-
摘要:
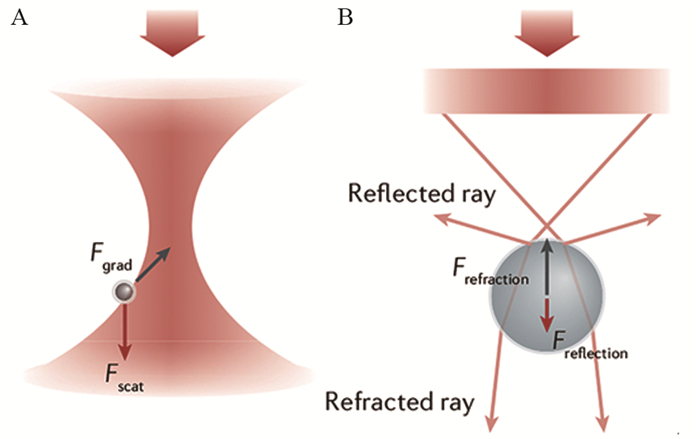
光镊用光操控物体,它可以在纳米空间、皮牛顿力和毫秒时间研究从单细胞到单分子的生物过程。光镊能够鉴别肿瘤细胞、操控肿瘤细胞、分析单分子和辅助肿瘤治疗,正深刻改变着肿瘤的研究和治疗。
Abstract:Optical tweezers are a means to manipulate objects with light. Optical tweezers can obtain a nanometer space, piconewton force, and a millisecond resolution; thus, they are excellently suited for studying biological processes from the single-cell to the single-molecule level. Optical tweezers can screen and manipulate tumor cells, study single molecule, and monitor cancer treatments. Thus, optical tweezers will promote the progress of cancer research and treatments.
-
Key words:
- Cancer /
- Optical tweezers /
- Cell /
- Single molecule
-
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:张百红:检索文献、文章撰写岳红云:文章审校
-
[1] Ashkin A, Dziedzic JM. Optical trapping and manipulation of viruses and bacteria[J]. Science, 1987, 235(4795): 1517-1520. doi: 10.1126/science.3547653
[2] Svoboda K, Schmidt CF, Schnapp BJ, et al. Direct observation of kinesin stepping by optical trapping interferometry[J]. Nature, 1993, 365(6448): 721-727. doi: 10.1038/365721a0
[3] Finer JT, Simmons RM, Spudich JA. Single myosin molecule mechanics: piconewton forces and nanometer steps[J]. Nature, 1994, 368(6467): 113-119. doi: 10.1038/368113a0
[4] Smith SB, Cui Y, Bustamante C. Overstretching B-DNA: the elastic response of individual double-stranded and single-stranded DNA molecules[J]. Science, 1996, 271: 795-799. doi: 10.1126/science.271.5250.795
[5] Wang MD, Schnitzer MJ, Yin H, et al. Force and velocity measured for single molecules of RNA polymerase[J]. Science, 1998, 282: 902-907. doi: 10.1126/science.282.5390.902
[6] Killian JL, Ye F, Wang MD. Optical Tweezers: A Force to Be Reckoned With[J]. Cell, 2018, 175(6): 1445-1448. doi: 10.1016/j.cell.2018.11.019
[7] Ozcelik A, Rufo J, Guo F, et al. Acoustic tweezers for the life sciences[J]. Nat Methods, 2018, 15(12): 1021-1028. doi: 10.1038/s41592-018-0222-9
[8] Bustamante CJ, Chemla YR, Liu SX, et al. Optical tweezers in single-molecule biophysics[J]. Nat Rev Methods Primers, 2021, 1: 25. doi: 10.1038/s43586-021-00021-6
[9] Wang X, Ho C, Tsatskis Y, et al. Intracellular manipulation and measurement with multipole magnetic tweezers[J]. Sci Robot, 2019, 4(28): eaav6180. doi: 10.1126/scirobotics.aav6180
[10] Varol R, Karavelioglu Z, Omeroglu S, et al. Acousto-holographic reconstruction of whole-cell stiffness maps[J]. Nat Commun, 2022, 13(1): 7351. doi: 10.1038/s41467-022-35075-x
[11] Sirotin MA, Romodina MN, Lyubin EV, et al. Single-cell all-optical coherence elastography with optical tweezers[J]. Biomed Opt Express, 2021, 13(1): 14-25.
[12] Marzo A, Drinkwater BW. Holographic acoustic tweezers[J]. Proc Natl Acad Sci U S A, 2019, 116(1): 84-89. doi: 10.1073/pnas.1813047115
[13] Terrasson A, Madsen L, Waleed M, et al. Ultrafast viscosity measurement with ballistic optical tueezers[C]//Optical Trapping and Optical Micromanipulation XVlll. 2021.
[14] Duś-Szachniewicz K, Drobczyński S, Woźniak M, et al. Differentiation of single lymphoma primary cells and normal B-cells based on their adhesion to mesenchymal stromal cells in optical tweezers[J]. Sci Rep, 2019, 9(1): 9885. doi: 10.1038/s41598-019-46086-y
[15] Li X, Chen Z, Li Y, et al. Optical tweezers study of membrane fluidity in small cell lung cancer cells[J]. Opt Express, 2021, 29(8): 11976-11986. doi: 10.1364/OE.420288
[16] Tsujita K, Satow R, Asada S, et al. Homeostatic membrane tension constrains cancer cell dissemination by counteracting BAR protein assembly[J]. Nat Commun, 2021, 12(1): 5930. doi: 10.1038/s41467-021-26156-4
[17] Lee OJ, Lim HG, Shung KK, et al. Automated estimation of cancer cell deformability with machine learning and acoustic trapping[J]. Sci Rep, 2022, 12(1): 6891. doi: 10.1038/s41598-022-10882-w
[18] Zhao Q, Wang HW, Yu PP, et al. Trapping and Manipulation of Single Cells in Crowded Environments[J]. Front Bioeng Biotechnol, 2020, 8: 422. doi: 10.3389/fbioe.2020.00422
[19] Kollipara PS, Li X, Li J, et al. Hypothermal opto-thermophoretic tweezers[J]. Nat Commun, 2023, 14(1): 5133. doi: 10.1038/s41467-023-40865-y
[20] Mondal PP, Baro N, Singh A, et al. Lightsheet optical tweezer (LOT) for optical manipulation of microscopic particles and live cells[J]. Sci Rep, 2022, 12(1): 10229. doi: 10.1038/s41598-022-13095-3
[21] Gupta K, Moon HR, Chen ZW, et al. Optically induced electrothermal microfluidic tweezers in bio-relevant media[J]. Sci Rep, 2023, 13(1): 9819. doi: 10.1038/s41598-023-35722-3
[22] Zhang SL, Scott EY, Singh J, et al. The optoelectronic microrobot: A versatile toolbox for micromanipulation[J]. Proc Natl Acad Sci U S A, 2019, 116(30): 14823-14828. doi: 10.1073/pnas.1903406116
[23] Xu T, Li YD, Han X, et al. Versatile, facile and low-cost single-cell isolation, culture and sequencing by optical tweezer-assisted pool-screening[J]. Lab Chip, 2022, 23(1): 125-135.
[24] Tian ZH, Wang ZY, Zhang P, et al. Generating multifunctional acoustic tweezers in Petri dishes for contactless, precise manipulation of bioparticles[J]. Sci Adv, 2020, 6(37): eabb0494. doi: 10.1126/sciadv.abb0494
[25] Li JG, Chen ZH, Liu YR, et al. Opto-refrigerative tweezers[J]. Sci Adv, 2021, 7(26): eabh1101. doi: 10.1126/sciadv.abh1101
[26] Vogt N. High-resolution optical tweezers[J]. Nat Methods, 2021, 18(4): 333. doi: 10.1038/s41592-021-01121-7
[27] Zhang YQ, Min CJ, Dou XJ, et al. Plasmonic tweezers: for nanoscale optical trapping and beyond[J]. Light Sci Appl, 2021, 10(1): 59. doi: 10.1038/s41377-021-00474-0
[28] Nadappuram BP, Cadinu P, Barik A, et al. Nanoscale tweezers for single-cell biopsies[J]. Nat Nanotechnol, 2019, 14(1): 80-88. doi: 10.1038/s41565-018-0315-8
[29] Shrestha P, Yang D, Tomov TE, et al. Single-molecule mechanical fingerprinting with DNA nanoswitch calipers[J]. Nat Nanotechnol, 2021, 16(12): 1362-1370. doi: 10.1038/s41565-021-00979-0
[30] Takezawa Y, Mori K, Huang WE, et al. Metal-mediated DNA strand displacement and molecular device operations based on base-pair switching of 5-hydroxyuracil nucleobases[J]. Nat Commun, 2023, 14(1): 4759. doi: 10.1038/s41467-023-40353-3
[31] Meijering AEC, Sarlós K, Nielsen CF, et al. Nonlinear mechanics of human mitotic chromosomes[J]. Nature, 2022, 605(7910): 545-550. doi: 10.1038/s41586-022-04666-5
[32] Kalluri P, LeBleu VS. The biology, function, and biomedical applications of exosomes[J]. Science, 2020, 367(6478): eaau6977. doi: 10.1126/science.aau6977
[33] Hong CC, Ndukaife JC, et al. Scalable trapping of single nanosized extracellular vesicles using plasmonics[J]. Nat Commun, 2023, 14(1): 4801. doi: 10.1038/s41467-023-40549-7
[34] Bolognesi G, Friddin M, Salehi-Reyhani A, et al. Sculpting and fusing biomimetic vesicle networks using optical tweezers[J]. Nat Commun, 2018, 9(1): 1882. doi: 10.1038/s41467-018-04282-w
[35] Bodescu MA, Aretz J, Grison M, et al. Kindlin stabilizes the talin·integrin bond under mechanical load by generating an ideal bond[J]. Proc Natl Acad Sci U S A, 2023, 120(26): e2218116120. doi: 10.1073/pnas.2218116120
[36] Lin L, Wang M, Peng X, et al. Opto-thermoelectric nanotweezers[J]. Nat Photonics, 2018, 12(4): 195-201. doi: 10.1038/s41566-018-0134-3
[37] Hao Y, Maillard R. Using Optical Tweezers to Dissect Allosteric Communication Networks in Protein Kinases[J]. Methods Mol Biol, 2022, 2394: 485-498.
[38] Milic B, Chakraborty A, Han K, et al. KIF15 nanomechanics and kinesin inhibitors, with implications for cancer chemotherapeutics[J]. Proc Natl Acad Sci U S A, 2018, 115(20): E4613-E4622.
[39] Patrick EM, Slivka JD, Payne B, et al. Observation of processive telomerase catalysis using high-resolution optical tweezers[J]. Nat Chem Biol, 2020, 16(7): 801-809. doi: 10.1038/s41589-020-0478-0
[40] Feng Y, Reinherz EL, Lang MJ. αβ T Cell Receptor Mechanosensing Forces out Serial Engagement[J]. Trends Immunol, 2018, 39(8): 596-609. doi: 10.1016/j.it.2018.05.005
[41] Duś-Szachniewicz K, Gdesz-Birula K, Nowosielska E, et al. Formation of Lymphoma Hybrid Spheroids and Drug Testing in Real Time with the Use of Fluorescence Optical Tweezers[J]. Cells, 2022, 11(13): 2113. doi: 10.3390/cells11132113



 下载:
下载:

