-
摘要:目的
探讨二甲双胍对卵巢癌细胞增殖、周期、凋亡和迁移的影响及其作用机制。
方法选取卵巢癌A2780、CAOV3、SKOV3细胞为研究对象,MTT和克隆形成实验检测二甲双胍对细胞增殖的影响,划痕实验和Transwell实验检测二甲双胍对细胞迁移能力的影响,流式细胞术检测二甲双胍对细胞周期和凋亡的影响,Western blot法检测二甲双胍对细胞AMPK磷酸化、mTOR磷酸化、CXCR4和Wnt/β-catenin蛋白表达的影响。
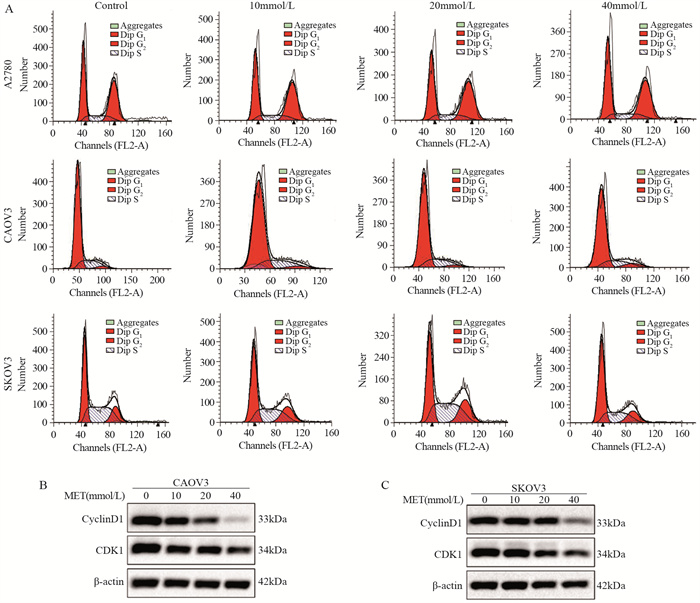
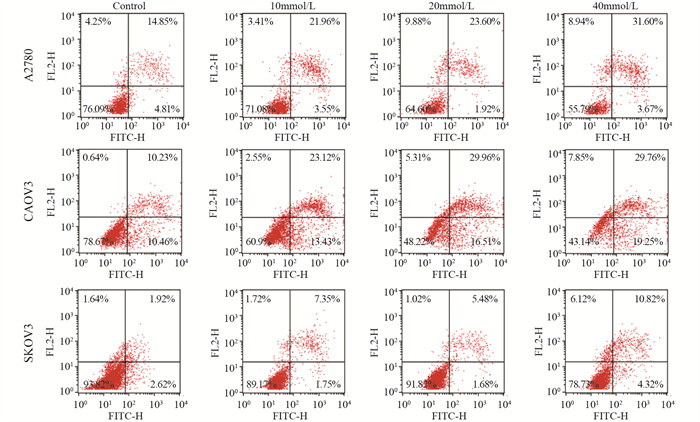
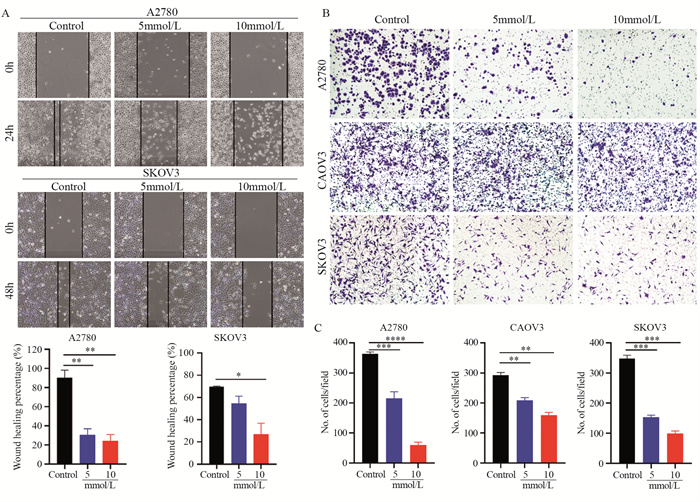
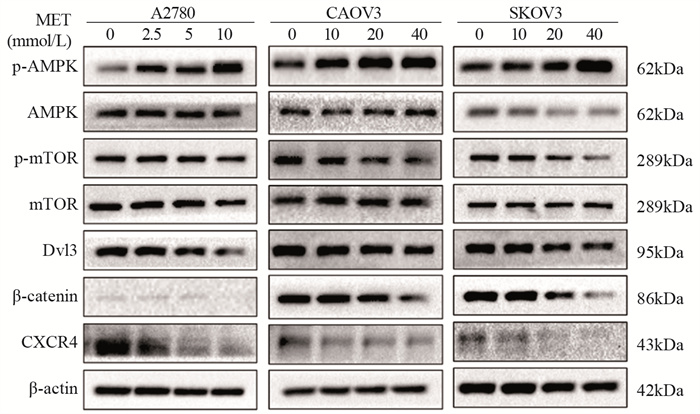
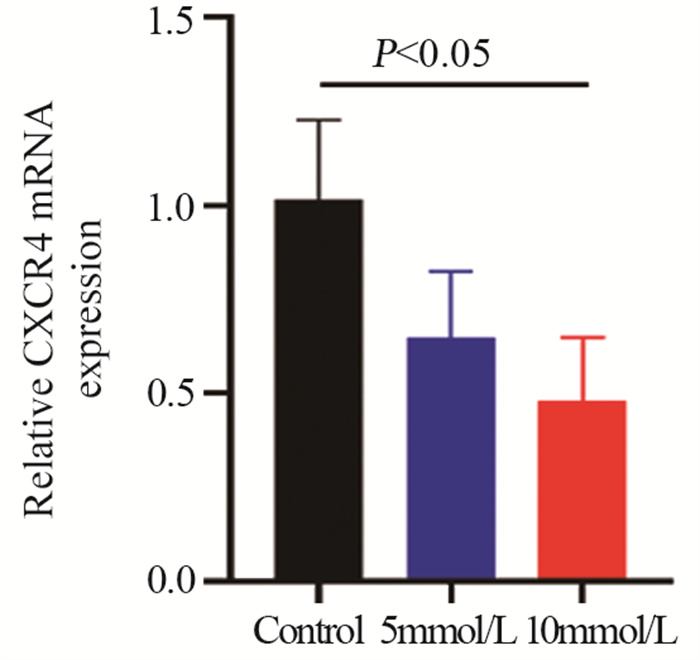
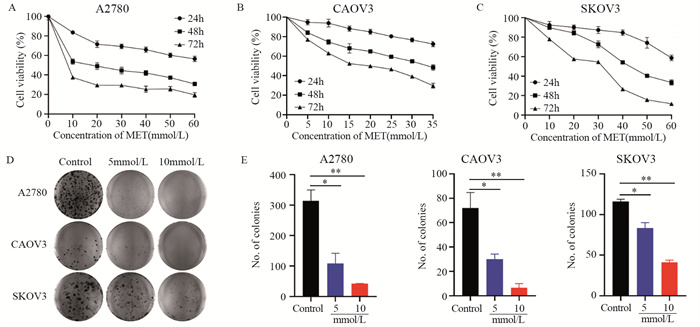
结果随着二甲双胍浓度增加和作用时间延长,卵巢癌细胞存活率明显下降。A2780、CAOV3及SKOV3细胞48 h的IC50分别为16.36、36.65和43.44 mmol/L。与对照组相比,二甲双胍处理后,细胞克隆形成能力和迁移能力被明显抑制,细胞周期阻滞于G0/G1期,细胞凋亡率增加。随着二甲双胍浓度增加,磷酸化AMPK蛋白表达逐渐增加,磷酸化mTOR、CXCR4、Dvl3、β-catenin、CyclinD1、CDK1蛋白表达逐渐降低。
结论二甲双胍对卵巢癌细胞具有抗肿瘤作用,其机制可能与激活AMPK抑制CXCR4介导的Wnt/β-catenin信号通路有关。
Abstract:ObjectiveTo explore the antitumor effects of metformin on ovarian cancer cells in vitro, particularly on tumor cell proliferation, cell cycle, apoptosis, migration, and possible mechanism.
MethodsOvarian cancer cell lines (A2780, CAOV3, and SKOV3) were treated with different concentrations of metformin. Their proliferation was explored using the MTT and clone formation assays, cell migration was examined using the scratch and Transwell assays, and cell cycle and apoptosis were examined using flow cytometry. In addition, metformin’s effects on the phosphorylation of AMPK and mTOR and the expression of CXCR4 and Wnt/β-catenin protein was measured by Western blot.
ResultsThe survival rates of ovarian cancer cells decreased significantly with increasing metformin concentration and metformin treatment time. The IC50 values of metformin at 48 h for A2780, CAOV3, and SKOV3 cells were 16.36, 36.65, and 43.44 mmol/L, respectively. Compared with the control group, the clone formation ability and cell migration ability of ovarian cancer cells were significantly inhibited by metformin treatment and cell cycle arrested at the G0/G1 phase, and the apoptosis rate increased. As metformin concentration increased, the expression of phosphorylated AMPK protein gradually increased, and the expression levels of phosphorylated mTOR, CXCR4, Dvl3, β-catenin, cyclin D1, and CDK1 decreased.
ConclusionMetformin exerts an antitumor effect on ovarian cancer cells, which is related to the activation of AMPK to inhibit CXCR4-mediated Wnt/β-catenin signaling pathway.
-
Key words:
- Metformin /
- Ovarian cancer /
- Proliferation /
- Migration /
- CXCR4
-
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:余慧:实验实施、数据分析、论文撰写和修改李臻:实验构思、方法设计、论文审阅王佳:实验指导、论文修改、基金支持
-
-
[1] Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023[J]. CA Cancer J Clin, 2023, 73(1): 17-48. doi: 10.3322/caac.21763
[2] Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger[J]. Ann Oncol, 2019, 30(5): 672-705. doi: 10.1093/annonc/mdz062
[3] Buczyńska A, Sidorkiewicz I, Krętowski AJ, et al. Metformin Intervention-A Panacea for Cancer Treatment?[J]. Cancers (Basel), 2022, 14(5): 1336. doi: 10.3390/cancers14051336
[4] Kim YS, Choi EA, Lee JW, et al. Metformin use reduced the overall risk of cancer in diabetic patients: A study based on the Korean NHIS-HEALS cohort[J]. Nutr Metab Cardiovasc Dis, 2020, 30(10): 1714-1722. doi: 10.1016/j.numecd.2020.05.010
[5] Hua Y, Zheng Y, Yao Y, et al. Metformin and cancer hallmarks: shedding new lights on therapeutic repurposing[J]. J Transl Med, 2023, 21(1): 403. doi: 10.1186/s12967-023-04263-8
[6] Wen KC, Sung PL, Wu A, et al. Neoadjuvant metformin added to conventional chemotherapy synergizes anti-proliferative effects in ovarian cancer[J]. J Ovarian Res, 2020, 13(1): 95. doi: 10.1186/s13048-020-00703-x
[7] Lu MZ, Li DY, Wang XF. Effect of metformin use on the risk and prognosis of ovarian cancer: an updated systematic review and meta-analysis[J]. Panminerva Med, 2023, 65(3): 351-361.
[8] Li C, Liu VW, Chiu PM, et al. Reduced expression of AMPK-beta1 during tumor progression enhances the oncogenic capacity of advanced ovarian cancer[J]. Mol Cancer, 2014, 13: 49.
[9] Wang J, Cai J, Han F, et al. Silencing of CXCR4 blocks progression of ovarian cancer and depresses canonical Wnt signaling pathway[J]. Int J Gynecol Cancer, 2011, 21(6): 981-987. doi: 10.1097/IGC.0b013e31821d2543
[10] Nurzhan S, Bekezhankyzy Z, Ding H, et al. The Effect of Different Glucose Concentrations on the Antiproliferative Activity of Metformin in MCF-7 Breast Cancer Cells[J]. Pharmaceutics, 2023, 15(9): 2186. doi: 10.3390/pharmaceutics15092186
[11] Papachristodoulou A, Heidegger I, Virk RK, et al. Metformin Overcomes the Consequences of NKX3.1 Loss to Suppress Prostate Cancer Progression[J]. Eur Urol, 2023. S0302-2838(23)03106-6. Online ahead of print.
[12] Zhang J, Kuang L, Li Y, et al. Metformin Regulates TET2 Expression to Inhibit Endometrial Carcinoma Proliferation: A New Mechanism[J]. Front Oncol, 2022, 12: 856707. doi: 10.3389/fonc.2022.856707
[13] Chen YH, Yang SF, Yang CK, et al. Metformin induces apoptosis and inhibits migration by activating the AMPK/p53 axis and suppressing PI3K/AKT signaling in human cervical cancer cells[J]. Mol Med Rep, 2021, 23(1): 88.
[14] Urpilainen E, Puistola U, Boussios S, et al. Metformin and ovarian cancer: the evidence[J]. Ann Transl Med, 2020, 8(24): 1711. doi: 10.21037/atm-20-1060
[15] Steinberg GR, Carling D. AMP-activated protein kinase: the current landscape for drug development[J]. Nat Rev Drug Discov, 2019, 18(7): 527-551. doi: 10.1038/s41573-019-0019-2
[16] Gwak H, Kim Y, An H, et al. Metformin induces degradation of cyclin D1 via AMPK/GSK3beta axis in ovarian cancer[J]. Mol Carcinog, 2017, 56(2): 349-358. doi: 10.1002/mc.22498
[17] Min X, Zhang T, Lin Y, et al. Metformin inhibits the growth of ovarian cancer cells by promoting the Parkin-induced p53 ubiquitination[J]. Biosci Rep, 2020. BSR20200679. Online ahead of print. doi: 10.1042/BSR20200679
[18] Yang Y, Li J, Lei W, et al. CXCL12-CXCR4/CXCR7 Axis in Cancer: from Mechanisms to Clinical Applications[J]. Int J Biol Sci, 2023, 19(11): 3341-3359. doi: 10.7150/ijbs.82317
[19] Scotton CJ, Wilson JL, Milliken D, et al. Epithelial cancer cell migration: a role for chemokine receptors?[J]. Cancer Res, 2001, 61(13): 4961-4965.
[20] Zhang J, Huang S, Quan L, et al. Determination of Potential Therapeutic Targets and Prognostic Markers of Ovarian Cancer by Bioinformatics Analysis[J]. Biomed Res Int, 2021, 2021: 8883800.
[21] Lim H, Kim SI, Kim EN, et al. Tissue Expression and Prognostic Role of CXCL12 and CXCR4 in High-grade Serous Ovarian Carcinoma[J]. Anticancer Res, 2023, 43(7): 3331-3340. doi: 10.21873/anticanres.16509
[22] Liu CF, Liu SY, Min XY, et al. The prognostic value of CXCR4 in ovarian cancer: a meta-analysis[J]. PLoS One, 2014, 9(3): e92629. doi: 10.1371/journal.pone.0092629
[23] Huang X, Hao J, Tan YQ, et al. CXC Chemokine Signaling in Progression of Epithelial Ovarian Cancer: Theranostic Perspectives[J]. Int J Mol Sci, 2022, 23(5): 2642. doi: 10.3390/ijms23052642
[24] Zheng N, Liu W, Chen J, et al. CXCR7 is not obligatory for CXCL12-CXCR4-induced epithelial-mesenchymal transition in human ovarian cancer[J]. Mol Carcinog, 2019, 58(1): 144-155. doi: 10.1002/mc.22916
[25] Luo N, Chen DD, Liu L, et al. CXCL12 promotes human ovarian cancer cell invasion through suppressing ARHGAP10 expression[J]. Biochem Biophys Res Commun, 2019, 518(3): 416-422. doi: 10.1016/j.bbrc.2019.07.098
[26] Liu Y, Ren CC, Yang L, et al. Role of CXCL12-CXCR4 axis in ovarian cancer metastasis and CXCL12-CXCR4 blockade with AMD3100 suppresses tumor cell migration and invasion in vitro[J]. J Cell Physiol, 2019, 234(4): 3897-3909. doi: 10.1002/jcp.27163
[27] Lai X, Chen J. C-X-C motif chemokine ligand 12: a potential therapeutic target in Duchenne muscular dystrophy[J]. Bioengineered, 2021, 12(1): 5428-5439. doi: 10.1080/21655979.2021.1967029
[28] Stuermer EK, Besser M, Terberger N, et al. Side effects of frequently used oral antidiabetics on wound healing in vitro[J]. Naunyn Schmiedebergs Arch Pharmacol, 2019, 392(3): 371-380. doi: 10.1007/s00210-018-01597-9
[29] Dirat B, Ader I, Golzio M, et al. Inhibition of the GTPase Rac1 mediates the antimigratory effects of metformin in prostate cancer cells[J]. Mol Cancer Ther, 2015, 14(2): 586-596. doi: 10.1158/1535-7163.MCT-14-0102
[30] Zheng G, Shen Z, Xu A, et al. Synergistic Chemopreventive and Therapeutic Effects of Co-drug UA-Met: Implication in Tumor Metastasis[J]. J Agric Food Chem, 2017, 65(50): 10973-10983. doi: 10.1021/acs.jafc.7b04378



 下载:
下载: