Therapeutic Effect of Savolitinib in Patients with Stage Ⅲ/Ⅳ Non-small Cell Lung Cancer
-
摘要:目的
分析赛沃替尼在Ⅲ/Ⅳ期非小细胞肺癌(NSCLC)患者中的治疗效果。
方法采用随机数字表法将95例MET 14外显子(METex14)跳跃突变的Ⅲ/Ⅳ期NSCLC患者分为对照组(47例)与观察组(48例)。对照组给予克唑替尼治疗,观察组给予赛沃替尼治疗。采用卡方检验分析两组临床疗效及不良反应发生情况,Kaplan-Meier生存分析评估两组患者生存情况。
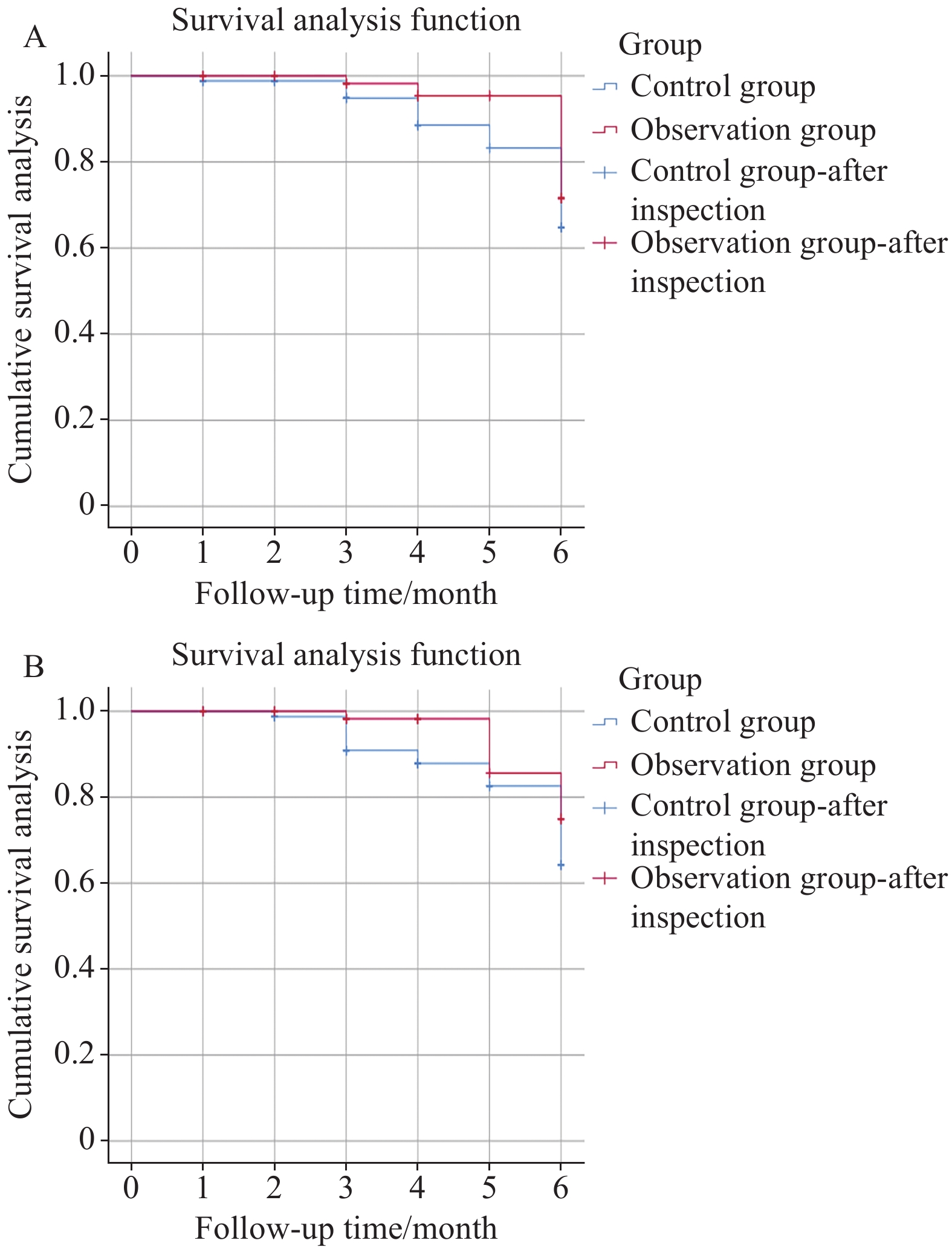
结果观察组客观缓解率(ORR)和疾病控制率(DCR)分别为52.08%和87.50%,均高于对照组的31.91%和70.21%(P<0.05);Kaplan-Meier生存分析显示,观察组总生存期(OS)和无进展生存期(PFS)长于对照组(Log rank χ2=8.003、4.528,P=0.005、0.033);两组不良反应程度比较差异无统计学意义(P>0.05)。
结论赛沃替尼可提高Ⅲ/Ⅳ期METex14跳跃突变NSCLC患者的治疗效果,延长患者的生存期,且患者对赛沃替尼的耐受性较好,不良反应可控。
-
关键词:
- 非小细胞肺癌 /
- 间质-上皮细胞转化因子 /
- 赛沃替尼
Abstract:ObjectiveTo analyze therapeutic effect of savolitinib in patients with stage Ⅲ/Ⅳ non-small cell lung cancer (NSCLC).
MethodsA total of 95 patients with MET 14 exon (METex14) jumping mutation in stage Ⅲ/Ⅳ NSCLC were divided into a control group (47 cases) and an observation group (48 cases) through a random-number table method. The patients in the control group were treated with crizotinib, whereas those in the observation group were treated with savolitinib. The clinical efficacy and incidence of toxic side effects in both groups were evaluated through a chi-square test, and survival was evaluated through Kaplan-Meier survival analysis.
ResultsCompared with control group (31.91% and 70.21%), the objective response rate and disease control rate of the observation group were 52.08% and 87.50%, respectively (P<0.05). According to Kaplan-Meier survival analysis, the overall survival and progression free survival rates in the observation group were higher than those in the control group (Log rank χ2=8.003, 4.528; P=0.005, 0.033). No statistically significant difference in the degree of toxic side effects was found between the groups (P>0.05).
ConclusionSavolitinib can improve the efficacy of treatment for stage Ⅲ/Ⅳ METex14 skip mutation NSCLC patients, prolong survival, enhance the tolerance of patients to savolitinib, and facilitate the management of adverse reactions.
-
0 引言
胃癌是常见的消化道恶性肿瘤,约占胃全部恶性肿瘤的95%,进展快,预后差,严重影响患者身心健康和生活质量,其发病率呈逐年上升趋势[1-2]。胃癌根治术联合D2淋巴结清扫术是当前外科治疗局部进展期胃癌的标准术式之一[3-5]。随着手术技术的日益完善,以及新型手术器械广泛应用,胃癌根治术后的并发症显著下降,但术后短期并发症严重影响患者住院时间、出血风险和生活质量,因此术后出现的短期并发症仍是备受关注的问题[6-8]。目前对于术后短期并发症影响患者远期生存率的分析报道较少。本研究旨在探讨胃癌D2根治术后短期并发症对患者远期生存率的影响,并分析相关影响因素。
1 资料与方法
1.1 临床资料
收集2010年1月—2011年12月于福建医科大学附属第一医院接受胃癌D2根治手术治疗的421例胃癌患者。纳入标准:(1)所有患者经临床表现、影像学资料及病理首次确诊为胃癌(Ⅰa~Ⅲc期),未进行术前相关治疗;(3)临床资料和生存数据完整,Clavien-Dindo(CD)评分系统进行并发症分级评估(CD评分≥Ⅱ级);(3)所有患者均接受胃癌D2根治术。排除标准:(1)患者合并其他恶性肿瘤;(2)有精神障碍、意识障碍;(3)合并严重肝肾功能和心肺功能障碍; (4)肿瘤复发或者转移;(5)失访的患者及意外事件引起的死亡。按照术后短期有无并发症分为:实验组(并发症组)76例,其中男58例,女18例,年龄45~75岁;对照组(无并发症组)345例,其中男256例,女89例,年龄45~89岁。倾向性得分匹配法(propensity score matching, PSM)平衡各变量,剔除未匹配的患者后,144例胃癌术后患者纳入本研究,其中76例为实验组,68例为对照组。本研究符合医学伦理学要求,患者及家属对本次研究知情并签署知情同意书。
1.2 手术方法
421例患者行根治性全胃切除或者根治性远端胃切除。根据第13版日本胃癌规约,所有患者按照标准的D2手术范围清除区域淋巴结。根治性远端胃切除患者清除淋巴结范围为第1、3、4、5、6、7、8、9、11、12a组淋巴结,根治性全胃清除的淋巴结范围为第1、2、3、4、5、6、7、8a、9、10、11、12a组淋巴结。根治性远端胃癌患者的消化道重建方式为布朗吻合;根治性全胃患者的消化道重建方式为Roux-en-Y吻合,所有吻合口均以丝线加固,残端包埋,术毕创面彻底止血,大量温热蒸馏水冲洗腹腔,放置引流管,逐层关腹。
1.3 观察指标
(1) 纳入分析的因素有年龄、性别、肿瘤最大径、pTNM分期、淋巴结转移、组织分化、脉管癌栓、神经侵犯和淋巴管侵犯等;(2)采用CD评分系统进行并发症分级评估观察患者术后短期并发症情况,包括肺部感染、腹腔感染、切口感染、吻合口出血、吻合口瘘、吻合口狭窄、胃排空延迟、肠梗阻和多器官衰竭等;(3)两组患者随访5年的生存情况。
1.4 随访情况
采用门诊随访、信件回访及电话问询等方式进行随访。随访截至2016年3月2日。患者的生存时间(月数)为自手术日期起至死亡日期或随访截止日期。对于失访和意外事故死亡的患者予以剔除。
1.5 统计学方法
采用SPSS24.0版统计软件处理数据。PSM通过软件中PS最邻近匹配法获得,其中最近距离与倾向得分对数的标准偏差的卡尺距离为0.02。实验组和对照组的基线资料比较采用卡方检验,生存分析采用Kaplan-Meier法,协变量与预后之间的关联分析采用Log rank单因素分析和Cox多因素分析。P < 0.05为差异有统计学意义。
2 结果
2.1 临床基线参数
PSM前共纳入的421例患者中术后短期并发症(76例)有肺部感染、腹腔感染、切口感染、吻合口出血、吻合口瘘、吻合口狭窄、胃排空延迟、肠梗阻和多器官衰竭等。76例(18.05%)术后出现短期并发症为实验组、345例(81.95%)未出现明显并发症为对照组。两组患者基线参数比较结果显示,仅术后住院时间差异有统计学意义(P < 0.001)。为进一步减小选择性偏倚,本研究对421例患者通过PSM按照1:1匹配,均衡所有基线特征,最终144例纳入本研究,其中实验组76例,对照组68例,见表 1。
表 1 基于PSM前后患者的临床病理特征Table 1 Clinicopathological characteristics of patients before and after PSM
2.2 生存分析
2.2.1 术后短期并发症生存分析
PSM前,实验组相对于对照组的远期生存率(HR=1.324, 95%CI: 0.942~1.860, p=0.106)差异无统计学意义。PSM后,与对照组比较,术后短期并发症患者的远期生存率(HR=1.175, 95%CI: 0.746~1.850, p=0.486)差异仍无统计学意义,结果进一步表明胃癌术后短期并发症对远期生存率影响结果无统计学意义,见图 1。
2.2.2 PSM后术后短期并发症亚组生存分析
PSM后亚组分析结果显示,肺部感染(HR=1.087, 95%CI: 0.679~1.741, p=0.728)、切口感染(HR=1.234, 95%CI: 0.679~2.244, p=0.490)、腹腔感染(HR=0.846, 95%CI: 0.421~1.697, p=0.637)和其他并发症(吻合口出血、吻合口瘘、吻合口狭窄、胃排空延迟、肠梗阻和多器官衰竭等)(HR=1.009, 95%CI: 0.503~2.204, p=0.980)的胃癌患者的远期生存率均无统计学意义(均P > 0.05),见图 2。进一步表明胃癌D2根治术后短期并发症对患者远期生存时间无明显影响。
![]() 图 2 PSM后患者术后短期并发症亚组的生存曲线Figure 2 Survival curves of gastric cancer patients in subgroups of short-term postoperative complications after PSMA: pulmonary infection; B: incision infection; C: abdominal infection; D: other complications (anastomotic bleeding, anastomotic leakage, anastomotic stenosis, delayed gastric emptying, intestinal obstruction and multiple organ failure, etc.)
图 2 PSM后患者术后短期并发症亚组的生存曲线Figure 2 Survival curves of gastric cancer patients in subgroups of short-term postoperative complications after PSMA: pulmonary infection; B: incision infection; C: abdominal infection; D: other complications (anastomotic bleeding, anastomotic leakage, anastomotic stenosis, delayed gastric emptying, intestinal obstruction and multiple organ failure, etc.)2.3 Log rank单因素和Cox多因素分析
PSM后,采用Log rank单因素分析144例患者远期生存率的影响因素,其中肿瘤的最大径、组织学类型、pTNM分期、分化程度、淋巴结转移率、脉管内癌栓、神经侵犯和淋巴管侵犯与胃癌术后的远期生存率显著相关(均P < 0.05)。将上述有统计学意义的参数进行Cox多因素分析,结果表明,仅组织学类型、淋巴结转移率和pTNM分期是患者远期生存率的独立预后影响因素,见表 2~3。
表 2 PSM后影响患者预后的单因素分析Table 2 Univariate analysis of prognostic factors of gastric cancer patients after PSM 表 3 PSM后影响预后的Cox多因素分析Table 3 Cox multivariate analysis of prognostic factors of gastric cancer patients after PSM
表 3 PSM后影响预后的Cox多因素分析Table 3 Cox multivariate analysis of prognostic factors of gastric cancer patients after PSM
3 讨论
在我国各种恶性肿瘤中发病率占第二位,死亡率居第三位[9]。目前,胃癌根治术联合D2淋巴结清扫术是当前外科治疗局部进展期胃癌的标准术式之一。在不影响胃癌根治疗效的前提下,减少术后短期并发症的发生,成为国内外胃恶性肿瘤治疗关注的热点问题[10],术后短期并发症加剧疾病痛苦,延缓患者术后恢复,延长住院天数,增加医疗资源负担及患者家庭的经济负担,因此控制和降低术后短期并发症的发生率具有重要临床意义。现对于术后短期并发症对于患者远期生存时间的影响研究尚有争议。
本研究中患者术后短期并发症主要以肺部感染为首,其发生率情况与既往研究胃癌术后远期生存率[11]的结果符合。
PSM前,对421例患者进行生存分析,结果显示胃癌D2根治术后的短期并发症未能对患者的远期生存率产生明显的影响。为减少选择偏倚,进行PSM平衡各变量。PSM后的结果表明,术后短期并发症仍未对患者的远期生存时间产生明显的影响,但根据Kaplan-Meier生存曲线描绘得到,无术后短期并发症的患者生存时间优于术后短期并发症患者[12],可能与术后短期并发症引起的炎性反应有关,并影响后续的首次化疗效果,表明控制和降低胃癌术后短期并发症仍具有一定的临床意义。我们分别对主要并发症包括肺部感染、切口感染、腹腔感染和其他症状(吻合口出血、吻合口瘘、吻合口狭窄、胃排空延迟、肠梗阻和多器官衰竭等)进行远期生存率分析,结果显示差异均无统计学意义(均P > 0.05),进一步表明术后短期并发症均对患者术后远期生存时间无显著影响。
探讨胃癌D2根治术后对患者远期生存时间的影响因素,对PSM后纳入的144例患者进行Log rank单因素分析患者远期生存率的影响因素,结果显示肿瘤的最大径、组织学类型、pTNM分期、分化程度、淋巴结转移率、脉管内癌栓、神经侵犯和淋巴管侵犯与胃癌术后的远期生存率显著相关[13]。Cox多因素分析结果表明,组织学类型、淋巴结转移率和pTNM分期是患者远期生存率的独立预后影响因素。有研究表明对于胃癌患者术后进行组织学类型、淋巴结转移率和pTNM分期的诊断具有良好指示预后的指标[14]。
胃癌患者临床症状较为隐匿,通常就诊时已属中晚期,失去了最佳治疗时机[15];早期胃癌的5年生存率可达95%,而进展期胃癌的生存率低于20%[16],因此提高胃癌的早期诊断率对胃癌的早期治疗至关重要。要根本上提高患者术后的远期生存率,通过早发现、早诊断和早治疗,将癌症尽量控制在早期,减低胃癌的晚期发生率。
本研究采用生存情况预测模型,结合有效检验,得到单个预测因子,并通过多因素校正等评价了该预测模型,有效地将临床各个病理特征及其所占的权重体现出来,具有较高、较强的预测水平,探讨对临床上行胃癌D2根治术后短期并发症对远期生存时间的影响,并提供精确的评估和预后判断的依据,为将来提高患者远期生存率提供新的改善方法与思路。
综上所述,胃癌D2根治术后的短期并发症对患者远期生存率没有显著性影响,但仍会降低患者术后的生活质量,加剧医疗资源压力,积极预防并控制并发症具有一定的临床意义。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:钟 诚:选题设计、资料与数据收集、文章撰写和修改张 扬:选题设计、数据收集储子昂:数据收集钱 洪:指导选题设计、文章写作及修改 -
表 1 两组一般资料比较[n(%),$\bar x \pm s $]
Table 1 Comparison of general information between two groups (n(%), $\bar x \pm s $)
General
informationObservation
group
(n=48)Control
group
(n=47)χ2/t P Gender 0.436 0.509 Male 13(27.08) 10(21.28) Female 35(72.92) 37(78.72) Age (years) 70.54 ± 5.22 70.43 ± 5.19 0.103 0.918 Smoking 6(12.50) 7(14.89) 0.115 0.734 Clinical stages 0.085 0.770 Ⅲ 30(62.50) 28(59.57) Ⅳ 18(37.50) 19(40.43) Pathological type 1.009 0.849 Sarcomatoid
carcinoma16(33.33) 14(29.79) Squamous
carcinoma5(10.42) 6(12.77) Adenocarcinoma 25(52.08) 23(48.94) Other 2(4.17) 4(8.51) 表 2 两组临床疗效比较[n(%)]
Table 2 Comparison of clinical efficacy between two groups (n(%))
Clinical
efficacyObservation
group (n=48)Control group
(n=47)χ2 P CR 1(2.08) 0 PR 24(50.00) 15(31.91) PD 6(12.50) 14(29.79) SD 17(35.42) 18(38.30) ORR 25(52.08) 15(31.91) 3.963 0.047 DCR 42(87.50) 33(70.21) 4.270 0.039 表 3 两组患者不良反应程度比较[n(%)]
Table 3 Comparison of degree of toxic side effects between two groups (n(%))
Toxic and
side reactionObservation
group (n=48)Control
group (n=47)χ2 P Nausea and
vomiting0.100 0.752 Level 1-2 40(83.33) 38(80.85) Level 3-4 8(16.67) 9(19.15) Liver and kidney
function impairment0.137 0.712* Level 1-2 44(91.67) 41(87.23) Level 3-4 4(8.33) 6(12.77) Edema 0.128 0.720 Level 1-2 43(89.58) 41(87.23) Level 3-4 5(10.42) 6(12.77) Rash 0.080 0.777 Level 1-2 42(87.50) 42(89.36) Level 3-4 6(12.50) 5(10.64) Note: *: continuity correction Chi-square test. -
[1] 王秋, 林川. ALDH1和AQP-5蛋白在非小细胞肺癌组织中的表达及与病理学参数, 预后的关系[J]. 河北医药, 2021, 43(4): 548-551. [Wang Q, Lin C. Expression of ALDH1 and AQP-5 proteins in NSCLC tissues and their relationship with clinical pathological parameters and prognosis of patients[J]. Hebei Yi Yao, 2021, 43(4): 548-551.] Wang Q, Lin C. Expression of ALDH1 and AQP-5 proteins in NSCLC tissues and their relationship with clinical pathological parameters and prognosis of patients[J]. Hebei Yi Yao, 2021, 43(4): 548-551.
[2] Xu H, Wang J, Al-Nusaif M, et al. CCL2 promotes metastasis and epithelial-mesenchymal transition of non-small cell lung cancer via PI3K/Akt/mTOR and autophagy pathways[J]. Cell Prolif, 2023, 18: e13560.
[3] Gohlke L, Alahdab A, Oberhofer A, et al. Loss of Key EMT-Regulating miRNAs Highlight the Role of ZEB1 in EGFR Tyrosine Kinase Inhibitor-Resistant NSCLC[J]. Int J Mol Sci, 2023, 24(19): 14742.
[4] Mahrous M, Omar Jebriel A, Allehebi A, et al. Consensus Recommendations for the Diagnosis, Biomarker Testing, and Clinical Management of Advanced or Metastatic Non-small Cell Lung Cancer With Mesenchymal-Epithelial Transition Exon 14 Skipping Mutations in the Middle East, Africa, and Russia[J]. Cureus, 2023, 15(7): e41992.
[5] 郭宗儒. 我国自行研制的抗癌药物赛沃替尼[J]. 药学学报, 2022, 57(3): 839-844. [Guo ZR. China's self-developed anti-cancer drug, Sevotinib[J]. Yao Xue Xue Bao, 2022, 57(3): 839-844.] Guo ZR. China's self-developed anti-cancer drug, Sevotinib[J]. Yao Xue Xue Bao, 2022, 57(3): 839-844.
[6] 葛均波, 徐永健, 王辰. 内科学[M]. 第9版. 北京: 人民卫生出版社, 2018: 75-86. [Ge JB, Xu YJ, Wang C. Internal medicine[M]. 9th edition. Beijing: People's Medical Publishing House Co. , Ltd, 2018: 75-86.] Ge JB, Xu YJ, Wang C. Internal medicine[M]. 9th edition. Beijing: People's Medical Publishing House Co. , Ltd, 2018: 75-86.
[7] 曹珍, 李增军, 单军奇, 等. 调控肝细胞生长因子受体c-Met表达对结肠癌细胞生物学潜能的影响[J]. 中华肿瘤防治杂志, 2022, 29(11): 816-823. [Cao Z, Li ZJ, Shan JQ, et al. Effect of regulation of hepatocyte growth factor receptor c-Met expression on the biological potentiality of colon cancer cells[J]. Zhonghua Zhong Liu Fang Zhi Za Zhi, 2022, 29(11): 816-823.] Cao Z, Li ZJ, Shan JQ, et al. Effect of regulation of hepatocyte growth factor receptor c-Met expression on the biological potentiality of colon cancer cells[J]. Zhonghua Zhong Liu Fang Zhi Za Zhi, 2022, 29(11): 816-823.
[8] 陈恒屹, 何勇. 肝细胞生长因子/间质上皮转化因子信号通路与非小细胞肺癌靶向治疗耐药的关系及机制研究进展[J]. 中国医学科学院学报, 2021, 43(2): 259-264. [Chen HY, He Y. Research Progress in Hepatocyte Growth Factor/Mesenchymal-epithelial Transition Factor Signaling Pathway: Effects and Mechanisms on Resistance to Targeted Therapy for Non-small Cell Lung Cancer[J]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao, 2021, 43(2): 259-264.] Chen HY, He Y. Research Progress in Hepatocyte Growth Factor/Mesenchymal-epithelial Transition Factor Signaling Pathway: Effects and Mechanisms on Resistance to Targeted Therapy for Non-small Cell Lung Cancer[J]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao, 2021, 43(2): 259-264.
[9] Recondo G, Che J, Jänne PA, et al. Targeting MET dysregulation in cancer[J]. Cancer Discov, 2020, 10(7): 922-934. doi: 10.1158/2159-8290.CD-19-1446
[10] 周晔, 于雁. MET14外显子跳跃突变与非小细胞肺癌[J]. 国际肿瘤学杂志, 2021, 48(6): 366-369. [Zhou Y, Yu Y. MET14 exon skipping mutation and non-small cell lung cancer[J]. Guo Ji Zhong Liu Xue Za Zhi, 2021, 48(6): 366-369.] Zhou Y, Yu Y. MET14 exon skipping mutation and non-small cell lung cancer[J]. Guo Ji Zhong Liu Xue Za Zhi, 2021, 48(6): 366-369.
[11] 钱伟玲, 吴沙沙, 赵红梅, 等. 克唑替尼胶囊联合NP方案治疗晚期非小细胞肺癌的效果及对血清NSE, CEA, Cyfra21-1水平的影响[J]. 临床误诊误治, 2022, 35(10): 26-32. [Qian WL, Wu SS, Zhao HM, et al. Effect of Crizotinib Capsules Combined with NP Regimen in the Treatment of Advanced Non-small Cell Lung Cancer and Its Influence on the Levels of Serum NSE, CEA and Cyfra21-1[J]. Lin Chuang Wu Zhen Wu Zhi, 2022, 35(10): 26-32.] Qian WL, Wu SS, Zhao HM, et al. Effect of Crizotinib Capsules Combined with NP Regimen in the Treatment of Advanced Non-small Cell Lung Cancer and Its Influence on the Levels of Serum NSE, CEA and Cyfra21-1[J]. Lin Chuang Wu Zhen Wu Zhi, 2022, 35(10): 26-32.
[12] Engstrom LD, Aranda R, Lee M, et al. Glesatinib exhibits antitumor activity in lung cancer models and patients harboring MET exon 14 mutations and overeomes mutation-mediated resistance to type I MET inbibitors in nonclinical models[J]. Clin Cancer Res, 2017, 23(21): 6661-6672.
[13] Barry E, Maloney E, Henry R, et al. Abstract 1150: Targeting MET Exon 14 mutations with the selective small molecule inhibitor Savolitinib[J]. Cancer Res, 2016, 76(14 Suppl): 1150.
[14] 刘海旺, 张宏旭, 刘燃, 等. 肝细胞生长因子/酪氨酸蛋白激酶MET信号通路与乳腺癌侵袭转移的相关性分析[J]. 安徽医药, 2021, 25(3): 516-519. [Liu HW, Zhang HX, Liu R, et al. Analysis of correlation between HGF/c-met signaling pathway and invasion and metastasis of breast cancer[J]. Anhui Yi Yao, 2021, 25(3): 516-519.] Liu HW, Zhang HX, Liu R, et al. Analysis of correlation between HGF/c-met signaling pathway and invasion and metastasis of breast cancer[J]. Anhui Yi Yao, 2021, 25(3): 516-519.
[15] Jones RDO, Grondine M, Borodovsky A, et al. A pharmacokinetic-pharmacodynamic model for the MET tyrosine kinase inhibitor, savolitinib, to explore target inhibition requirements for anti-tumour activity[J]. Br J Pharmacol, 2021, 178(3): 600-613. doi: 10.1111/bph.15301
[16] Huang G, Liu X, Jiang T, et al. Luteolin overcomes acquired resistance to osimertinib in non-small cell lung cancer cells by targeting the HGF-MET-Akt pathway[J]. Am J Cancer Res, 2023, 13(9): 4145-4162.



 下载:
下载: