + + + - 80% + + + + undetected 11 Liver + + - + + + - 90% + + + + undetected Notes: +: Positive; −: Negative 3 讨论
SWI/SNF复合物是一类多亚基构成的染色质重塑复合物[5],包含ATP酶催化亚基SMARCA4(BRG1)/SMARCA2(BRM);高度保守核心亚基SMARCB 1(INI1、SNF5和BAF47)、SMARCC1(BAF155)和SMARCC2(BAF170);功能特异性辅助亚基PBRM 1(BAF180)和ARID1A(BAF250A)。研究发现,SWI/SNF复合物参与DNA复制、转录和修复,调控胚胎发育、组织再生、细胞衰老及凋亡等过程,在抑制肿瘤发生发展中发挥着重要作用[6]。当SWI/SNF复合物亚单位发生异常时,SWI/SNF复合物功能受到影响,可能就导致肿瘤的形成。
大家所熟知的因核心亚基 SMARCB1(INI1)突变而发生的肿瘤包括儿童中枢神经系统的不典型畸胎样/横纹肌样肿瘤、肾脏的横纹肌样肿瘤和软组织的上皮样肉瘤[7],而对催化亚基SMARCA4(BRG1)突变所致的病变研究相对较少,SMARCA4是继ARID1A之后恶性肿瘤中突变第二频繁的SWI/SNF基因,其代表性肿瘤为卵巢高钙血症小细胞癌(small cell carcinoma of ovary of hypercalcemic type,SCCOHT)。据新近研究发现,约有 5%~10%的非小细胞肺癌(non-small cell lung cancer,NSCLC)存在SMARCA4(BRG1)丢失[5]。随后,陆续报道发生于其他部位的SMARCA4(BRG1)缺失性癌,包括食管、胃、子宫等,但均十分少见。
本文共收集了11例SMARCA4(BRG1)缺失性癌,发生部位包括子宫、胃、结直肠和肝脏。本组病例显示肿瘤好发于中老年人(中位年龄60岁),性别无明显差异,就诊时肿瘤体积较大,患者大多处于中晚期。镜下形态:肿瘤组织内见多灶状凝固性坏死,显示大量脉管侵犯,肿瘤细胞呈弥漫片状、巢状排列。大部分肿瘤细胞形态一致,显示失黏附性,胞浆丰富、红染,呈现上皮样或横纹肌样形态,但少数病例显示肿瘤细胞具多形性,或呈梭形细胞形态,或形成腺样结构,肿瘤间质可发生黏液样变性。
免疫组织化学标记显示,11例均失表达SMARCA4(BRG1),SMARCB1(INI1)表达保留,1例SMARCA2(BRM)失表达,肿瘤细胞同时表达上皮源性和间叶源性标记。值得指出的是,上皮源性标记在子宫及胃肠道SMARCA4缺失性癌中表达明显减少,甚至为阴性,而发生于肝脏者为强阳性。本文3例子宫SMARCA4(BRG1)缺失性癌中2例为阴性,1例仅少许细胞表达;6例胃肠道SMARCA4(BRG1)缺失性癌中3例局灶细胞表达,3例部分细胞表达,其他上皮性标记物CK7、CK20也常为阴性。朱培培等[8]报道9例胃肠道SMARCA4缺失性未分化癌中3例上皮性标记为阴性,5例仅个别细胞表达。Chang等[9]报道的19例胃肠道SMARCA4缺失性未分化癌也呈现同样的结果,其中11例上皮性标记不表达或表达明显减低。本文11例波形蛋白均为强阳性表达,给诊断带来了很大的挑战,尤其对于活检标本,易误诊为肉瘤或其他恶性肿瘤。SMARCA4(BRG1)缺失性癌常表达原始细胞标记,本文7例表达SALL4,2例表达CD34,2例均为阴性。有趣的是SALL4和CD34并不同时表达,临床工作中需同时检测两者。神经内分泌标志物在SMARCA4(BRG1)缺失性癌中有不同程度的表达,11例均检测到Syn表达,细胞呈弱-中等强度阳性,而其他神经内分泌标记CD56、CgA仅散在或少数细胞表达。发生于子宫的SMARCA4(BRG1)缺失性癌,细胞缺乏ER、PR表达,本文3例激素标记均为阴性。发生于胃肠道者缺乏SATB2、CDX2表达。
DNA错配修复蛋白检测显示,本文8例消化系统SMARCA4缺失性癌均为pMMR(MMR-proficient)。既往文献报道2例肠道SMARCA4缺失肠癌二代测序显示1例存在BRAF V600E和p53突变,2例均为MSS[10]。Tsuruta等[11]检测了54例胃低分化实性腺癌SWI/SNF复合物表达状态,结果显示26例SMARCA4缺失,且其中13例呈dMMR(MMR-deficient),缺失率达50%,提示SMARCA4缺失更易引起dMMR,该结果与本文检测结果相差较大,可能与本文入组病例数量较少有关,后期会收集更多样本进行检测。本文3例子宫SMARCA4(BRG1)缺失性癌均出现一个或两个错配修复蛋白的缺失,其中1例经Sanger测序检测证实为高度微卫星不稳定型(microsatellite instable-high, MSI-H)。以上结果与Tessier-Cloutier等[12]研究一致,其检测了44例子宫SWI/SNF复合物表达缺失的未分化癌,结果显示38例呈dMMR,其中17例SMARCA4缺失。
需要与本病鉴别诊断的肿瘤包括:(1)SMARCB1(INI1)缺失性癌:两者形态学表现有重叠,最主要的鉴别在于SMARCA4(BRG1)标志物检测。(2)神经内分泌癌:肿瘤细胞常弥漫性表达2个以上的神经内分泌标志物,而SMARCA4(BRG1)缺失性癌虽不同程度表达神经内分泌标志物,但多为灶性或部分表达,而且很少同时表达两种及两种以上神经内分泌标记。(3)上皮样胃肠道间质瘤:发生于胃肠道的SMARCA4(BRG1)缺失需与之鉴别,典型病例表达CD34、CD117、DOG-1,且大多数病例具有C-Kit、PDGFRA基因活化突变。(4)淋巴造血系统肿瘤:如间变性大细胞淋巴瘤、弥漫性大B细胞淋巴瘤:SMARCA4(BRG1)缺失性癌呈弥漫分布,黏附性差,需与之鉴别。肿瘤细胞表达T细胞或B细胞标记,不表达上皮标记物,必要时可行T或B细胞受体基因重排检测辅助诊断。(5)黑色素瘤:消化系统及子宫原发黑色素瘤少见,多为转移来源,结合病史及相关免疫组织化学标志物(MelanA、HMB45、SOX10等)可以鉴别。
目前对SMARCA4(BRG1)缺失性癌缺乏有效的治疗手段,常规治疗包括积极手术治疗,辅助传统的放化疗及靶向药物治疗等,但效果较差,患者常在短期内死亡。Machado等[13]回顾性分析41例未分化胃癌病例,其中5例SWI/SNF缺失性癌存在大量淋巴细胞浸润,提示其对免疫治疗可能更加敏感。有研究发现SMARCA4缺失性肺癌可以从免疫检查点抑制剂[14]和以铂类为主的化疗[15]中获益。Schoenfeld等[16]观察接受免疫检查点抑制剂治疗的445例SMARCA4缺失性肺癌患者,发现SMARCA4突变的患者较SMARCA4野生型具有更高的客观缓解率,生存率有显著提高。部分胸腔SMARCA4缺失性肿瘤病例也在此治疗中获得了一定的疗效[17]。其他针对SMARCA4表达改变的肿瘤治疗热点还包括氧化磷酸化抑制剂和CDK4/6抑制剂,且现有多项涉及SWI/SNF突变型肿瘤相关治疗的药物正处于临床试验阶段,相信在未来会有更多的治疗手段来改善患者预后。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:江晓珍:论文撰写郭峰棻:临床资料收集、影像学资料复查及论文撰写盛以芸:免疫组织化学染色梁圣楠:病理信息收集、切片扫描万红萍:复阅病理切片邓 葵:数据分析、论文指导 -
表 1 11例SMARCA4(BRG1)缺失性癌患者临床特征
Table 1 Clinical characteristics of 11 cases of SMARCA4(BRG1)-deficient carcinoma
Patients Age (year)
/GenderPosition Diameter (cm) Pathological diagnosis Therapeutic method 1 53/F Uterus 2.0 Undifferentiated endometrial carcinoma Surgery+chemotherapy 2 52/F Uterus 1.2 Endometrioid adenocarcinoma Ⅰ+
Undifferentiated carcinomaSurgery+chemotherapy+
radiotherapy3 60/F Uterus 2.1 Endometrioid adenocarcinoma Ⅱ+
Undifferentiated carcinomaSurgery+chemotherapy 4 71/M Distal stomach 9.0 Predisposed to undifferentiated carcinoma Surgery+chemotherapy 5 73/M Stomach - Malignant tumor Surgery+chemotherapy+
Targeted therapy6 62/F Small intestine 9.0 Undifferentiated carcinoma Surgery+chemotherapy 7 66/F Rectum 11.0 Mixed carcinoma Surgery+chemotherapy 8 48/M Right semicolon 8.5 60% adenocarcinoma
+40% undifferentiated carcinomaSurgery+chemotherapy 9 58/F Rectum - Undifferentiated carcinoma Chemotherapy+
Targeted therapy10 66/F Liver - Malignant tumor Targeted therapy+
immunotherapy11 45/F Liver - Undifferentiated carcinoma Chemotherapy Notes: M: male; F: female; -: not available. 表 2 11例SMARCA4(BRG1)缺失性癌免疫组织化学表型
Table 2 Immunohistochemical phenotype of 11 cases of SMARCA4(BRG1)-deficient carcinoma
No Position CK Vim BRG1 INI1 Syn SALL4 CD34 Ki-67 MMR Molecular detection MLH1 MSH2 MSH6 PMS2 1 Uterus - + - + + + - 70% - + + - undetected 2 Uterus focal + + - + + - + 70% + + + - MSI-H 3 Uterus - focal + - + + + - 80% + + - + undetected 4 Stomach + + - + + - - 90% + + + + undetected 5 Stomach + + - + + - - 70% + + + + undetected 6 Small intestine focal + + - + + - + 95% + + + + undetected 7 Rectum focal + + - + + + - 60% + + + + undetected 8 Right semicolon + + - + + + - 70% + + + + undetected 9 Rectum focal + + - + + + - 80% + + + + undetected 10 Liver + + - + + + - 80% + + + + undetected 11 Liver + + - + + + - 90% + + + + undetected Notes: +: Positive; −: Negative -
[1] Jelinic P, Mueller JJ, Olvera N, et al. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary[J]. Nat Genet, 2014, 46(5): 424-426. doi: 10.1038/ng.2922
[2] La Fleur L, Falk-Sörqvist E, Smeds P, et al. Mutation patterns in apopulation based non-small cell lung cancer cohort and prognostic impact of concomitant mutations in KRAS and TP53 or STK11[J]. Lung Cancer, 2019, 130: 50-58. doi: 10.1016/j.lungcan.2019.01.003
[3] Sirohi D MD, Ohe C, Smith SC, et al. SWI/SNF-deficient neoplasms of the genitourinary tract[J]. Semin Diagn Pathol, 2021, 38(3): 212-221. doi: 10.1053/j.semdp.2021.03.007
[4] Fernando TM, Piskol R, Bainer R, et al. Functional characterization of SMARCA4 variants identified by targeted exome-sequencing of 131, 668 cancer patients[J]. Nat Commun, 2020, 11(1): 5551. doi: 10.1038/s41467-020-19402-8
[5] Peterson CL, Dingwall A, Scott MP. Five SWI/SNF gene products are components of a large multisubunit complex required for transcriptional enhancement[J]. Proc Natl Acad Sci U S A, 1994, 91(8): 2905-2908. doi: 10.1073/pnas.91.8.2905
[6] Masliah-Planchon J, Bièche I, Guinebretière JM, et al. SWI/SNF chromatin remodeling and human malignancies[J]. Annu Rev Pathol, 2015, 10: 145-171. doi: 10.1146/annurev-pathol-012414-040445
[7] Agaimy A. The expanding family of SMARCB1(INI1)-deficient neoplasia: implications of phenotypic, biological, and molecular heterogeneity[J]. Adv Anat Pathol, 2014, 21(6): 394-410. doi: 10.1097/PAP.0000000000000038
[8] 朱培培, 李新星, 刘佳涵, 等. 胃肠道SMARCA4缺失性未分化癌9例临床病理学分析[J]中华病理学杂志, 2022, 51(9): 868-874. [Zhu PP, Li XX, Liu JH, et al. SMARCA4-deficient undifferentiated carcinoma of the gastrointestinal tract: a clinicopathological and immunohistochemical study of nine cases[J]. Zhonghua Bing Li Xue Za Zhi, 2022, 51(9): 868-874.] Zhu PP, Li XX, Liu JH, et al. SMARCA4-deficient undifferentiated carcinoma of the gastrointestinal tract: a clinicopathological and immunohistochemical study of nine cases[J]. Zhonghua Bing Li Xue Za Zhi, 2022, 51(9): 868-874.
[9] Chang B, Sheng W, Wang L, et al. SWI/SNF complex-deficient undifferentiated carcinoma of the gastrointestinal tract: clinicopathologic study of 30 cases with an emphasis on variable morphology, immune features, and the prognostic significance of different SMARCA4 and SMARCA2 subunit deficiencies[J]. Am J Surg Pathol, 2021, 46(7): 889-906.
[10] 黄榕芳, 何诚, 朱伟峰, 等. SMARCA4缺失肠癌的临床病理学及分子特征[J]中华病理学杂志, 2021, 50(4): 382-384. [Huang RF, He C, Zhu WF, et al. Clinicopathological and molecular features of SMARCA4-deficient carcinoma of the intestinal tract[J]. Zhonghua Bing Li Xue Za Zhi, 2021, 50(4): 382-384.] Huang RF, He C, Zhu WF, et al. Clinicopathological and molecular features of SMARCA4-deficient carcinoma of the intestinal tract[J]. Zhonghua Bing Li Xue Za Zhi, 2021, 50(4): 382-384.
[11] Tsuruta S, Kohashi K, Yamada1 Y, et al. Solid-type poorly differentiated adenocarcinoma of the stomach: Deficiency of mismatch repair and SWI/SNF complex[J]. Cancer Sci, 2020, 111(3): 1008-1019. doi: 10.1111/cas.14301
[12] Tessier-Cloutier B, Kang EY, Alex D, et al. Endometrial neuroendocrine carcinoma and undifferentiated carcinoma are distinct entities with overlap in neuroendocrine marker expression[J]. Histopathology, 2022, 81(1): 45-54.
[13] Machado I, Yoshida A, Morales MGN, et al. Review with novel markers facilitates precise categorization of 41 cases of diagnostically challenging, "undifferentiated small round cell tumors". A clinicopathologic, immunophenotypic and molecular analysis[J]. Ann Diagn Pathol, 2018, 34(7): 1-12.
[14] Naito T, Udagawa H, Umemura S, et al. Non-small cell lung cancer with loss of expression of the SWI/SNF complex is associated with aggressive clinicopathological features, PD-L1-positive status, and high tumor mutation burden[J]. Lung Cancer, 2019, 138: 35-42. doi: 10.1016/j.lungcan.2019.10.009
[15] Bell EH, Chakraborty AR, Mo X, et al. SMARCA4/BRG1 is a novel prognostic biomarker predictive of cisplatin-based chemotherapy outcomes in resected non-small cell lung cancer[J]. Clin Cancer Res, 2016, 22(10): 2396-2404. doi: 10.1158/1078-0432.CCR-15-1468
[16] Schoenfeld AJ, Bandlamudi C, Lavery JA, et al. The genomic landscape of SMARCA4 alterations and associations with outcomes in Patients with lung cancer[J]. Clin Cancer Res, 2020, 26(21): 5701-5708. doi: 10.1158/1078-0432.CCR-20-1825
[17] Takada K, Sugita S, Murase K, et al. Exceptionally rapid response to pembrolizumab in a SMARCA4-deficient thoracic sarcoma overexpressing PD-L1: a case report[J]. Thorac Cancer, 2019, 10(12): 2312-2315. doi: 10.1111/1759-7714.13215



 下载:
下载: