Research Progress of Osimertinib Supported by Nanodrug Delivery System Against Non-small Cell Lung Cancer
-
摘要:
奥希替尼是不可逆的第三代表皮生长因子受体酪氨酸激酶抑制剂(EGFR-TKI),用于治疗经典EGFR突变和T790M耐药突变的非小细胞肺癌(NSCLC)。然而,与其他EGFR-TKIs一样,奥希替尼不可避免地存在获得性耐药、水溶性差、肿瘤累积率低等问题,限制了其治疗效果。纳米递药系统可增加药物的溶解度和稳定性,延长药物血液循环时间,提高细胞摄取率,增加在肿瘤组织中的聚集改善药物耐药问题,已成为解决传统靶向药物弊端的有效手段。本文综述了第三代EGFR-TKI奥希替尼的作用机制,重点阐述了奥希替尼纳米递药系统抗NSCLC的研究进展,并对该领域面临的挑战和未来发展方向进行了展望。
Abstract:Osimertinib is an irreversible third representative epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) for the treatment of non-small cell lung cancer (NSCLC) with T790M resistance and classical EGFR mutations. However, the therapeutic effectiveness of osimertinib is limited by acquired drug-resistance, poor water solubility and low tumor accumulation rates. Nanodrug delivery systems can increase the solubility and stability of drugs, prolong the blood circulation time of drugs, improve the uptake rate of cells, promote drug accumulation in tumor tissues, and improve drug resistance. Thus, they are effective in overcoming the limitations of traditional targeted drugs. In this study, we reviewed the mechanism of action of the third-generation EGFR-TKI osimertinib, focused on research advances in osimertinib nanodrug delivery systems against NSCLC, and explored the challenges and future development direction in this field.
-
Key words:
- NSCLC /
- Osimertinib /
- Nanodrug delivery system
-
0 引言
多形性腺瘤(pleomorphic adenoma, PA)是腮腺中最常见的肿瘤,也被称为混合瘤,占所有腮腺肿瘤的60%~70%,世界卫生组织根据PA的组织病理学及生物学特性,将其界定为临界瘤[1]。据统计,每10万人就有1.4~2.3人发生该病,且PA发病率呈逐年递增趋势[2]。虽然PA生长比较缓慢,但其术后复发率可达到20%~45%,且多次复发可致恶变[3]。PA最常见的类型是癌在多形性腺瘤(carcinoma in pleomorphic adenoma, CA-EX-PA),约占涎腺恶性肿瘤的10%~20%,且90%以上都是由PA恶变所致,可危及生命,尽早发现两者的生物学行为、治疗方法及判断预后一直是医学界研究的热点[2]。
线粒体在细胞生存和死亡中发挥重要作用。为维持细胞的正常活动状态, 细胞要选择性地清除受损伤或不需要的线粒体, 这一过程被称为线粒体自噬(mitochondrial autophagy或mitophagy)[4]。线粒体自噬与肿瘤的关系成为医学研究的热点,作为诱导线粒体自噬的关键基因,PINK1/Parkin通路是主要分子调控机制之一,也是线粒体自噬的经典途径之一[5]。PINK1和Parkin具有协同作用,当它们任何一方表达水平异常或功能受到抑制时,清除受损线粒体的自噬能力会明显下降,使细胞正常生理功能进一步受到损害[6-7]。
目前,线粒体自噬活性的改变与恶性肿瘤的发生、发展密切相关, 且线粒体自噬在肿瘤发生发展的不同阶段所发挥的作用也不同。肿瘤发生早期,线粒体自噬维持细胞正常的新陈代谢,抑制肿瘤的发生;肿瘤发生后期,线粒体自噬的发生则会提高细胞的耐受,促进肿瘤的发展[8]。在卵巢癌、乳腺癌等多种肿瘤中也发现线粒体自噬基因出现等位缺失现象,但其在腮腺肿瘤的发生发展阶段,线粒体活性是否发生变化目前尚不清楚[9]。因此,本研究拟通过免疫组织化学的方法检测正常腮腺组织、腮腺多形性腺瘤及癌在多形性腺瘤组织中PINK1和Parkin的蛋白表达差异,探讨线粒体自噬在多形性腺瘤及其恶变中的作用,为进一步了解多形性腺瘤及其恶变提供理论依据。
1 资料与方法
1.1 资料来源
收集2016年4月—2018年4月间郑州大学附属肿瘤医院确诊的PA组织32例、CA-EX-PA组织42例。所有组织标本均未行术前放疗和化疗,并收集24例正常腮腺组织作为对照组。4%甲醛溶液常规固定,石蜡包埋,4 μm厚度连续切片,HE染色。所有患者事前均已签署《生物标本二次利用知情同意书》。
1.2 主要试剂
兔抗人PINK1和Parkin单克隆抗体购自武汉三鹰生物技术有限公司;免疫组织化学检测试剂盒、磷酸盐缓冲溶液、苏木素染色试剂购于上海碧云天生物技术有限公司,其余试剂购于中国医药上海化学试剂公司。
1.3 免疫组织化学检测PINK1和Parkin的蛋白表达差异
10%福尔马林固定标本,常规石蜡包埋,4 μm厚连续切片,脱蜡,依次经100%、95%、80%、70%及50%梯度浓度酒精水化,每个浓度5 min,PBS缓冲液冲洗,热抗原修复,滴加5%的山羊血清,室温下放置20~30 min,封闭非特异性抗原,滴加一抗PINK1(1:2 000)、Parkin(1:2 000)并置于4℃的冰箱过夜,取出切片,37℃孵育箱复温30 min后,PBS冲洗,滴加二抗37℃孵育30 min后PBS冲洗,加辣根过氧化物酶工作液反应15 min,DAB显色。苏木素对比染色,常规脱水、透明、干燥、封片,并拍照观察。
1.4 免疫组织化学结果评价标准
当细胞质染成棕褐色、棕黄色、黄色或淡黄色时,认为PINK1和Parkin两种蛋白阳性表达。利用二级计分法对两种蛋白的表达进行评定:随机选取每个切片10个200×的显微镜视野,通过阳性细胞百分率和染色深度进行评分。阳性率评分:阳性细胞数占总细胞数 < 5%计为0分,5%~20%计为1分,≥20%~50%计为2分,≥50%计为3分。染色深度评分:无染色计为0分,淡黄色计为1分,黄色/棕黄色计为2分,褐色/棕褐色计为3分。两项评分相乘即为总评分:0~2分计为阴性(-),3~4分计为弱阳性(+),5~6分计为中强阳性(++),6分以上计为强阳性(+++)。
1.5 统计学方法
应用Image-Pro Plus 6.0系统软件分析阳性细胞的吸光度(IOD),采用χ2检验和Spearman等级分析进行数据分析,实验结果采用统计学软件SPSS22.0进行分析,P < 0.05表示差异有统计学意义。
2 结果
2.1 PINK1和Parkin蛋白在三种组织中的表达
正常腮腺组织、PA组织和CA-EX-PA组织中PINK1蛋白阳性表达率依次为100%、91%和67%,PA和CA-EX-PA组织中PINK1的表达率显著低于正常组织(均P < 0.05)。Parkin蛋白在三种组织中阳性表达率依次为100%、88%和52%,PA和CA-EX-PA组织中Parkin的表达率同样显著低于正常组织(均P < 0.05),见表 1、图 1。
表 1 PINK1和Parkin在不同组织中的表达(n)Table 1 Expression of PINK1 and Parkin in different tissues (n)
2.2 CA-EX-PA组织中PINK1和Parkin蛋白表达的相关性
Spearman等级分析结果显示PINK1和Parkin蛋白在CA-EX-PA组织中的表达具有正相关性(r=0.877, P < 0.05),见表 2。
表 2 PINK1和Parkin在CA-EX-PA组织中表达的相关性Table 2 Correlation between PINK1 and Parkin expression in CA-EX-PA tissues
2.3 PINK1和Parkin蛋白在CA-EX-PA组织中表达与临床病理特征的关系
CA-EX-PA患者的年龄、性别和肿瘤直径与PINK1和Parkin蛋白表达不具有相关性(P > 0.05),CA-EX-PA患者的肿瘤侵袭性、TNM分期和淋巴结转移与PINK1和Parkin蛋白的表达显著相关(P < 0.05),见表 3。
表 3 CA-EX-PA患者中PINK1和Parkin表达与临床病理因素的关系Table 3 Correlation of PINK1 and Parkin expression with clinicopathological of CA-EX-PA patients
3 讨论
线粒体自噬能够选择性清除多余或受损线粒体,在维持细胞内线粒体数量以及线粒体的正常功能方面发挥重要的作用[10]。正常情况下,自噬体的生成和降解之间保持动态平衡,以维持细胞内环境的稳定;病理状态下,自噬体生成过多或清除过少,会引起细胞内受损线粒体大量堆积,导致细胞线粒体自噬异常,最终细胞功能产生紊乱甚至凋亡[11]。PINK1和Parkin是诱导线粒体自噬的重要因子,两者的表达缺失能够引起细胞线粒体自噬活性降低,可能导致肿瘤的发生[12-13]。研究显示在人类多种肿瘤中均有线粒体自噬活性改变的现象。例如,利用致癌物质诱导鼠的胰腺细胞后,线粒体自噬在癌变前期活性升高,但是当完成癌变以后,线粒体自噬活性能力反而减弱,提示线粒体自噬活性的降低可能促进一些肿瘤的恶化。腮腺肿瘤的发生是否与线粒体自噬相关因子的表达发生变化相关,是否参与了腮腺肿瘤的病理进程,目前相关方面报道甚少。
PINK1和Parkin蛋白可能具有抑制肿瘤功能,在乳腺癌、肺癌、直肠癌、卵巢癌等肿瘤中均表现为缺失表达,两者的表达水平显著低于正常组织[14]。Jeong等研究证实在小鼠胰腺癌癌变早期线粒体自噬水平明显升高,而晚期则显著降低[15]。另有实验研究显示,在敲除PINK1等位基因的小鼠中,乳腺癌、肺癌、腮腺肿瘤等癌症的发病率均明显高于正常小鼠[16]。本研究实验结果提示在PA和CA-EX-PA组织中PINK1和Parkin蛋白表达显著低于正常组织。PINK1基因在PA和CA-EX-PA组织中低表达可能是因为PA组织的迅速生长,细胞需要降低线粒体自噬水平来适应低氧和营养缺乏等环境。
Spearman相关系数检验结果显示,在癌组织中PINK1和Parkin在CA-EX-PA中表达呈正相关(r=0.691, P=0.010),说明两者的低表达可以降低CA-EX-PA线粒体自噬活性,与CA-EX-PA的发生、发展密切相关,两者的联合检测对CA-EX-PA的治疗效果及预后具有重要的参考价值。淋巴转移是判断腮腺恶性肿瘤预后的重要指标,本研究证实,在CA-EX-PA组织中,PINK1和Parkin蛋白的表达与淋巴结转移、TNM分期、肿瘤侵袭性显著相关。
在PA和CA-EX-PA组织中PINK1和Parkin蛋白表达明显降低,说明腮腺肿瘤的发生、演进和恶化可能和线粒体自噬活性降低相关,因此同时检测PINK1和Parkin蛋白表达水平可以更加客观准确地评估腮腺恶性肿瘤患者的预后,从而制定合理、有效的治疗方法,以提高患者的生存率。线粒体自噬的具体调控机制尚未完全明确,仍需进一步研究,为腮腺肿瘤的治疗提供新的靶点和新思路。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:刘汝贵、赵瑞瑞:总结和归纳文献、文章撰写刘春朝、武晓:文章选题、指导、审校 -
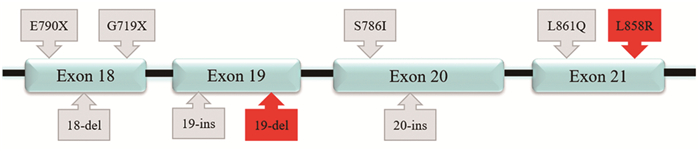
表 1 奥希替尼相关纳米递药系统
Table 1 Osimertinib-related nanodrug delivery systems

-
[1] Bade BC, Dela Cruz CS. Lung Cancer 2020 Epidemiology, Etiology, and Prevention[J]. Clin Chest Med, 2020, 41(1): 1-24. doi: 10.1016/j.ccm.2019.10.001
[2] Howlader N, Forjaz G, Mooradian MJ, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality[J]. N Engl J Med, 2020, 383(7): 640-649. doi: 10.1056/NEJMoa1916623
[3] Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC)[J]. Lung Cancer, 2019, 137: 113-122. doi: 10.1016/j.lungcan.2019.09.017
[4] Hayashi H, Nadal E, Gray JE, et al. Overall Treatment Strategy for Patients With Metastatic NSCLC With Activating EGFR Mutations[J]. Clin Lung Cancer, 2022, 23(1): E69-E82. doi: 10.1016/j.cllc.2021.10.009
[5] Leonetti A, Sharma S, Minari R, et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer[J]. Br J Cancer, 2019, 121(9): 725-737. doi: 10.1038/s41416-019-0573-8
[6] Yang J, Li YB, Sun JM, et al. An Osimertinib-Perfluorocarbon Nanoemulsion with Excellent Targeted Therapeutic Efficacy in Non-small Cell Lung Cancer: Achieving Intratracheal and Intravenous Administration[J]. ACS Nano, 2022, 16(8): 12590-12605. doi: 10.1021/acsnano.2c04159
[7] Khan KU, Minhas MU, Badshah SF, et al. Overview of nanoparticulate strategies for solubility enhancement of poorly soluble drugs[J]. Life Sci, 2022, 291: 120301. doi: 10.1016/j.lfs.2022.120301
[8] Peng SJ, Xiao FF, Chen MW, et al. Tumor-Microenvironment-Responsive Nanomedicine for Enhanced Cancer Immunotherapy[J]. Adv Sci, 2022, 9(1): 2103836. doi: 10.1002/advs.202103836
[9] Carcereny E, Moran T, Capdevila L, et al. The epidermal growth factor receptor (EGRF) in lung cancer[J]. Transl Respir Med, 2015, 3: 1. doi: 10.1186/s40247-015-0013-z
[10] Hassanein SS, Ibrahim SA, Abdel-Mawgood AL. Cell Behavior of Non-Small Cell Lung Cancer Is at EGFR and MicroRNAs Hands[J]. Int J Mol Sci, 2021, 22(22): 12496. doi: 10.3390/ijms222212496
[11] Da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer[J]. Annu Rev Pathol, 2011, 6: 49-69. doi: 10.1146/annurev-pathol-011110-130206
[12] Uribe ML, Marrocco I, Yarden Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance[J]. Cancers, 2021, 13(11): 2748. doi: 10.3390/cancers13112748
[13] Wu SG, Chang YL, Hsu YC, et al. Good Response to Gefitinib in Lung Adenocarcinoma of Complex Epidermal Growth Factor Receptor (EGFR) Mutations with the Classical Mutation Pattern[J]. Oncologist, 2008, 13(12): 1276-1284. doi: 10.1634/theoncologist.2008-0093
[14] Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer[J]. Semin Cancer Biol, 2020, 61: 167-179. doi: 10.1016/j.semcancer.2019.09.015
[15] Castellanos E, Feld E, Horn L. Driven by Mutations: The Predictive Value of Mutation Subtype in EGFR-Mutated Non-Small Cell Lung Cancer[J]. J Thorac Oncol, 2017, 12(4): 612-623. doi: 10.1016/j.jtho.2016.12.014
[16] Mithoowani H, Febbraro M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology[J]. Curr Oncol, 2022, 29(3): 1828-1839. doi: 10.3390/curroncol29030150
[17] Ramalingam SS, Yang JCH, Lee CK, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer[J]. J Clin Oncol, 2018, 36(9): 841-849. doi: 10.1200/JCO.2017.74.7576
[18] Lu S, Dong X R, Jian H, et al. AENEAS: A Randomized Phase Ⅲ Trial of Aumolertinib Versus Gefitinib as First-Line Therapy for Locally Advanced or Metastatic Non-Small-Cell Lung Cancer With EGFR Exon 19 Deletion or L858R Mutations[J]. J Clin Oncol, 2022, 40(27): 3162-3171. doi: 10.1200/JCO.21.02641
[19] Piper-Vallillo AJ, Sequist LV, Piotrowska Z. Emerging Treatment Paradigms for EGFR-Mutant Lung Cancers Progressing on Osimertinib: A Review[J]. J Clin Oncol, 2020, 38(25): 2926-2936. doi: 10.1200/JCO.19.03123
[20] Zeng Y, Yu DL, Tian WT, et al. Resistance mechanisms to osimertinib and emerging therapeutic strategies in nonsmall cell lung cancer[J]. Curr Opin Oncol, 2022, 34(1): 54-65. doi: 10.1097/CCO.0000000000000805
[21] He J, Huang Z, Han L, et al. Mechanisms and management of 3rd-generation EGFR-TKI resistance in advanced non-small cell lung cancer (Review)[J]. Int J Oncol, 2021, 59(5): 90. doi: 10.3892/ijo.2021.5270
[22] Liu Q, Yu S, Zhao W, et al. EGFR-TKIs resistance via EGFR-independent signaling pathways[J]. Mol Cancer, 2018, 17(1): 53. doi: 10.1186/s12943-018-0793-1
[23] Etheridge ML, Campbell SA, Erdman AG, et al. The big picture on nanomedicine: the state of investigational and approved nanomedicine products[J]. Nanomedicine, 2013, 9(1): 1-14. doi: 10.1016/j.nano.2012.05.013
[24] Ramasamy T, Ruttala HB, Gupta B, et al. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review[J]. J Control Release, 2017, 258. 226-253. doi: 10.1016/j.jconrel.2017.04.043
[25] Xu XY, Ho W, Zhang XQ, et al. Cancer nanomedicine: from targeted delivery to combination therapy[J]. Trends Mol Med, 2015, 21(4): 223-232. doi: 10.1016/j.molmed.2015.01.001
[26] Jahangirian H, Lemraski EG, Webster TJ, et al. A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine[J]. Int J Nanomedicine, 2017, 12. 2957-2978. doi: 10.2147/IJN.S127683
[27] Caster JM, Patel AN, Zhang T, et al. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials[J]. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2017, 9(1).
[28] Soejima K, Yasuda H, Hirano T. Osimertinib for EGFR T790M mutation-positive non-small cell lung cancer[J]. Expert Rev Clin Pharmacol, 2017, 10(1): 31-38. doi: 10.1080/17512433.2017.1265446
[29] Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study[J]. Lancet Oncol, 2016, 17(12): 1643-1652. doi: 10.1016/S1470-2045(16)30508-3
[30] Hu XC, Chen SM, Yin H, et al. Chitooligosaccharides-modified PLGA nanoparticles enhance the antitumor efficacy of AZD9291 (Osimertinib) by promoting apoptosis[J]. Int J Biol Macromol, 2020, 162: 262-272. doi: 10.1016/j.ijbiomac.2020.06.154
[31] Guardiola S, Sanchez-Navarro M, Rosell R, et al. Anti-EGF nanobodies enhance the antitumoral effect of osimertinib and overcome resistance in non-small cell lung cancer (NSCLC) cellular models[J]. Med Oncol, 2022, 39(12): 195. doi: 10.1007/s12032-022-01800-1
[32] Izci M, Maksoudian C, Manshian BB, et al. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors[J]. Chem Rev, 2021, 121(3): 1746-1803. doi: 10.1021/acs.chemrev.0c00779
[33] Kang H, Rho S, Stiles WR, et al. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting[J]. Adv Healthc Mater, 2020, 9(1): 1901223. doi: 10.1002/adhm.201901223
[34] Ulldemolins A, Seras-Franzoso J, Andrade F, et al. Perspectives of nano-carrier drug delivery systems to overcome cancer drug resistance in the clinics[J]. Cancer Drug Resist, 2021, 4(1): 44-68.
[35] Kaushik N, Borkar SB, Nandanwar SK, et al. Nanocarrier cancer therapeutics with functional stimuli-responsive mechanisms[J]. J Nanobiotechnol, 2022, 20(1): 152. doi: 10.1186/s12951-022-01364-2
[36] Kravanja G, Primozic M, Knez Z, et al. Chitosan-Based (Nano)Materials for Novel Biomedical Applications[J]. Molecules, 2019, 24(10): 960.
[37] Cao Y, Tan YF, Wong YS, et al. Recent Advances in Chitosan-Based Carriers for Gene Delivery[J]. Mar Drugs, 2019, 17(6): 381. doi: 10.3390/md17060381
[38] Kumar SK, Choppala AD. Development and Optimization of Osimertinib-loaded Biodegradable Polymeric Nanoparticles Enhance In-vitro Cytotoxicity in Mutant EGFR NSCLC Cell Models and In-vivo Tumor Reduction in H1975 Xenograft Mice Models[J]. Aaps Pharmscitech, 2022, 23(5): 159. doi: 10.1208/s12249-022-02314-9
[39] Gu JJ, Yao WL, Shi PY, et al. MEK or ERK inhibition effectively abrogates emergence of acquired osimertinib resistance in the treatment of epidermal growth factor receptor-mutant lung cancers[J]. Cancer, 2020, 126(16): 3788-3799. doi: 10.1002/cncr.32996
[40] Li YT, Zang HJ, Qian GQ, et al. ERK inhibition effectively overcomes acquired resistance of epidermal growth factor receptor-mutant non-small-cell lung cancer cells to osimertinib[J]. Cancer, 2020, 126(6): 1339-1350. doi: 10.1002/cncr.32655
[41] Chen W, Yu DL, Sun SY, et al. Nanoparticles for co-delivery of osimertinib and selumetinib to overcome osimertinib-acquired resistance in non-small cell lung cancer[J]. Acta Biomater, 2021, 129: 258-268. doi: 10.1016/j.actbio.2021.05.018
[42] Zhang QN, Yie GQ, Li Y, et al. Studies on the cyclosporin A loaded stearic acid nanoparticles[J]. Int J Pharm, 2000, 200(2): 153-159. doi: 10.1016/S0378-5173(00)00361-6
[43] Trombino S, Russo R, Mellace S, et al. Solid lipid nanoparticles made of trehalose monooleate for cyclosporin-A topic release[J]. J Drug Deliv Sci Technol, 2019, 49: 563-569. doi: 10.1016/j.jddst.2018.12.026
[44] Chen SM, Ji XG, Zhao MY, et al. Construction of chitooligosaccharide-based nanoparticles of pH/redox cascade responsive for co-loading cyclosporin A and AZD9291[J]. Carbohydr Polym, 2022, 291: 1119619.
[45] Chen Q, Huang GJ, Wu WT, et al. A Hybrid Eukaryotic-Prokaryotic Nanoplatform with Photothermal Modality for Enhanced Antitumor Vaccination[J]. Adv Mater, 2020, 32(16): 1908185. doi: 10.1002/adma.201908185
[46] Xu B, Zeng FJ, Deng JL, et al. A homologous and molecular dual-targeted biomimetic nanocarrier for EGFR-related non-small cell lung cancer therapy[J]. Bioact Mater, 2023, 27: 337-347.
[47] Ren MX, Li YZ, Zhang H, et al. An oligopeptide/aptamer-conjugated dendrimer-based nanocarrier for dual-targeting delivery to bone[J]. J Mat Chem B, 2021, 9(12): 2831-2844. doi: 10.1039/D0TB02926B
[48] Shen SH, Wu YS, Liu YC, et al. High drug-loading nanomedicines: progress, current status, and prospects[J]. Int J Nanomed, 2017, 12. 4085-4109. doi: 10.2147/IJN.S132780
[49] Vega-Villa KR, Takemoto JK, Yanez JA, et al. Clinical toxicities of nanocarrier systems[J]. Adv Drug Deliv Rev, 2008, 60(8): 929-938. doi: 10.1016/j.addr.2007.11.007
[50] Pingale P, Kendre P, Pardeshi K, et al. An emerging era in manufacturing of drug delivery systems: Nanofabrication techniques[J]. Heliyon, 2023, 9(3): e14247. doi: 10.1016/j.heliyon.2023.e14247
[51] Huang L, Zhao SJ, Fang F, et al. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy[J]. Biomaterials, 2021, 268. 120557. doi: 10.1016/j.biomaterials.2020.120557
[52] Yang MY, Zhao RR, Fang YF, et al. Carrier-free nanodrug: A novel strategy of cancer diagnosis and synergistic therapy[J]. Int J Pharm, 2019, 570: 118663. doi: 10.1016/j.ijpharm.2019.118663
[53] Liu LH, Zhang XZ. Carrier-free nanomedicines for cancer treatment[J]. Prog Mater Sci, 2022, 125: 100919. doi: 10.1016/j.pmatsci.2021.100919
[54] Fu SW, Li GT, Zang WL, et al. Pure drug nano-assemblies: A facile carrier-free nanoplatform for efficient cancer therapy[J]. Acta Pharm Sin B, 2022, 12(1): 92-106. doi: 10.1016/j.apsb.2021.08.012
[55] Mei H, Cai SS, Huang DN, et al. Carrier-free nanodrugs with efficient drug delivery and release for cancer therapy: From intrinsic physicochemical properties to external modification[J]. Bioact Mater, 2022, 8: 220-240.
[56] Han HS, Choi KY. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications[J]. Biomedicines, 2021, 9(3): 305. doi: 10.3390/biomedicines9030305
[57] Correia JH, Rodrigues JA, Pimenta S, et al. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions[J]. Pharmaceutics, 2021, 13(9): 1332. doi: 10.3390/pharmaceutics13091332
[58] Yang L, Huang B, Hu SQ, et al. Indocyanine green assembled free oxygen-nanobubbles towards enhanced near-infrared induced photodynamic therapy[J]. Nano Res, 2022, 15(5): 4285-4293. doi: 10.1007/s12274-022-4085-0
[59] Hu XY, Li JW, Chen YL, et al. A Self-Assembly ICG Nanoparticle Potentiating Targeted Photothermal and Photodynamic Therapy in NSCLC[J]. ACS Biomater Sci Eng, 2022, 8(10): 4535-4546. doi: 10.1021/acsbiomaterials.2c00620



 下载:
下载: