-
摘要:
放射性脑损伤是鼻咽癌放疗的常见远期并发症,严重影响患者的生活质量和总生存。在调强放疗时代,越来越多经治鼻咽癌患者获得长期生存,因此放疗后的远期并发症尤其是放射性脑损伤愈发引人关注。目前关于放射性脑损伤的认识仍在不断探索和更新中。本文就鼻咽癌放射性脑损伤的研究进展进行综述。
Abstract:Radiation-induced brain injury (RBI) is a common long-term complication of radiotherapy for nasopharyngeal carcinoma (NPC) and seriously affects the quality of life and overall survival of patients. In the era of intensity-modulated radiation therapy (IMRT), the long-term complications after radiotherapy, especially RBI, are becoming increasingly concerning because a number of treated patients with NPC obtain long-term survival. At present, the understanding of RBI is still being explored, and its pathogenesis and treatment methods are continuously updated. This article reviews the research progress of RBI in patients with NPC.
-
Key words:
- Nasopharyngeal carcinoma /
- Radiotherapy /
- Radiation-induced brain injury /
- Pathogenesis /
- Treatment
-
0 引言
调强放射治疗是当前鼻咽癌放射治疗最重要的治疗手段,局部晚期鼻咽癌经放化疗综合治疗后总体5年生存率可达80%以上,10年生存率将近70%以上[1]。随着进入长期生存的患者不断增多,放疗相关的远期并发症问题也日益凸显。放射性脑损伤是放疗后的一种慢性、不可逆的神经系统损害,是鼻咽癌放疗后长期生存患者最为严重的并发症之一。目前,人们对于放射性脑损伤的认识仍不充分,研究人员在放射性脑损伤的分子病理机制及临床诊疗手段上做了大量的工作,本文将对近年来关于放射性脑损伤的研究进展进行综述。
1 放射性脑损伤的定义
放射性脑损伤(radiation-induced brain injury, RBI)最早在1930年由Fischer等提出,在文献中报道了1例45岁男性患者因太阳穴癌肿接受X射线照射出现放射性脑损伤的病理依据。根据中国专家共识,放射性脑损伤是指“电离辐射后出现的脑部损伤,可以发生在电离辐射后的任何时间,以照射结束后6~47个月最为常见。从广义上来说,放射性脑损伤是放射治疗后神经细胞和颅内血管受损后出现的一系列病理生理改变,影像学可见脑部病灶”[2]。放射性脑损伤根据出现的时间分为急性型(放疗过程中或放疗后1个月内)、早迟发反应型(放疗结束后1~6个月)和晚迟发反应型(放疗结束6个月后),可能因脑细胞为晚反应细胞,放射性脑损伤的最常见类型为晚迟发反应型,此时患者可表现头痛、认知功能障碍、癫痫等症状。Na等[3]统计鼻咽癌放疗后放射性脑损伤的4年累计发生率为1.9%~5%。麦春平等[4]在对74例鼻咽癌患者进行临床分析,结果发现放射性脑损伤发生在鼻咽癌放疗后11~56个月,平均发生时间为37.5个月。
2 放射性脑损伤的发病机制
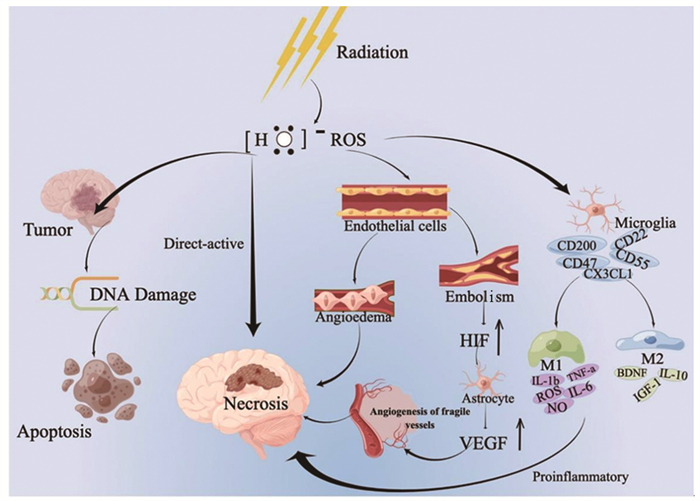
放射性脑损伤是动态演变的过程,大多数学者认为可能是以下因素共同作用的结果:放射线对脑组织的直接损伤、血管损伤和免疫炎性反应,见图 1。
2.1 放射线对脑组织的直接损伤
神经胶质细胞具有分裂增殖能力,对射线比较敏感,放射线直接作用于射野区的脑组织区域引发电离和激发,产生氧自由基,导致单链和双链DNA损伤,可导致脑白质脱髓鞘、软化和萎缩。Andrews等[5]评估大脑照射恒河猴的后期影响,结果发现白质是迟发性辐射脑损伤的好发部位,并分析谷氨酸能神经传递受损可能发生在白质中最为突出。
2.2 血管损伤
在放疗后的最初24 h内,RBI始于放射诱发的血管损伤,随后是脑实质的损伤[6]。放射线主要作用于颅内中小动脉的内皮细胞,导致脑血管的通透性增加、血管周围水肿,是急性反应期的主要发病机制。损伤的血管因内皮细胞暴露启动凝血途径形成血栓,可导致细胞缺血缺氧。近年来发现血管内皮生长因子(VEGF)和缺氧诱导因子-1α(HIF-1α)在放射性脑损伤的发病机制中越来越突出。HIF-1α是VEGF的反式激活因子,其上调可导致星形胶质细胞产生VEGF增多,诱导血管生成,然而源自这种反应生成的血管不仅通透性大而且容易被破坏,易诱发二次损伤[7]。此外,Zhou等[8]研究表明VEGF的过表达可导致血管生成素-2(Ang-2)的上调,Ang-2可能是作为VEGF和微血管损伤之间的介导细胞因子。
2.3 免疫炎性反应
辐射诱导的神经炎症是多种促炎和抗炎细胞因子的交叉网络。Andrews等[9]在2017年评估放射性脑损伤的分子效应时证明巨噬细胞/小胶质细胞介导的神经炎症可能通过增加巨噬细胞趋化因子CCL2和巨噬细胞/小胶质细胞相关CD68的基因表达而导致放射性脑损伤。在2019年Andrews等[5]通过转录组学分析再次证实巨噬细胞/小胶质细胞介导的神经炎症确实存在于放射性脑损伤中,而且在神经炎症通路分析表明MHCⅡ类肽抗原呈递和补体系统在放射性脑损伤中发挥作用。研究表明10 Gy是激活小胶质细胞的最佳辐射剂量,此时激活的小胶质细胞有两种不同类型[10-11]:经典活化型M1和旁路活化型M2。M1型小胶质细胞不仅上调吞噬细胞机制而且能够诱导NF-κB等途径释放促炎分子如IL-1b、TNF-a、IL-6、ROS以及NO[12-13]。另一方面,M2型小胶质细胞与抗炎细胞因子相关,例如IL-10、胰岛素生长因子-1(IGF-1)和脑源性神经营养因子(BDNF),这些抗炎细胞因子限制神经元损伤并促进愈合。已有相关研究表明不规则趋化因子(FKN)CX3CL1与小胶质细胞上的CX3CR1结合后,小胶质细胞向M2型极化,对放射性脑损伤进行神经保护[14]。近期研究发现脑源性微粒(BDMP)可激活小胶质细胞,然后促进炎症因子的释放,至于具体的信号转导通路仍未明确[15]。
综上所述,放射线对脑组织的直接损伤、血管损伤、免疫炎性反应是放射性脑损伤的主要发病机制。随着不断地探索以及研究设备的升级,研究进入分子水平,目前免疫炎性反应更是研究热点,这对于放射性脑损伤的治疗也提供了新的思路。
3 放射性脑损伤的相关影响因素
放射性脑损伤的相关影响因素包括除了遗传易感性、年龄、动脉硬化、化疗等,更多的影响因素归因于接受放疗的照射剂量,接受的总剂量越多、发生放射性脑损伤的概率越大,见表 1。
表 1 放射性脑损伤的影响因素Table 1 Influencing factors of radiation-induced brain injury
4 放射性脑损伤的诊断方法
放射性脑损伤的诊断方法首先是患者的主诉,特别要注意患者的认知功能是否下降。目前临床上最常用的量表有蒙特利尔认知评估(MoCA)、简易精神状态量表(MMSE)。其次最常见的是以影像学作为诊断依据,主要有CT检查、磁共振成像(MRI)检查、正电子发射体层显像术(PET-CT)。目前MRI发展迅速,除了常规MRI检查,还有弥散加权成像(DWI)、扩散张量成像(DTI)、扩散峰度成像(DKI)、灌注加权成像(PWI)、磁共振波谱(MRS)和血氧水平依赖功能磁共振成像(BOLD-fMRI)。总结相关文献可知DTI和DKI对检测早期鼻咽癌放射性脑损伤的微观结构更敏感。DWI用参数表观扩散系数(ADC)表示,当出现弥散受限时,ADC值降低,但当病灶部位有出血或胶质增生时,ADC值也会减低,难以明确是鼻咽癌放射性脑损伤还是胶质瘤复发[28]。此时PWI、MRS可补充DWI的不足,在鉴别脑胶质瘤复发或假性进展和鼻咽癌放射性脑损伤有较高的诊断价值[29-30]。BOLD-fMRI是无创性的活体脑功能成像技术,通过该技术发现的“脑功能连接改变”可能成为发现放疗后出现脑功能损害以及认知障碍的潜在生物标志物[31]。
病理活检仍是诊断放射性脑损伤的金标准,但因脑组织活检困难,因此病理活检并非是诊断放射性脑损伤的必要条件。现诸多证明液体活检可能是颅内活检的替代方法,在外周血或脑脊液中通过检测生物标志物(包括循环肿瘤细胞、循环肿瘤核酸、microRNA等)可用于区分脑胶质瘤和鼻咽癌放射性脑损伤[32-34]。
近年来也有文章报道关于使用机器学习和影像组学方法来研究放射性脑损伤,如Yang等研究基于剂量分布和CT影像学提出用于预测辐射诱导的颞叶损伤和指导个体调强放疗的剂量组学风险模型,该剂量组学风险模型在预测放射性脑损伤风险方面优于传统模型(QUANTEC模型和Wen模型)[26]。
根据专家共识和临床经验总结诊断标准如下:(1)有头颈部放疗病史;(2)有相应的临床表现;(3)影像学检查支持;(4)排除颅内供血血管闭塞、颅内恶性肿瘤、血源性脑转移和脑脓肿等感染性病变。
5 放射性脑损伤的治疗
对于小而无症状的病变,通常建议刚发现时(每6~8周)较短的间隔内进行密切的影像学随访,直到病情稳定或病灶缩小为止,可根据具体情况增加或减少随访频率[35]。对于有症状的放射性脑损伤,其经典的治疗方案为手术治疗、糖皮质激素、抗凝剂[36]。随着对放射性脑损伤发病机制的进一步了解,其治疗方法也越来越多。
5.1 清除活性氧和抗血管损伤治疗
(1)自由基清除剂如维生素E、艾地苯醌、依达拉奉,可保护细胞膜免受脂质过氧化;(2)抗凝剂如肝素,可抑制放疗后导致血管损伤后的血小板聚集,减轻血管的狭窄;(3)高压氧:通过提高氧浓度,可刺激脑损伤处的血管生成,促进修复;(4)贝伐珠单抗:唯一有Ⅰ类医学证据的药物。从Anderson癌症中心Ⅱ期研究[37]到2011年Levin等[38]进行的随机双盲安慰剂对照研究证实了贝伐珠单抗的疗效。2018年Xu等[39]随机单盲前瞻性临床研究发现治疗8周后,贝伐珠单抗组患者与激素组患者的有效率为65.5%和31.5%。贝伐珠单抗治疗标准的治疗方案为4个疗程的5~7.5 mg/kg体重,每两周给药一次,Voss等[40]研究结果证明单次给药(定义为在至少6周的间隔内单次给药贝伐珠单抗而不进行第二次给药)和Tijtgat等[41]研究结果表明低剂量方案(总400 mg静脉负荷剂量,每4周一次静脉注射100 mg)均有可能是治疗RBI有效且降低成本的替代方案;(5)阿帕替尼:有研究表明可用于治疗放射性脑损伤,但有必要开展进一步的随机对照研究[42];(6)安罗替尼:可下调星形胶质细胞的活化,改善脑缺氧,减轻脑水肿,明显减轻急性RBI的不良影响,且呈剂量依赖性,但缺乏临床验证[43]。
5.2 促神经元修复和再生治疗
(1)神经生长因子(nerve growth factor, NGF):NGF可促进受损的神经元功能修复和再生。首例经NGF成功逆转的脑损伤病例是1例55岁的中国女性鼻咽癌患者(T3N1M0,Ⅲ期,AJCC 2002)接受放化疗,3年后复查发现双侧颞叶放射性脑损伤,该患者接受了鼠神经生长因子治疗(18 µg/d,肌肉注射),连续应用2个月,3个月后复查MRI,双侧颞叶损伤灶完全恢复,神经症状完全消失,随访时间3年以上,未见肿瘤复发、新病灶出现[44]。不久后Wang等[45]开展一项前瞻性随机对照Ⅱ期临床研究,研究最终入组共28例患者(NGF组14例和糖皮质激素对照组14例),结果发现NGF组治疗有效率高于对照组(85.7%和14.2%),该研究认为NGF能够有效地逆转鼻咽癌放疗后的颞叶损伤;(2)神经节苷脂:是一类含唾液酸的酸性糖鞘脂,与神经细胞的再生和转导有着密切的关系。目前最新研究表明神经节苷脂也是小胶质细胞炎性反应的重要调节剂,可用于治疗[46]。
5.3 抗免疫炎性反应治疗
(1)最经典的传统治疗:激素。激素对放射性脑损伤的治疗多基于临床经验、回顾性分析,缺乏大型的临床随机对照研究。糖皮质激素的作用是暂时的而非治愈性,长期使用可引发一系列的不良反应,可根据个体化调整剂量;(2)参芪扶正注射液可通过调节炎症因子减轻放射性脑损伤,特别是伴有记忆能力受损治疗效果更好[47];(3)电针:在中医方面,放射线相当于“热邪”。针灸可活血化瘀、疏通经络、消肿止痛,起到改善血液循环,达到调理气机。电针具有可控性强、刺激均匀持久等特点,可起到镇痛作用[48]。相关研究表明[49]辐射对海马CA1区突触结构和突触功能相关蛋白表达均有损伤,而电针干预可明显改善辐射引起的突触结构和功能损伤。
综上所述,放射性脑损伤的治疗主要包括提高放疗的收益比、清除活性氧、抗血管损伤治疗、促神经元恢复和再生以及抗免疫炎性反应治疗。随着对免疫炎性机制的进一步探索,近年针对抗免疫炎性机制研究出很多治疗药物,现主要在动物实验阶段,有待进一步临床试验,如槲皮素包涵体复合物[50]、双去甲氧基姜黄素[51]、普瑞巴林[52]、金纳米颗粒(AuNPs)和a-硫辛酸(ALA)混合物[53]、3-甲基腺嘌呤[54]、选择性COX-2塞来昔布[55]、褪黑素(MLT)[56]、人重组内皮抑素[57]、硫酸镁[58]、重组蛋白类rt-03[59]。其中槲皮素[50]属于类黄酮化合物,具有抗氧化、抗炎、抑菌特性,可调节肠道微生物。菌群-肠-脑轴调节肠道微生物治疗RBI是一个新概念。王怀清等[59]自主研发的rt-03重组蛋白类药物,具有抗炎和抗凋亡作用,可削弱巨噬细胞分泌TNF-α,缓解小鼠急性脑损伤。
6 总结和展望
放射性脑损伤是放疗剂量累积导致的脑损伤,照射剂量每增加1 Gy,出现放射性脑损伤的概率将增加4.4%[60]。提高肿瘤照射剂量,同时保护危及器官是放疗一直努力的方向。相对于二维时代、三维时代,调强放疗的出现使放射性脑损伤的发生率减少。目前粒子束治疗(质子、重离子)是最先进的放疗治疗系统,但因设备昂贵和治疗费用高,该技术在国内未获得广泛应用。提高放疗技术、勾画靶区时危及器官的处理亟待进一步优化。对于头颈部放疗的患者,要做到早发现、早诊断、早治疗。近年来根据中国专家的共识结合患者的身体情况进行个体化处理,取得一定的疗效。随着对其发病机制的深入了解,探索出更多的治疗药物,但很多药物还处于动物实验期,应鼓励更多的临床试验。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:赖静:文章构思,文献获取及解读,论文撰写与修改林培欣:文章构思,文献获取及解读黄静:论文审校 -
表 1 放射性脑损伤的影响因素
Table 1 Influencing factors of radiation-induced brain injury

-
[1] Tian YM, Liu MZ, Zeng L, et al. Long-term outcome and pattern of failure for patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy[J]. Head Neck, 2019, 41(5): 1246-1252. doi: 10.1002/hed.25545
[2] 中国放射性脑损伤多学科协作组, 中国医师协会神经内科分会脑与脊髓损害专业委员会. 放射性脑损伤诊治中国专家共识[J]. 中华神经医学杂志, 2019, 18(6): 541-549. https://www.cnki.com.cn/Article/CJFDTOTAL-NKLL201905001.htm Chinese Radiation Brain Injury Multidisciplinary Collaboration Group, Brain and Spinal Cord Injury Professional Committee, Neurology Branch, Chinese Medical Doctor Association. Diagnoses and treatments of radiation-induced brain injury: an expert consensus[J]. Zhonghua Shen Jing Yi Xue Za Zhi, 2019, 18(6): 541-549. https://www.cnki.com.cn/Article/CJFDTOTAL-NKLL201905001.htm
[3] Na A, Haghigi N, Drummond KJ. Cerebral radiation necrosis[J]. Asia Pac J Clin Oncol, 2014, 10(1): 11-21. doi: 10.1111/ajco.12124
[4] 麦春平, 魏展福, 黄金杜. 鼻咽癌调强放疗后放射性脑病的临床特征及危险因素分析[J]. 医学食疗与健康, 2021, 19(23): 51, 78. Mai CP, Wei ZF, Huang JD. Clinical features and risk factors of radiation encephalopathy after intensity-modulated radiotherapy for nasopharyngeal carcinoma[J]. Yi Xue Shi Liao Yu Jian Kang, 2021, 19(23): 51, 78.
[5] Andrews RN, Dugan GO, Peiffer AM, et al. White Matter is the Predilection Site of Late-Delayed Radiation-Induced Brain Injury in Non-Human Primates[J]. Radiat Res, 2019, 191(3): 217-231. doi: 10.1667/RR15263.1
[6] Abdulla S, Saada J, Johnson G, et al. Tumour progression or pseudoprogression? A review of post-treatment radiological appearances of glioblastoma[J]. Clin Radiol, 2015, 70(11): 1299-1312. doi: 10.1016/j.crad.2015.06.096
[7] Nordal RA, Nagy A, Pintilie M, et al. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor[J]. Clin Cancer Res, 2004, 10(10): 3342-3353. doi: 10.1158/1078-0432.CCR-03-0426
[8] Zhou DX, Huang XR, Xie Y, et al. Astrocytes-derived VEGF exacerbates the microvascular damage of late delayed RBI[J]. Neuroscience, 2019, 408: 14-21. doi: 10.1016/j.neuroscience.2019.03.039
[9] Andrews RN, Metheny-Barlow LJ, Peiffer AM, et al. Cerebrovascular Remodeling and Neuroinflammation is a Late Effect of Radiation-Induced Brain Injury in Non-Human Primates[J]. Radiat Res, 2017, 187(5): 599-611. doi: 10.1667/RR14616.1
[10] Dong XR, Luo M, Huang GD, et al. Relationship between irradiation-induced neuro-inflammatory environments and impaired cognitive function in the developing brain of mice[J]. Int J Radiat Biol, 2015, 91(3): 224-239. doi: 10.3109/09553002.2014.988895
[11] Xue J, Dong JH, Huang GD, et al. NF-κB signaling modulates radiation-induced microglial activation[J]. Oncol Rep, 2014, 31(6): 2555-2560. doi: 10.3892/or.2014.3144
[12] Wang HX, Liu C, Han M, et al. TRAM1 Promotes Microglia M1 Polarization[J]. J Mol Neurosci, 2016, 58(2): 287-296. doi: 10.1007/s12031-015-0678-3
[13] 黄蓉蓉, 丁桂荣. 小胶质细胞在放射性脑损伤中的作用及其机制研究进展[J]. 国际放射医学核医学杂志, 2021, 45(2): 124-131. Huang RR, Ding GR. The role and mechanism of microglia in radiation-induced brain injury[J]. Guo Ji Fang She Yi Xue He Yi Xue Za Zhi, 2021, 45(2): 124-131.
[14] Wu J, Ding DH, Li QQ, et al. Lipoxin A4 Regulates Lipopolysaccharide-Induced BV2 Microglial Activation and Differentiation via the Notch Signaling Pathway[J]. Front Cell Neurosci, 2019, 13: 19.
[15] 杜家虞, 唐洁, 张倩, 等. 脑源性微粒在头颈部肿瘤放射性脑损伤中对小胶质细胞极化作用的研究进展[J]. 临床耳鼻咽喉头颈外科杂志, 2021, 35(1): 88-91. https://www.cnki.com.cn/Article/CJFDTOTAL-LCEH202101025.htm Du JY, Tang J, Zhang Q, et al. Research progress of brain-derived microparticles on microglia polarization in radiation-induced brain injury of head and neck tumors[J]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi, 2021, 35(1): 88-91. https://www.cnki.com.cn/Article/CJFDTOTAL-LCEH202101025.htm
[16] Wang TM, Shen GP, Chen MY, et al. Genome-Wide Association Study of Susceptibility Loci for Radiation-Induced Brain Injury[J]. J Natl Cancer Inst, 2019, 111(6): 620-628. doi: 10.1093/jnci/djy150
[17] 李川. 鼻咽癌放疗后放射性脑病的影响因素回归分析[J]. 实用癌症杂志, 2018, 33(3): 392-394. https://www.cnki.com.cn/Article/CJFDTOTAL-SYAZ201803014.htm Li C. Regression Analysis of Influencing Factors of Radiation Encephalopathy of Nasopharyngeal Carcinoma after Radiotherapy[J]. Shi Yong Ai Zheng Za Zhi, 2018, 33(3): 392-394. https://www.cnki.com.cn/Article/CJFDTOTAL-SYAZ201803014.htm
[18] 罗琼. 鼻咽癌放疗后致放射性脑病的影响因素分析[J]. 实用癌症杂志, 2017, 32(5): 859-861. https://www.cnki.com.cn/Article/CJFDTOTAL-SYAZ201705052.htm Luo Q. Analysis of the Influencing Factors of Radiation Encephalopathy in Nasopharyngeal Carcinoma after Radiotherapy[J]. Shi Yong Ai Zheng Za Zhi, 2017, 32(5): 859-861. https://www.cnki.com.cn/Article/CJFDTOTAL-SYAZ201705052.htm
[19] Zhang YM, Yi XP, Gao GY, et al. Chemotherapy Potentially Facilitates the Occurrence of Radiation Encephalopathy in Patients With Nasopharyngeal Carcinoma Following Radiotherapy: A Multiparametric Magnetic Resonance Imaging Study[J]. Front Oncol, 2019, 9: 567. doi: 10.3389/fonc.2019.00567
[20] Ng WT, Ngan RKC, Kwong DLW, et al. Prospective, Multicenter, Phase 2 Trial of Induction Chemotherapy Followed by Bio-Chemoradiotherapy for Locally Advanced Recurrent Nasopharyngeal Carcinoma[J]. Int J Radiat Oncol Biol Phys, 2018, 100(3): 630-638. doi: 10.1016/j.ijrobp.2017.11.038
[21] Feng M, Huang YC, Fan XG, et al. Prognostic variables for temporal lobe injury after intensity modulated-radiotherapy of nasopharyngeal carcinoma[J]. Cancer Med, 2018, 7(3): 557-564. doi: 10.1002/cam4.1291
[22] 孙丕云. 不同放射治疗方式治疗局部晚期鼻咽癌的临床观察及预后分析[D]. 南宁: 广西医科大学, 2019. Sun PY. Clinical observation and prognosis analysis of different radiotherapy for locally advanced nasopharyngeal carcinoma[D]. Nanning: Guangxi Medical University, 2019.
[23] 章国芬, 贺蓓娃. 鼻咽癌后程加速超分割致放射性脑病4例报道[J]. 镇江: 江苏大学学报(医学版), 2002, 12(3): 290-291. doi: 10.3969/j.issn.1671-7783.2002.03.059 Zhang GF, He BW. Radiation encephalopathy due to late course accelerated hyperfractionation of nasopharyngeal carcinoma: a report of 4 cases[J]. Zhenjiang: Jiangsu Da Xue Xue Bao (Yi Xue Ban), 2002, 12(3): 290-291. doi: 10.3969/j.issn.1671-7783.2002.03.059
[24] Huang XD, Li YC, Chen FP, et al. Evolution and Dosimetric Analysis of Magnetic Resonance Imaging-Detected Brain Stem Injury After Intensity Modulated Radiation Therapy in Nasopharyngeal Carcinoma[J]. Int J Radiat Oncol Biol Phys, 2019, 105(1): 124-131. doi: 10.1016/j.ijrobp.2019.04.032
[25] Fan X, Huang Y, Xu P, et al. Dosimetric analysis of radiation-induced brainstem necrosis for nasopharyngeal carcinoma treated with IMRT[J]. BMC Cancer, 2022, 22(1): 178. doi: 10.1186/s12885-022-09213-z
[26] Yang SS, OuYang PY, Guo JG, et al. Dosiomics risk model for predicting radiation induced temporal lobe injury and guiding individual intensity-modulated radiation therapy[J]. Int J Radiat Oncol Biol Phys, 2023, 115(5): 1291-1300. doi: 10.1016/j.ijrobp.2022.11.036
[27] 李坊铭, 陈国健, 林剑毅, 等. 鼻咽癌放疗后放射性脑病影响因素分析[J]. 中国肿瘤外科杂志, 2016, 8(6): 402-404. doi: 10.3969/j.issn.1674-4136.2016.06.016 Li FM, Chen GJ, Lin JY, et al. Radiation encephalopathy after radiotherapy influencing factors[J]. Zhongguo Zhong Liu Wai Ke Za Zhi, 2016, 8(6): 402-404. doi: 10.3969/j.issn.1674-4136.2016.06.016
[28] 黎成, 卜超, 黄穗乔. 放射性脑病的影像学诊断进展[J]. 岭南现代临床外科, 2019, 19(1): 11-18. https://www.cnki.com.cn/Article/CJFDTOTAL-LNWK201901004.htm Li C, Bu C, Huang HQ. Advance of the imaging diagnosis of radiation encephalopathy[J]. Lingnan Xian Dai Lin Chuang Wai Ke, 2019, 19(1): 11-18. https://www.cnki.com.cn/Article/CJFDTOTAL-LNWK201901004.htm
[29] Zhang J, Wu Y, Wang YL, et al. Diffusion-weighted imaging and arterial spin labeling radiomics features may improve differentiation between radiation-induced brain injury and glioma recurrence[J]. Eur Radiol, 2022, 33(5): 3332-3342. doi: 10.1007/s00330-022-09365-3
[30] 孙飞月, 李云. MRI联合MRS在脑胶质瘤复发、假性进展和放射性脑损伤中鉴别诊断中的运用价值[J]. 罕少疾病杂志, 2022, 29(6): 22-25. https://www.cnki.com.cn/Article/CJFDTOTAL-HSJB202206008.htm Sun FY, Li Y. The Application Value of MRI Combined with MRS in the Differential Diagnosis of Recurrence, Pseudo-Progression and Radiation-Induced Brain Injury in Glioma[J]. Han Shao Ji Bing Za Zhi, 2022, 29(6): 22-25. https://www.cnki.com.cn/Article/CJFDTOTAL-HSJB202206008.htm
[31] 宋晓涵, 杨金荣, 王丽君. 鼻咽癌放疗后脑损伤MR功能成像研究进展[J]. 磁共振成像, 2021, 12(1): 96-99. https://www.cnki.com.cn/Article/CJFDTOTAL-CGZC202101024.htm Song XH, Yang JR, Wang LJ. Research progress of functional magnetic resonance imaging in radiation-induced brain injury after radiotherapy of nasopharyngeal carcinoma[J]. Ci Gong Zhen Cheng Xiang, 2021, 12(1): 96-99. https://www.cnki.com.cn/Article/CJFDTOTAL-CGZC202101024.htm
[32] Gatto L, Franceschi E, Nunno VD, et al. Liquid biopsy in gliobastoma management: from current research to future perspectives[J]. Oncologist, 2021, 26(10): 865-878. doi: 10.1002/onco.13858
[33] Balana C, Castañer S, Carrato C, et al. Preoperative Diagnosis and Molecular Characterization of Gliomas With Liquid Biopsy and Radiogenomics[J]. Front Neurol, 2022, 13: 865171. doi: 10.3389/fneur.2022.865171
[34] Sultana N, Sun C, Katsube T, et al. Biomarkers of Brain Damage Induced by Radiotherapy[J]. Dose Response, 2020, 18(3): 1559325820938279.
[35] Ali FS, Arevalo O, Zorofchian S, et al. Cerebral Radiation Necrosis: Incidence, Pathogenesis, Diagnostic Challenges, and Future Opportunities[J]. Curr Oncol Rep, 2019, 21(8): 66. doi: 10.1007/s11912-019-0818-y
[36] Zhou X, Liu PY, Wang XS, et al. Temporal Lobe Necrosis Following Radiotherapy in Nasopharyngeal Carcinoma: New Insight Into the Management[J]. Front Oncol, 2021, 10: 593487. doi: 10.3389/fonc.2020.593487
[37] Gonzalez J, Kumar JA, Conrad AC, et al. Effect of bevacizumab on radiation necrosis of the brain[J]. Int J Radiat Oncol Biol Phys, 2007, 67(2): 323-326. doi: 10.1016/j.ijrobp.2006.10.010
[38] Levin VA, Bidaut L, Hou P, et al. Randomized Double-Blind Placebo-Controlled Trial of Bevacizumab Therapy for Radiation Necrosis of the Central Nervous System[J]. Int J Radiat Oncol Biol Phys, 2011, 79(5): 1487-1495. doi: 10.1016/j.ijrobp.2009.12.061
[39] Xu YT, Rong XM, Hu WH, et al. Bevacizumab Monotherapy Reduces Radiation-induced Brain Necrosis in Nasopharyngeal Carcinoma Patients: A Randomized Controlled Trial[J]. Int J Radiat Oncol Biol Phys, 2018, 101(5): 1087-1095. doi: 10.1016/j.ijrobp.2018.04.068
[40] Voss M, Wenger KJ, Fokas E, et al. Single-shot bevacizumab for cerebral radiation injury[J]. BMC Neurol, 2021, 21(1): 77. doi: 10.1186/s12883-021-02103-0
[41] Tijtgat J, Schwarze JK, Awada G, et al. 371P Low-dose bevacizumab for the treatment of focal post-radiation necrosis of the brain[J]. Ann Oncol, 2021, 32(Suppl 5): S526.
[42] He L, Pi YX, Li Y, et al. Efficacy and safety of apatinib in radiation-induced brain injury among head and neck cancer: an open-label, single-arm, phase 2 study[J]. Int J Radiat Oncol Biol Phys, 2022, 113(4): 796-804. doi: 10.1016/j.ijrobp.2022.03.027
[43] Gao XH, Zheng J, Ma L, et al. Mitigation of acute radiation-induced brain injury in a mouse model using anlotinib[J]. Ann Palliat Med, 2021, 10(1): 312-322.
[44] Wang XS, Ying HM, Zhou ZR, et al. Successful treatment of radiation-induced temporal lobe necrosis with mouse nerve growth factor[J]. J Clin Oncol, 2011, 29(7): e166-e168.
[45] Wang XS, Ying HM, He XY, et al. Treatment of cerebral radiation necrosis with nerve growth factor: A prospective, randomized, controlled phase Ⅱ study[J]. Radiother Oncol, 2016, 120(1): 69-75.
[46] Galleguillos D, Wang Q, Steinberg N, et al. Anti-inflammatory role of GM1 and other gangliosides on microglia[J]. J Neuroinflammation, 2022, 19(1): 9.
[47] Chen LJ, Zhang RG, Yu DD, et al. Shenqi Fuzheng Injection Ameliorates Radiation-induced Brain Injury[J]. Curr Med Sci, 2019, 39(6): 965-971.
[48] 武鑫, 李炎辉, 高剑峰. 电针对放射性脑损伤作用及机制研究进展[J]. 生理学报, 2023, 75(1): 108-114. https://www.cnki.com.cn/Article/CJFDTOTAL-SLXU202301012.htm Wu X, Li YH, Gao JF. Research progress on the effect and mechanism of electroacupuncture on radiation-induced brain injury[J]. Sheng Li Xue Bao, 2023, 75(1): 108-114. https://www.cnki.com.cn/Article/CJFDTOTAL-SLXU202301012.htm
[49] 武鑫, 李炎辉, 张文靖, 等. 不同疗程电针对放射性脑损伤小鼠突触超微结构及突触功能相关蛋白的影响[J]. 生理学报, 2021, 73(6): 909-916. https://www.cnki.com.cn/Article/CJFDTOTAL-SLXU202106006.htm Wu X, Li YH, Zhang WJ, et al. Effects of electroacupuncture with different courses on the synaptic structure and synaptic function-related proteins in mice with radiation-induced brain injury[J]. Sheng Li Xue Bao, 2021, 73(6): 909-916. https://www.cnki.com.cn/Article/CJFDTOTAL-SLXU202106006.htm
[50] Hu JL, Jiao WC, Tang ZY, et al. Quercetin inclusion complex gels ameliorate radiation-induced brain injury by regulating gut microbiota[J]. Biomed Pharmacother, 2023, 158: 114142.
[51] Chang YQ, Zhou GJ, Wen HM, et al. Treatment of radiation-induced brain injury with bisdemethoxycurcumin[J]. Neural Regen Res, 2023, 18(2): 416-421.
[52] Zhang Z, Jiang JR, He Y, et al. Pregabalin mitigates microglial activation and neuronal injury by inhibiting HMGB1 signaling pathway in radiation-induced brain injury[J]. J Neuroinflammation, 2022, 19(1): 231.
[53] Abdelkader NF, EI-Batal AI, Amin YM, et al. Neuroprotective Effect of Gold Nanoparticles and Alpha-Lipoic Acid Mixture against Radiation-Induced Brain Damage in Rats[J]. Int J Mol Sci, 2022, 23(17): 9640.
[54] Feng HC, Cui YH, Liu J, et al. Effects of 3-Methyladenine on Microglia Autophagy and Neuronal Apoptosis After Radiation-Induced Brain Injury[J]. Dose Response, 2022, 20(2): 15593258221100593.
[55] Xu XT, Huang H, Tu Y, et al. Celecoxib Alleviates Radiation-Induced Brain Injury in Rats by Maintaining the Integrity of Blood-Brain Barrier[J]. Dose Response, 2021, 19(2): 15593258211024393.
[56] Elmissiry MA, Shabana S, Ghazala SJ, et al. Melatonin exerts a neuroprotective effect against γ-radiation-induced brain injury in the rat through the modulation of neurotransmitters, inflammatory cytokines, oxidative stress, and apoptosis[J]. Environ Sci Pollut Res Int, 2021, 28(24): 31108-31121.
[57] Huang XR, Li MP, Zhou DX, et al. Endothelial progenitor cell transplantation restores vascular injury in mice after whole-brain irradiation[J]. Brain Res, 2020, 1746: 147005.
[58] Chen N, Xu RJ, Wang LL, et al. Protective effects of magnesium sulfate on radiation induced brain injury in rats[J]. Curr Drug Deliv, 2018, 15(8): 1159-1166.
[59] 王怀清, 李杨, 王加宇, 等. 重组蛋白类药物RT-03对小鼠急性放射性脑损伤的治疗作用[J]. 国际药学研究杂志, 2019, 46(1): 65-70. https://www.cnki.com.cn/Article/CJFDTOTAL-GWYZ201901019.htm Wang HQ, Li Y, Wang JY, et al. Therapeutic effect of a recombinant protein drug RT-03 on acute radiation-induced brain injury in mice[J]. Guo Ji Yao Xue Yan Jiu Za Zhi, 2019, 46(1): 65-70. https://www.cnki.com.cn/Article/CJFDTOTAL-GWYZ201901019.htm
[60] Lee AW, Ng WT, Hung WM, et al. Major Late Toxicities After Conformal Radiotherapy for Nasopharyngeal Carcinoma-Patient-and Treatment-Related Risk Factors[J]. Int J Radiat Oncol Biol Phys, 2009, 73(4): 1121-1128.



 下载:
下载:

