Effect of LncRNA GHET1 on Proliferation, Apoptosis, and Metastasis of Gallbladder Cancer Cells by Targeting miR-27b
-
摘要:目的
探讨GHET1对胆囊癌细胞生物学行为的影响及其对miR-27b的调控机制。
方法采用实时荧光定量PCR检测50例胆囊癌样本中GHET1与miR-27b的表达情况。利用si-NC载体、si-GHET1载体、miR-27b抑制物以及si-GHET1载体+miR-27b抑制物转染至SGC-996细胞,并分别作为对照组、GHET1干扰组、miR-27b干扰组以及GHET1+miR-27b干扰组。采用MTT法、流式细胞术以及Transwell实验检测各组细胞增殖、凋亡以及转移情况,以荧光素酶报告基因实验验证GHET1对miR-27b的调控作用。
结果GHET1在癌组织中的表达较癌旁组织升高,而miR-27b在癌组织中的表达较癌旁组织降低,GHET1与miR-27b表达呈负相关(P<0.05)。GHET1表达与TNM分期和淋巴结转移相关(P<0.05)。高GHET1表达与胆囊癌患者预后较差相关(P<0.05)。与对照组比较,GHET1干扰组细胞增殖能力降低、凋亡率升高且细胞转移数量减少,而miR-27b干扰组细胞增殖能力升高、凋亡率降低且细胞转移数量增加(P<0.05)。与GHET1干扰组比较,GHET1+miR-27b干扰组细胞增殖能力升高、凋亡率降低且细胞转移数量增加(P<0.05)。GHET1可以海绵吸附miR-27b并抑制其表达。
结论GHET1靶向miR-27b促进胆囊癌细胞增殖和转移并抑制细胞凋亡,提示GHET1/miR-27b轴在胆囊癌进展中具有一定的作用。
-
关键词:
- 胃癌增殖增强转录物1 /
- miR-27b /
- 胆囊癌 /
- 增殖 /
- 转移
Abstract:ObjectiveTo investigate the effect of GHET1 on the biological behavior of gallbladder cancer cells and the regulatory mechanism of GHET1 on miR-27b.
MethodsThe expression of GHET1 and miR-27b in 50 samples of gallbladder cancer was detected by real-time quantitative PCR. The si-NC vector, si-GHET1 vector, miR-27b inhibitor, and si-GHET1 vector+miR-27b inhibitor were transfected into SGC-996 cells and set as the control group, GHET1 interference group, miR-27b interference group, and GHET1+miR-27b interference group. Cell proliferation, apoptosis, and metastasis in each group were detected by MTT, flow cytometry, and Transwell assays. The regulatory effect of GHET1 on miR-27b was validated by luciferase reporter gene assay.
ResultsGHET1 expression was higher in cancer tissues than that in paracancerous ones. miR-27b expression was lower in cancer tissues than that in paracancerous tissues. GHET1 was negatively correlated with miR-27b expression (P<0.05), and GHET1 expression was associated with TNM staging and lymph node metastasis (P<0.05). High GHET1 expression was associated with poor prognosis of patients with gallbladder cancer (P<0.05). Compared with the control group, the GHET1 interference group showed decreased cell-proliferation ability, increased apoptosis rate, and reduced number of cell metastasis. The miR-27b interference group showed increased cell-proliferation ability, decreased apoptosis rate, and increased number of cell metastasis (P<0.05). Compared with the GHET1 interference group, the GHET1+miR-27b interference group showed increased cell-proliferation ability, decreased apoptosis rate, and increased number of cell metastasis (P<0.05). GHET1 inhibited miR-27b expression by acting as a sponge of miR-27b.
ConclusionGHET1 promotes the proliferation and metastasis and inhibits the apoptosis of gallbladder cancer cells by targeting miR-27b, suggesting that GHET1/miR-27b axis plays a role in gallbladder cancer progression.
-
Key words:
- GHET1 /
- miR-27b /
- Gallbladder cancer /
- Proliferation /
- Metastasis
-
0 引言
胆囊癌(gallbladder cancer, GBC)是一种极具转移性的胆道恶性肿瘤,也是全球第五常见的消化道肿瘤[1-2]。近年来,尽管高通量芯片发现了上千种lncRNAs在胆囊癌样本中呈差异表达[3],但仅有少数lncRNAs的功能得到了比较详细的阐明。胃癌增殖增强转录物1(gastric carcinoma proliferation enhancing transcript 1, GHET1)于2014年首次在胃癌研究中被发现具有促进细胞增殖的作用,随后证实其在膀胱癌、乳腺癌以及宫颈癌中也发挥促癌作用[4]。miR-27b在多种肿瘤中低表达并发挥抑癌因子功能[5],经相关公共数据库与生物信息学分析,发现miR-27b可能受到GHET1的调控。有关GHET1与miR-27b在胆囊癌中的作用报道较少。鉴于此,本研究将明确GHET1对胆囊癌细胞生物学行为的影响及其对miR-27b的调控机制,以期为胆囊癌的诊疗提供一定的实验基础。
1 材料与方法
1.1 主要试剂
RNA提取试剂(Tiangen,北京),miRNA反转录试剂盒(Clontech,美国),普通反转录与实时荧光定量PCR试剂盒(Takara,日本),Dulbecco改良Eagle培养基(DMEM)(Thermo Fisher Scientific,美国),胎牛血清(FBS)(Cell-Box,香港),MTT(Beyotime Biotechnology,上海),Transwell小室和基质胶(Corning,美国),荧光素酶检测试剂盒(GeneCopoeia,美国),lipofectamine 2000转染试剂(Invitrogen,美国),si-NC载体、si-GHET1载体以及miR-27b抑制物(Bersinbio,广州),GHET1-wt和GHET1-mut报告载体(Ribobio,广州)。
1.2 临床标本
收集2016年1月—2019年12月在湖南省人民医院肝胆外科行手术治疗的50例胆囊癌患者的癌组织与癌旁组织。其中男21例,女29例,年龄39~77岁(55.18±11.69岁)。所有患者术前均未接受放疗、化疗或其他抗癌治疗。纳入标准:原发性胆囊癌患者,经影像学检查与病理检查确诊,并具有手术治疗指征。排除标准:其他类型胆道肿瘤患者,严重心、肝、肾功能衰竭患者,出血性疾病或凝血功能严重异常患者,精神障碍疾病患者,伴有其他手术禁忌证患者。收集的组织存入液氮中备用。所有患者均签署知情同意书。本研究通过湖南省人民医院医学研究伦理委员会审批(伦理号2019009)。
1.3 细胞培养
SGC-996细胞来自中国科学院上海研究所细胞培养库。采用DMEM培养基和10%FBS培养细胞,并加入100 U/ml青霉素和100 μg/ml链霉素进行抑菌处理。细胞培养箱温度设定为37℃,含5%CO2和95%空气。每隔两天更换一次培养基,并进行常规传代。待细胞状态到达对数生长期时,进行体外细胞实验。
1.4 实验分组和细胞转染
体外细胞实验分成对照组、GHET1干扰组、miR-27b干扰组以及GHET1+miR-27b干扰组,分别向SGC-996细胞中转染si-NC载体、si-GHET1载体、miR-27b抑制物以及si-GHET1载体+miR-27b抑制物。将SGC-996细胞均匀接种至6孔板,使每孔细胞数量约为5×106个。上述各组细胞培养24 h后,采用lipofectamine 2000转染试剂分别瞬时转染2.5 μg si-NC载体、2.5 μg si-GHET1载体、200 nmol miR-27b抑制物、2.5 μg si-GHET1载体+200 nmol miR-27b抑制物。24 h后1 200 r/min离心5 min,收集各组细胞,检测转染效率。
1.5 实时荧光定量PCR
采用RNA提取试剂提取组织与细胞中的总RNA。分别采用miRNA反转录与普通反转录试剂盒合成针对miR-27b和GHET1的cDNA。随后以各自cDNA作为模版进行实时荧光定量PCR。扩增条件设为:95℃ 10 min,之后95℃ 10 s、60℃ 30 s、72℃ 30 s、共40个循环,随后72℃ 5 min,最后4℃ 20 min。miR-27b引物:5’-TTCACAGTGGCTAAGTTCTGC-3’(正向),5’-CGCAGGGTCCGAGGTATTC-3’(反向)。GHET1引物:5’-CTTGAGCCTCAGTTTCTCCATC-3’(正向),5’-CTTGCTAATTTGAGTTCCTCGT-3’(反向)。采用2-ΔΔCt法,分别以U6和GAPDH为内参基因计算miR-27b和GHET1相对表达水平。
1.6 MTT法检测细胞增殖
将1.4方法中的细胞均匀接种至96孔板,使每孔细胞数量约为3×103个。在0、12、24和48 h时间点向对应孔中加入13 μl MTT(浓度:5 mg/ml)。培养4 h后,再向对应孔中加入150 μl二甲基亚砜。室温低速摇晃15 min致结晶完全溶解。采用酶标仪测量各孔在450 nm处的吸光度(OD)值,比较各组细胞增殖情况。
1.7 Transwell试验检测细胞转移
利用基质胶均匀覆盖Transwell上室,并将1.4方法中的细胞均匀接种至上室,细胞数量约为5×105个。下室中添加500 μl DMEM培养基+10%FBS充当引诱剂。48 h后使用无菌棉条轻轻擦掉上室中的细胞,将转移至下室中细胞用4%多聚甲醛室温固定15 min。随后采用0.1%结晶紫溶液室温染色10 min,并采用IX71倒置显微镜观察拍照,随机选择6处视野计算每个视野下转移细胞数。
1.8 流式细胞术检测细胞凋亡
将1.4方法中的细胞均匀接种至12孔板,使每孔细胞数量约为2.5×106个。48 h后1 200 r/min离心5 min收集细胞,以冷PBS洗涤三次。采用适量结合液将细胞重新悬浮,使其密度为1×106/ml。取1 ml细胞,加入5 μl Annexin Ⅴ-FITC室温避光染色30 min。再加入2 μl碘化丙啶(PI)室温避光染色20 min,采用流式细胞仪比较各组细胞凋亡率。
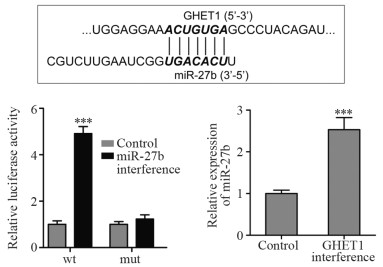
1.9 荧光素酶报告基因实验检测GHET1与miR-27b的结合位点情况
GHET1-wt报告载体(ACUGUGA)中包含miR-27b预测结合位点,而GHET1-mut报告载体中(将ACUGUGA突变成UGACACU)不含miR-27b预测结合位点。将SGC-996细胞均匀接种至24孔板,使每孔细胞数量约为4×105个。培养24 h后,采用Lipofectamine 2000转染试剂瞬时共转染50 nmol miR-27b抑制物和0.8 μg GHET1-wt报告载体,0 nmol miR-27b抑制物和0.8 μg GHET1-mut报告载体。48 h后1 200 r/min离心5 min收集细胞,采用荧光素酶检测试剂盒检测各组海肾和萤火虫的荧光值。以萤火虫的荧光值为参照,比较各组相对荧光素酶活性。
1.10 统计学方法
采用SPSS27.0与GraphPad Prism 5.0软件进行统计学分析,每种试验独立重复3次。计数资料结果用例(%)表示,以卡方检验进行统计比较。计量资料结果采用(x±s)表示,癌与癌旁组织中GHET1与miR-27b的表达比较采用配对t检验,其他两组之间的统计比较采用独立样本t检验。三组或三组以上之间的统计比较采用单因素方差分析,两两比较采用Bonferroni法。采用Spearman相关系数检验评估GHET1与miR-27b表达之间的相关性。采用Kaplan-Meier曲线评估GHET1表达与胆囊癌患者预后的关系。P<0.05为差异有统计学意义。
2 结果
2.1 GHET1与miR-27b在胆囊癌中的表达及其相关性
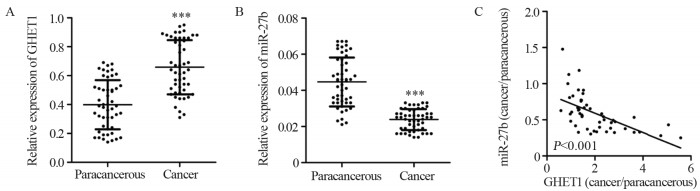
实时荧光定量PCR结果显示,癌组织与癌旁组织中GHET1的相对表达水平分别为0.66±0.19和0.40±0.17,癌组织中GHET1表达较癌旁组织高(P<0.001),见图 1A。癌组织与癌旁组织中miR-27b的相对表达水平分别为0.023±0.006和0.045±0.013,癌组织中miR-27b表达较癌旁组织低(P<0.001),见图 1B。GHET1与miR-27b表达呈负相关,相关系数为-0.59(P<0.001),见图 1C。
2.2 GHET1表达与胆囊癌患者临床病理特征以及预后的关系
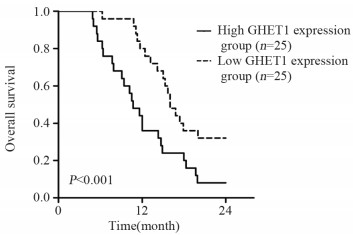
以癌组织中GHET1表达的中位数值(0.64)为临界点,将胆囊癌患者分为GHET1低表达组(n=25)与GHET1高表达组(n=25)。卡方检验结果显示,GHET1表达与淋巴结转移和TNM分期相关(P<0.05),但与年龄、性别、肿瘤大小、病理类型以及组织学等级不相关(P>0.05),见表 1。与低GHET1表达胆囊癌患者比较,高GHET1表达胆囊癌患者两年总体生存率较差(P<0.001),见图 2。
表 1 GHET1表达与胆囊癌患者临床病理特征的关系Table 1 Relationship between GHET1 expression and clinicopathological characteristics of GBC patients
2.3 细胞转染效率检测
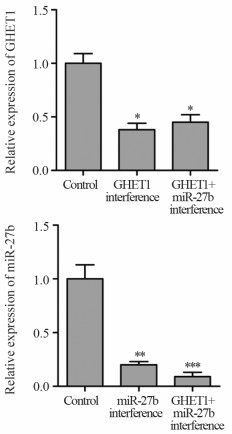
实时荧光定量PCR结果显示,与对照组比较,GHET1干扰组与GHET1+miR-27b干扰组中GHET1表达下降(P=0.012、0.015),表明si-GHET1载体对GHET1表达具有良好的抑制效果。与对照组比较,miR-27b干扰组与GHET1+miR-27b干扰组中miR-27b表达下降(P=0.007、<0.001),表明miR-27b抑制物对miR-27b表达具有良好的抑制效果,见图 3。
2.4 抑制GHET1和miR-27b对SGC-996细胞增殖的影响
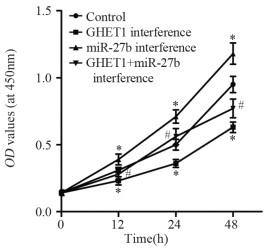
结果显示,与对照组比较,GHET1干扰组12、24以及48 h细胞OD值下降(P=0.034、0.018、0.012),miR-27b干扰组12、24以及48 h细胞OD值升高(P=0.029、0.011、0.025)。与GHET1干扰组比较,GHET1+miR-27b干扰组12、24以及48 h细胞OD值升高(P=0.042、0.015、0.037),见图 4。该结果表明,抑制GHET1降低SGC-996细胞增殖,而抑制miR-27b促进SGC-996细胞增殖,并且抑制miR-27b可以逆转抑制GHET1对SGC-996细胞增殖的影响。
2.5 抑制GHET1和miR-27b对SGC-996细胞凋亡的影响
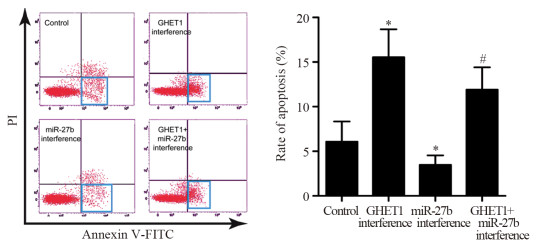
流式细胞术结果显示,与对照组比较,GHET1干扰组中细胞凋亡率升高(P=0.019),miR-27b干扰组中细胞凋亡率降低(P=0.046)。与GHET1干扰组比较,GHET1+miR-27b干扰组中细胞凋亡率降低(P=0.015),见图 5。该结果表明,抑制GHET1增加SGC-996细胞凋亡,而抑制miR-27b降低SGC-996细胞凋亡,并且抑制miR-27b可以逆转抑制GHET1对SGC-996细胞凋亡的影响。
2.6 抑制GHET1和miR-27b对SGC-996细胞转移的影响
Transwell试验结果显示,与对照组比较,GHET1干扰组中细胞转移数量降低(P=0.033),miR-27b干扰组中细胞转移数量升高(P=0.027)。与GHET1干扰组比较,GHET1+miR-27b干扰组中细胞转移数量升高(P=0.031),见图 6。该结果表明,抑制GHET1降低SGC-996细胞转移,而抑制miR-27b促进SGC-996细胞转移,并且抑制miR-27b可以逆转抑制GHET1对SGC-996细胞转移的影响。
2.7 GHET1对miR-27b的调控作用
生物信息学分析结果显示,GHET1中“ACUGUGA”序列与miR-27b中“UGACACU”序列完全互补配对;荧光素酶报告基因试验结果显示,与对照组比较,miR-27b干扰组中GHET1-wt报告载体的相对荧光素酶活性增加(P<0.001),GHET1-mut报告载体的相对荧光素酶活性无明显改变(P>0.05);实时荧光定量PCR结果显示,对照组和GHET1干扰组中miR-27b的相对表达水平分别为1.0±0.08和2.53±0.29,差异有统计学意义(P<0.001),见图 7。该结果表明,GHET1可以海绵吸附miR-27b并抑制其表达。
3 讨论
近年来,靶向表观遗传因子是治疗疾病的一种新思路,尤其在恶性肿瘤中具有较大潜力。越来越多的证据显示,lncRNAs作为一类表观遗传因子在胆囊癌中异常表达并影响肿瘤进展,了解其作用机制将为该病的治疗提供新的分子靶点[6-7]。例如,Zhang等[8]报道H19在胆囊癌中表达上调,过表达H19可促进胆囊癌细胞增殖和转移。Lu等[9]发现小核宿主基因1(small nucleolar RNA host gene 1, SNHG1)在胆囊癌中呈现高表达,抑制SNHG1可降低胆囊癌细胞增殖和转移。Gao等[10]通过一系列细胞实验证实沉默叉头框蛋白D2相邻相反链RNA 1(FOXD2 adjacent opposite strand RNA 1, FOXD2-AS1)可以使胆囊癌进展受到一定的抑制。此外,TTN反义RNA1(TTN antisense RNA 1, TTN-AS1)被报道在胆囊癌进展过程中发挥促癌因子作用[11]。GHET1定位于人染色体7q36.1位置,长度为1 903 nt。作为胃癌中研究最多的lncRNA之一,GHET1与细胞增殖、细胞周期、转移以及多重耐药密切相关[12-13]。本研究发现,GHET1促进胆囊癌细胞增殖和转移并抑制细胞凋亡,有助于进一步完善GHET1在肿瘤中的功能注释。
本研究首先分析GHET1在50例胆囊癌样本中的表达,发现癌组织中该基因的表达高于癌旁组织,与其他肿瘤样本中的表达基本一致[4]。随后通过分析GHET1表达与胆囊癌患者预后的关系,发现高GHET1表达预示不良预后,提示GHET1可能用于监测胆囊癌预后,但仍需要大样本、多中心试验验证。此外,还发现GHET1表达与TNM分期和淋巴结转移相关。该结果与已发表的Meta分析结果相符[14]。
GHET1相较其他的lncRNA表现出高稳定性和特异性,在肿瘤治疗方面显示出较大的潜力。通过MTT法、流式细胞术以及Transwell试验,本研究发现抑制GHET1可降低SGC-996细胞增殖和转移,并增加细胞凋亡。该结果与Liu等[15]和Han等[16]结果一致,初步揭示GHET1在胆囊癌进展中发挥促癌因子作用。后续研究将在其他的胆囊癌细胞株如GBC-SD和NOZ中验证该结果。
作为一种研究较多的miRNA,miR-27b在多种肿瘤中参与调控细胞生物学行为。例如,Bao等[17]报道miR-27b通过激活Hippo信号通路抑制胃癌细胞转移和上皮-间充质转化。Li等[18]发现miR-27b靶向Engrailed同源盒蛋白2(engrailed homeobox 2, EN2)抑制膀胱癌细胞增殖和侵袭。Miao等[19]发现miR-27b通过调控Yes相关蛋白1(Yes1 associated transcriptional regulator, YAP1)抑制胶质瘤细胞增殖和迁移并促进细胞凋亡。本研究发现miR-27b在胆囊癌组织的表达低于癌旁组织,与其他肿瘤中报道的结果一致[5]。随后一系列体外细胞实验结果证实,抑制miR-27b促进SGC-996细胞增殖和转移并抑制细胞凋亡。由此初步表明,miR-27b在胆囊癌进展中发挥抑癌因子作用。需要注意的是,该结果需在GBC-SD和NOZ细胞株中进一步验证。
研究表明,lncRNAs主要通过海绵吸附下游miRNAs而发挥转录调节作用[20]。鉴于GHET1与miR-27b在胆囊癌进展中的作用截然相反,且胆囊癌中GHET1与miR-27b表达呈负相关。另外,生物信息学预测显示,GHET1中“ACUGUGA“序列可以与miR-27b中“UGACACU”序列特异性结合。基于以上证据,我们推测miR-27b可能受到GHET1直接调控。随后经荧光素酶报告基因试验证实,GHET1可以通过海绵吸附方式下调miR-27b表达。为了明确GHET1是否通过靶向miR-27b影响胆囊癌进展,我们在SGC-996细胞中同时抑制GHET1和miR-27b。结果发现,细胞增殖和转移较单独抑制GHET1增加,而细胞凋亡较单独抑制GHET1降低,表明抑制miR-27b可以逆转抑制GHET1对SGC-996细胞增殖、转移以及凋亡的影响。后续将通过实验在胆囊癌细胞中上调GHET1的同时上调miR-27b,观察细胞增殖、转移以及凋亡的改变。同时在动物模型中验证GHET1/miR-27b轴对体内胆囊癌生长与转移的影响。如果体内与体外功能一致,将来研发靶向GHET1的小分子药物或许可以起到有效的抗癌作用。
综上所述,GHET1在胆囊癌中表达上调,GHET1表达与TNM分期和淋巴结转移相关,高GHET1表达预示不良预后。GHET1靶向miR-27b促进胆囊癌细胞增殖和转移并抑制细胞凋亡,表明GHET1/miR-27b轴在胆囊癌进展中具有一定的作用。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:阳镇、李文川:实验实施、数据整合和分析、论文撰写与修改周宁:选题与实验设计、论文审阅校正 -
表 1 GHET1表达与胆囊癌患者临床病理特征的关系
Table 1 Relationship between GHET1 expression and clinicopathological characteristics of GBC patients

-
[1] Halaseh SA, Halaseh S, Shakman R. A Review of the Etiology and Epidemiology of Gallbladder Cancer: What You Need to Know[J]. Cureus, 2022, 14(8): e28260.
[2] Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021[J]. CA Cancer J Clin, 2021, 71(1): 7-33. doi: 10.3322/caac.21654
[3] Zhu X, Shi C, Hou C. AFAP1-AS1/Hsa-miR-15a-5p/Bcl-2 Axis is a Potential Regulator of Cancer Cell Proliferation and Apoptosis in Gallbladder Carcinoma[J]. Nutr Cancer, 2022, 74(9): 3363-3374. doi: 10.1080/01635581.2022.2059090
[4] Jiang YF, Zhang HY, Ke J, et al. Overexpression of LncRNA GHET1 predicts an unfavourable survival and clinical parameters of patients in various cancers[J]. J Cell Mol Med, 2019, 23(8): 4891-4899. doi: 10.1111/jcmm.14486
[5] Martino E, Balestrieri A, Mele L, et al. Milk Exosomal miR-27b Worsen Endoplasmic Reticulum Stress Mediated Colorectal Cancer Cell Death[J]. Nutrients, 2022, 14(23): 5081. doi: 10.3390/nu14235081
[6] Rana V, Parama D, Khatoon E, et al. Reiterating the Emergence of Noncoding RNAs as Regulators of the Critical Hallmarks of Gall Bladder Cancer[J]. Biomolecules, 2021, 11(12): 1847. doi: 10.3390/biom11121847
[7] Yang M, Lu H, Liu J, et al. lncRNAfunc: a knowledgebase of lncRNA function in human cancer[J]. Nucleic Acids Res, 2022, 50(D1): D1295-D1306. doi: 10.1093/nar/gkab1035
[8] Zhang Y, Li H, Lv C, et al. HHLA2 promotes tumor progression by long non-coding RNA H19 in human gallbladder cancer[J]. Int J Oncol, 2022, 61(3): 112. doi: 10.3892/ijo.2022.5402
[9] Lu X, Hu K, Tan Q, et al. Silencing SNHG1 Suppresses Viability, Proliferation and Invasion of Gallbladder Carcinoma Cells via Targeting miR-194-5p[J]. Ann Clin Lab Sci, 2022, 52(5): 707-720.
[10] Gao J, Dai C, Yu X, et al. Silencing of long non-coding RNA FOXD2-AS1 inhibits the progression of gallbladder cancer by mediating methylation of MLH1[J]. Gene Ther, 2021, 28(6): 306-318. doi: 10.1038/s41434-020-00187-w
[11] Lin Z, Li Y, Shao R, et al. LncRNA TTN-AS1 acts as a tumor promoter in gallbladder carcinoma by regulating miR-107/HMGA1 axis[J]. World J Surg Oncol, 2021, 19(1): 163. doi: 10.1186/s12957-021-02279-2
[12] Liu H, Wu Y. Long non-coding RNA gastric carcinoma highly expressed transcript 1 promotes cell proliferation and invasion in human head and neck cancer[J]. Oncol Lett, 2018, 15(5): 6941-6946.
[13] Li B, Xie D, Zhang H. Long non-coding RNA GHET1 contributes to chemotherapeutic resistance to Gemcitabine in bladder cancer[J]. Cancer Chemother Pharmacol, 2019, 84(1): 187-194. doi: 10.1007/s00280-019-03873-8
[14] Poursheikhani A, Nokhandani N, Yousefi H, et al. Clinicopathological Significance of Long Non-Coding RNA GHET1 in Human Cancers: A Meta-Analysis[J]. Curr Pharm Biotechnol, 2020, 21(14): 1422-1432. doi: 10.2174/1389201021999200727163238
[15] Liu Z, Luo S, Wu M, et al. LncRNA GHET1 promotes cervical cancer progression through regulating AKT/mTOR and Wnt/beta-catenin signaling pathways[J]. Biosci Rep, 2020, 40(1): BSR20191265. doi: 10.1042/BSR20191265
[16] Han M, Wang Y, Gu Y, et al. lncRNA GHET1 knockdown suppresses breast cancer activity in vitro and in vivo[J]. Am J Transl Res, 2019, 11(1): 31-44.
[17] Bao CH, Guo L. miR-27b-3p Inhibits Invasion, Migration and Epithelial-mesenchymal Transition in Gastric Cancer by Targeting RUNX1 and Activation of the Hippo Signaling Pathway[J]. Anticancer Agents Med Chem, 2022, 22(5): 864-873. doi: 10.2174/1871520621666210707095833
[18] Li Y, Duan Q, Gan L, et al. microRNA-27b inhibits cell proliferation and invasion in bladder cancer by targeting engrailed-2[J]. Biosci Rep, 2021, 41(1): BSR20201000. doi: 10.1042/BSR20201000
[19] Miao W, Li N, Gu B, et al. MiR-27b-3p suppresses glioma development via targeting YAP1[J]. Biochem Cell Biol, 2020, 98(4): 466-473. doi: 10.1139/bcb-2019-0300
[20] Lin X, Zhuang S, Chen X, et al. lncRNA ITGB8-AS1 functions as a ceRNA to promote colorectal cancer growth and migration through integrin-mediated focal adhesion signaling[J]. Mol Ther, 2022, 30(2): 688-702. doi: 10.1016/j.ymthe.2021.08.011



 下载:
下载: