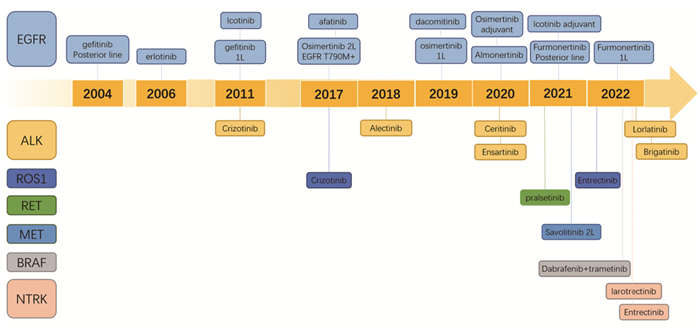
Perspective of Precision Therapy on Lung Cancer
-
摘要:
目前,肺癌仍然是我国发病率和死亡率较高的恶性肿瘤。传统放化疗疗效有限,肺癌治疗新药物和新方案的探索一直在进行中。过去20多年,随着分子靶向和免疫治疗的飞速发展,临床疗效不断刷新,越来越多的癌症患者从中获益。肺癌治疗方法日新月异,是实体肿瘤精准治疗领域的“排头兵”。本文梳理了肺癌精准靶向和免疫治疗的发展历程,并对存在的问题及未来发展方向进行探讨。
Abstract:Lung cancer remains to have the highest morbidity and mortality rates in China among known malignant tumors. Novel drugs and regimens have been sought because of the limited efficiency of traditional chemotherapy and radiotherapy in lung cancer treatment. In the last 20 years, rapid developments in molecular targeted therapy and immunotherapy have increased clinical efficacy and benefitted patients with cancer. Treatments for lung cancer are the most rapidly developed among treatments for solid tumors, pioneering tumor precision medicine. This manuscript reviews the evolution and development of targeted therapy and immunotherapy and discusses existing problems and future directions in the precision therapy of lung cancer.
-
Key words:
- Lung cancer /
- Precision medicine /
- Molecular targeted therapy /
- Immunotherapy
-
0 引言
原发性支气管肺癌是起源于支气管黏膜或腺体的恶性肿瘤,发病率和死亡率均居全球癌症首位。肺癌易发生颅内、骨骼、淋巴结等远处转移,骨骼是肺癌远处转移的常见部位,骨转移多以中轴骨等承重骨多见,是晚期肺癌疼痛的主要原因。Hong等[1]研究显示,恶性实体瘤从诊断开始到发生骨转移的中位时间为18.9个月,而骨转移相关事件大多在骨转移后1月发生,发生率大约为45.1%,肺癌骨转移患者的中位生存时间仅为6~10个月, 经过治疗后1年生存率也仅为40%~50%[2]。所以,寻找早期预测及诊断骨转移的指标显得尤为重要。近年来,多数学者专注探索预测肺癌骨转移的相关指标以及建立预测模型。既往的研究结果已证实,血钙、T4期、N3期、p-Ⅲ期、非鳞状细胞、骨唾液蛋白BSP表达、癌胚抗原水平升高、高碱性磷酸酶是肺癌骨转移的危险因素[3-5],但是对炎性反应与肺癌骨转移关系的研究甚少。炎性反应在肿瘤微环境中起着重要的作用,与肿瘤发生、发展、侵袭、转移密切相关。自1860年Virchow等观察到肿瘤细胞中有白细胞浸润,并首次提出慢性炎性反应迁延不愈可导致肿瘤的发生后,越来越多的学者开始研究炎性反应与肿瘤的相关性[6]。肿瘤相关性炎性反应已经被列为肿瘤的第七大特征。目前临床常用的炎性反应指标:C反应蛋白、中性粒细胞/淋巴细胞比值(NLR)、血小板/淋巴细胞比值(PLR)、淋巴细胞/单核细胞比值(LMR)、系统免疫炎症指数(systemic immune-inflammation index, SII)等指标已经在多种实体瘤中进行研究[7-12]。本研究从炎性反应指标、肿瘤标志物着手,进一步探讨免疫炎症指数、肿瘤标志物与肺癌骨转移之间的关系。
1 资料与方法
1.1 基本资料
收集兰州大学第二医院肿瘤中心、呼吸内科2015年1月1日—2020年12月31日未行手术、放化疗、免疫、靶向等任何抗肿瘤治疗的肺恶性肿瘤患者。纳入标准:(1)明确诊断为肺恶性肿瘤的初治患者;(2)首次来我院就诊,未行手术、放化疗、免疫、靶向等任何抗肿瘤治疗的患者;(3)病历资料完整者。排除标准:(1)病历资料不全者;(2)合并其他系统严重疾病;(3)合并骨相关疾病;(4)严重感染;(5)外院确诊或者已行抗肿瘤治疗;(6)影像检查提示骨破坏未进一步进行全身骨显像者。资料收集包括患者年龄、性别、吸烟状况、BMI、病理分型、中性粒细胞计数、淋巴细胞计数、外周血小板计数、CEA、Cyfra21-1、NSE值。本研究经本院医学伦理委员会批准(批号:2021A-119)。数据不会泄露任何个人隐私,已获得入组所有患者知情同意。
1.2 肺骨转移诊断标准
根据肺癌骨转移诊疗专家共识(2019版),肺癌骨转移的诊断应满足以下两个条件之一:(1)临床或病理诊断为肺癌,骨病变活检符合肺癌转移;(2)肺癌病理诊断明确,具有典型的骨转移影像学表现[2]。
1.3 数据定义及分组
数据定义:SII=血小板计数×中性粒细胞/淋巴细胞比值;
分组定义:预测组:由随访发生骨转移患者与随访未发生骨转移患者构成;诊断组:由初次就诊时已经发生骨转移患者和随访未发生骨转移患者构成。
1.4 统计学方法
所有数据采用SPSS25.0软件进行统计学分析。根据是否发生骨转移,进行骨转移和非骨转移患者数据分析,采用Kolmogorov-Smirnov检验计量资料是否服从正态分布,对于服从正态分布的计量资料以x±s描述,采用t检验比较;以M(P25~P75)描述不服从正态分布的计量资料,采用Mann-Whitney秩和检验;计数资料组间比较采用卡方检验;二元Logistic回归进行单因素及多因素分析,确定肺癌骨转移相关危险因素,P < 0.05为差异有统计学意义;ROC曲线、约登指数确定各因素对肺癌骨转移的预测及诊断价值并确定其最佳截点值。建立组合模型,评估其预测价值及诊断价值。
2 结果
2.1 预测组患者资料
2.1.1 预测组患者的一般临床特征
预测组共纳入肺癌患者425例,年龄最小者26岁,最大者79岁,平均年龄为58.96±9.29岁,卡方检验结果显示,在性别、年龄、吸烟、病理类型方面,骨转移与未发生骨转移患者无明显差异(P > 0.05),见表 1。
表 1 预测组肺癌患者的临床特征Table 1 Clinical characteristics of lung cancer patients in the predictive group
2.1.2 预测组实验室检查资料对比分析结果
SII、CEA、Cyfra21-1、NSE均不服从正态分布。秩和检验结果显示,两组数据在SII、CEA、Cyfra21-1、NSE方面均存在差异,骨转移患者均较非骨转移患者显著升高(P < 0.05),见表 2。
表 2 预测组肺癌患者实验室检查资料及特征Table 2 Laboratory data and characteristics of patients with lung cancer
2.1.3 Logistic回归单因素及多因素分析
对于上述检验差异的危险因素进行Logistic单因素及多因素回归分析。单因素分析结果显示:SII、NSE水平升高的肺癌患者更容易发生骨转移(P < 0.05)。多因素分析结果显示:SII、NSE是肺癌骨转移独立危险因素,是肺癌骨转移的独立预测因素(P < 0.05),见表 3。
表 3 预测组肺癌骨转移危险因素Logistic单因素及多因素分析结果Table 3 Results of Logistic univariate and multivariate analyses of risk factors of bone metastasis of lung cancer in the predictive group
2.1.4 绘制ROC曲线明确相关危险因素预测价值
将Logistic分析筛选的肺癌骨转移危险因素绘制ROC曲线,明确预测价值。结果显示:NSE、SII预测截断值分别为58.64 ng/ml、850。将二者组合为组合模型,结果显示组合模型的预测价值进一步提高,见表 4、图 1。
表 4 肺癌骨转移炎性反应指标预测效能评估Table 4 Evaluation of predictive efficacy of inflammatory markers for bone metastasis of lung cancer
2.2 诊断组患者资料
2.2.1 诊断组患者的一般临床特征
诊断组共纳入193例初诊时就发生骨转移的患者,结合随访未发生骨转移患者326例,共519例患者临床资料进行对比分析。其中年龄最小为24岁,最大为86岁,平均年龄为59.78±9.46岁,计数资料卡方检验结果显示,年龄、性别、吸烟两组之间差异无统计学意义(P > 0.05),病理类型两组之间差异有统计学意义(P < 0.001),表明年龄、性别、是否吸烟与肺癌是否发生骨转移无相关性,而在病理类型中,腺癌更易发生骨转移,见表 5。
表 5 诊断组肺癌患者临床特征Table 5 Basic clinical data and characteristics of patients with lung cancer in the diagnosis group
2.2.2 诊断组计数资料秩和检验分析结果
诊断组检验数据均不服从正态分布,采用秩和检验。结果显示:骨转移患者SII、CEA、Cyfra21-1、NSE水平较非骨转移患者均显著升高(P < 0.05),见表 6。
表 6 诊断组肺癌患者炎性反应指标对比(M(P25, P75)Table 6 Comparison of inflammatory parameters in patients with lung cancer in the diagnosis group (M(P25, P75)
2.2.3 肺癌骨转移危险因素Logistic单因素及多因素回归分析
对于上述检验差异的危险因素进行Logistic单因素及多因素回归分析。单因素分析显示:腺癌、高水平SII、CEA、Cyfra21-1、NSE的肺癌患者更容易发生骨转移。多因素分析显示:腺癌、高水平SII、CEA、Cyfra21-1、NSE均为肺癌骨转移的独立危险因素,是肺癌骨转移的独立诊断指标(P < 0.05),见表 7。
表 7 诊断组肺癌骨转移危险因素Logistic单因素及多因素分析Table 7 Results of Logistic univariate and multivariate analyses of risk factors of bone metastasis of lung cancer in the diagnosis group
2.2.4 绘制ROC曲线明确肺癌骨转移相关危险因素诊断价值
对上述Logistic回归分析结果提示的危险因素绘制ROC曲线,计算约登指数,明确截断值和预测价值。各危险因素的最佳截断值,见表 8,其中Cyfra21-1曲线下面积最大,AUC为0.78,诊断敏感度为0.824,诊断特异性为0.604。依次建立SII+腺癌、SII+CEA、SII+NSE、SII+Cyfra21-1组合模型,结果显示:SII+Cyfra21-1组合模型曲线下面积最大,AUC为0.820,敏感度为74.0%,特异性为78.5%,并优于任何单因素曲线下面积(P < 0.05),见表 8、图 2、3。
表 8 肺癌骨转移相关危险因素诊断效能评估Table 8 Evaluation of diagnostic efficacy of risk factors related to bone metastasis of lung cancer
3 讨论
骨转移是癌症进展的一种表现,同时也存在骨折、高钙血症等骨骼相关事件的风险,不仅降低患者的生活质量,同时也增加了医疗费用,增加了社会负担。随着肺癌诊疗技术进展,肺癌死亡率较前有所下降,但是骨转移的诊断及治疗仍显滞后,转移机制并未研究明确,相对生存率较低,所以及时地发现骨转移、寻找骨转移危险因素显得尤为重要。本研究通过对比分析618例肺癌患者的临床资料,从预测及诊断两部分寻找骨转移的危险因素,并建立肺癌骨转移预测及诊断模型。
3.1 一般临床特征与肺癌骨转移
本研究发现,性别、年龄、吸烟不是肺癌骨转移的危险因素,这与既往研究结果一致[13-15]。有研究发现,与从不吸烟者相比,既往吸烟者更容易发生骨骼相关事件(OR=2.80, 95%CI: 1.32~6.00),同时也发现既往吸烟患者到首次发现骨骼相关事件发生的中位时间较未吸烟者短(HR=1.75, 95%CI: 1.05~2.92)[16]。在肺癌病理类型中,腺癌患者更易发生骨转移(HR=1.51, 95%CI: 1.06~2.15, P=0.021)[13]。本研究发现,病理类型在预测组未表现出明显差异,在诊断组中,骨转移发生率腺癌为52%,鳞癌为22%,小细胞癌为27%,腺癌患者更易发生骨转移,是肺癌骨转移的独立危险因素,与既往研究结果一致[17]。腺癌骨转移风险较其余病理类型高的原因,可能与腺癌的发病率较高相关,也可能与腺癌发生部位及易血行转移相关[18]。
3.2 系统免疫炎症指数与肺癌转移
近来研究表明,SII与多种实体瘤的预后密切相关。Gao等[19]回顾性分析410例NSCLC患者的临床资料,以395.4为界限将患者分为高SII组和低SII组,发现高SII组患者具有更高的T分期及淋巴结转移阳性率。Wang[20]等通过对9项研究中的2441名肺癌患者数据进行荟萃分析,得出预处理前SII升高的患者OS较差,并通过对比得出SII的预测预后作用优于NLR、PLR。本研究数据显示,在预测组中,SII≥850的肺癌患者更易发生骨转移,是肺癌骨转移的独立预测因素;诊断组中,SII在两组患者中同样存在显著差异(P < 0.05),单因素及多因素分析显示SII≥951.6是肺癌骨转移独立危险因素,SII单独诊断肺癌骨转移的曲线下面积为0.754,联合Cyfra21-1组合模型曲线下面积最大,AUC为0.820,敏感度为74.0%,特异性为78.5%,并优于任何单因素曲线下面积(P < 0.05),其诊断肺癌骨转移价值可观。综上所述,高水平SII在肺癌骨转移的预测及诊断中均具有重要的临床意义,联合肺癌肿瘤标志物后诊断价值进一步提高。
3.3 肺癌肿瘤标志物与肺癌骨转移
肿瘤标志物对许多癌症的诊断、分期、治疗和复发的监测具有重要意义。在一项纳入113例间变性淋巴瘤激酶重排的NSCLC的临床研究中,研究者将CEA根据10 ng/ml分为高组和低组,发现CEA高组发生骨转移的比例更高(HR=4.002, 95%CI: 1.280-12.511, P=0.0171),提示在肺癌诊断时血清CEA≥10 ng/ml的ALK重排NSCLC患者可能有骨远处转移的风险,应该引起重视[21]。
NSE是糖酵解酶烯醇化酶的细胞特异性同工酶,是目前诊断、判断预后和随访小细胞肺癌最可靠的肿瘤标志物。研究发现,NSE在肺癌骨转移的诊断中存在一定的价值,NSE在骨转移组水平明显高于未骨转移及良性肿瘤组(P < 0.05),进一步分析发现NSE水平与肺癌骨转移数量正相关[22]。
Cyfra21-1是一种细胞角蛋白,同样作为一种肺癌血清学标志物,常规应用于肺癌的筛查、治疗及疗效观察中。在一项关于老年患者肺腺癌骨转移的相关危险因素研究中显示,Cyfra21-1在单因素及多因素分析中,均为肺腺癌骨转移的危险因素(P < 0.001),其敏感度为70.5%,这说明Cyfra21-1与肺癌远处转移密切相关[23]。
本研究分析了CEA、Cyfra21-1、NSE与肺癌骨转移的相关性,结果发现在预测组及诊断组中,骨转移组肿瘤标志物基线水平均高于非骨转移组(P < 0.001)。在预测组中,多因素校正分析显示,当NSE≥58.64 ng/ml时更易发生骨转移,是肺癌骨转移独立预测因素,而CEA、Cyfra21-1并未表现出预测价值;在诊断组中,Logistic回归分析提示CEA、Cyfra21-1、NSE均为肺癌患者发生骨远处转移的独立危险因素,是肺癌骨转移的独立诊断因素,当联合SII时,诊断价值均高于单一肿瘤标志物诊断价值。以上证据均说明,肺癌肿瘤标志物与肺癌骨转移密切相关,在肿瘤的诊疗过程中应密切关注肺癌肿瘤标志物变化,及时关注是否有骨转移的发生。
综上所述,SII、CEA、Cyfra21-1、NSE在骨转移组水平均显著高于非骨转移组,腺癌、高水平SII、CEA、Cyfra21-1、NSE均为肺癌骨转移的独立危险因素,当SII联合其他单一危险因素时,预测价值及诊断价值进一步提高,值得临床推广使用。本研究不足之处是未行多中心研究,对于SII及肺癌肿瘤标志物的截断值的选取与既往研究不一致,应扩大样本量及多中心研究。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:任胜祥:总体策划、论文构思、文稿撰写、修改与审校邱天羽:文献收集、论文构思、文稿撰写 -
[1] Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016[J]. J Natl Cancer Cent, 2022, 2(1): 1-9. doi: 10.1016/j.jncc.2022.02.002
[2] Yuan M, Huang L, Chen J, et al. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer[J]. Signal Transduct Target Ther, 2019, 4: 61. doi: 10.1038/s41392-019-0099-9
[3] Papadimitrakopoulou VA, Mok TS, Han JY, et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis[J]. Ann Oncol, 2020, 31(11): 1536-1544. doi: 10.1016/j.annonc.2020.08.2100
[4] Lu S, Wang QM, Zhang GJ, et al. Efficacy of Aumolertinib (HS-10296) in Patients With Advanced EGFR T790M+ NSCLC: Updated Post-National Medical Products Administration Approval Results From the APOLLO Registrational Trial[J]. J Thorac Oncol, 2022, 17(3): 411-422. doi: 10.1016/j.jtho.2021.10.024
[5] Shi YK, Hu XS, Zhang SC, et al. Efficacy, safety, and genetic analysis of furmonertinib (AST2818) in patients with EGFR T790M mutated non-small-cell lung cancer: a phase 2b, multicentre[J]. Lancet Respir Med, 2021, 9(8): 829-839. doi: 10.1016/S2213-2600(20)30455-0
[6] Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer[J]. N Engl J Med, 2018, 378(2): 113-125. doi: 10.1056/NEJMoa1713137
[7] Ramalingam SS, Gray JE, Ohe Y, et al. Osimertinib vs comparator EGFR-TKI as first-line treatment for EGFRm advanced NSCLC (FLAURA): Final overall survival analysis[J]. Ann Oncol, 2019, 30(Suppl_5): V851-V934. http://www.sciencedirect.com/science/article/pii/s0923753419604369
[8] Cho BC, Chewaskulyong B, Lee KH, et al. Osimertinib versus Standard of Care EGFR TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC: FLAURA Asian Subset[J]. J Thorac Oncol, 2019, 14(1): 99-106. doi: 10.1016/j.jtho.2018.09.004
[9] Shi Y, Chen G, Wang X, et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study[J]. Lancet Respir Med, 2022, 10(11): 1019-1028. doi: 10.1016/S2213-2600(22)00168-0
[10] Lu S, Dong X, Jian H, et al. AENEAS: A Randomized Phase ⅢTrial of Aumolertinib Versus Gefitinib as First-Line Therapy for Locally Advanced or Metastatic Non-Small-Cell Lung Cancer With EGFR Exon 19 Deletion or L858R Mutations[J]. J Clin Oncol, 2022, 40(27): 3162-3171. doi: 10.1200/JCO.21.02641
[11] Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS PhaseⅠ Study[J]. J Clin Oncol, 2021, 39(30): 3391-3402. doi: 10.1200/JCO.21.00662
[12] Han H, Li S, Chen T, et al. Targeting HER2 Exon 20 Insertion-Mutant Lung Adenocarcinoma with a Novel Tyrosine Kinase Inhibitor Mobocertinib[J]. Cancer Res, 2021, 81(20): 5311-5324. doi: 10.1158/0008-5472.CAN-21-1526
[13] Zhong WZ, Wang Q, Mao WM, et al. Gefitinib Versus Vinorelbine Plus Cisplatin as Adjuvant Treatment for Stage Ⅱ-ⅢA (N1-N2) EGFR-Mutant NSCLC: Final Overall Survival Analysis of CTONG1104 PhaseⅢ Trial[J]. J Clin Oncol, 2021, 39(7): 713-722. doi: 10.1200/JCO.20.01820
[14] Yue DS, Xu SD, Wang Q, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage ⅢA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, phase 2 trial[J]. Lancet Respir Med, 2018, 6(11): 863-873. doi: 10.1016/S2213-2600(18)30277-7
[15] Zhou C, He J, Su C, et al. Icotinib versus Chemotherapy as Adjuvant Treatment for Stage Ⅱ-ⅢA EGFR-Mutant NSCLC (EVIDENCE): A Randomized, Open-Label, Phase 3 Study[J]. J Thorac Oncol, 2021, 16(3): S232-S232.
[16] Wu Y, John T, Grohe C, et al. Postoperative Chemotherapy Use and Outcomes from ADAURA: Osimertinib as Adjuvant Therapy for Resected EGFR-Mutated NSCLC[J]. J Thorac Oncol, 2022, 17(3): 423-433. doi: 10.1016/j.jtho.2021.10.014
[17] Zhong WZ, Wu YL, Chen KN, et al. LBA48_PRCTONG 1103: Erlotinib versus gemcitabine plus cisplatin as neo-adjuvant treatment for stage ⅢA-N2 EGFR-mutation non-small cell lung cancer (EMERGING): A randomised study[J]. Ann Oncol, 2018, 29(suppl_8): 738-738. http://www.sciencedirect.com/science/article/pii/S0923753419504495
[18] Lyu C, Fang W, Jiao W, et al. Osimertinib as neoadjuvant therapy in patients with EGFR mutated resectable stage Ⅱ-ⅢB lung adenocarcinoma (NEOS): Updated results[J]. Ann Oncol, 2022, 33(suppl 2): S71-S72.
[19] Zhou CC, Kim SW, Reungwetwattana T, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study[J]. Lancet Respir Med, 2019, 7(5): 437-446. doi: 10.1016/S2213-2600(19)30053-0
[20] Mok T, Kim DW, Wu YL, et al. First-line crizotinib versus pemetrexed-cisplatin or pemetrexed-carboplatin in patients (pts) with advanced ALK-positive non-squamous non-small cell lung cancer (NSCLC): results of a phaseⅢ study (PROFILE 1014)[J]. J Clin Oncol, 2014, 32(15_Suppl): 8002. doi: 10.1200/jco.2014.32.15_suppl.8002
[21] Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study[J]. Ann Oncol, 2020, 31(8): 1056-1064. doi: 10.1016/j.annonc.2020.04.478
[22] Cai DJ, Hu CP, Li L, et al. The prevalence and prognostic value of KRAS co-mutation subtypes in Chinese advanced non-small cell lung cancer patients[J]. Cancer Med, 2020, 9(1): 84-93. doi: 10.1002/cam4.2682
[23] Tan DS, Geater S, Yu CJ, et al. First-line ceritinib versus chemotherapy in patients (pts) with advanced ALK rearranged (ALK+) non-small cell lung cancer (NSCLC): ASCEND-4 Asian subgroup analysis[J]. Ann Oncol, 2019, 30(suppl 5): 599.
[24] Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor-Naive ALK-Positive Non-Small Cell Lung Cancer: Second Interim Analysis of the PhaseⅢ ALTA-1L Trial[J]. J Clin Oncol, 2020, 38(31): 3592-3603. doi: 10.1200/JCO.20.00505
[25] Horn L, Wang ZP, Wu G, et al. Ensartinib vs. Crizotinib for Patients With Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer A Randomized Clinical Trial[J]. JAMA Oncol, 2021, 7(11): 1617-1625. doi: 10.1001/jamaoncol.2021.3523
[26] Shaw AT, Bauer TM, de Marinis F, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer[J]. N Engl J Med, 2020, 383(21): 2018-2029. doi: 10.1056/NEJMoa2027187
[27] Bergethon K, Shaw AT, Ou SHI, et al. ROS1 Rearrangements Define a Unique Molecular Class of Lung Cancers[J]. J Clin Oncol, 2012, 30(8): 863-870. doi: 10.1200/JCO.2011.35.6345
[28] Wu YL, Yang JCH, Kim DW, et al. PhaseⅡ Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer[J]. J Clin Oncol, 2018, 36(14): 1405-1411. doi: 10.1200/JCO.2017.75.5587
[29] D'Angelo A, Sobhani N, Chapman R, et al. Focus on ROS1-Positive Non-Small Cell Lung Cancer (NSCLC): Crizotinib, Resistance Mechanisms and the Newer Generation of Targeted Therapies[J]. Cancers (Basel), 2020, 12(11): 3293. doi: 10.3390/cancers12113293
[30] Shaw AT, Solomon BJ, Chiari R, et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1-2 trial[J]. Lancet Oncol, 2019, 20(12): 1691-1701. doi: 10.1016/S1470-2045(19)30655-2
[31] Jaenne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRASG12C Mutation[J]. N Engl J Med, 2022, 387(2): 120-131. doi: 10.1056/NEJMoa2204619
[32] Mazieres J, Peters S, Lepage B, et al. Lung Cancer That Harbors an HER2 Mutation: Epidemiologic Characteristics and Therapeutic Perspectives[J]. J Clin Oncol, 2013, 31(16): 1997-2003. doi: 10.1200/JCO.2012.45.6095
[33] Song ZB, Lv DQ, Chen SQ, et al. Pyrotinib in Patients with HER2-Amplified Advanced Non-Small Cell Lung Cancer: A Prospective, Multicenter, Single-Arm Trial[J]. Clin Cancer Res, 2022, 28(3): 461-467. doi: 10.1158/1078-0432.CCR-21-2936
[34] Elamin YY, Robichaux JP, Carter BW, et al. Poziotinib for Patients With HER2 Exon 20 Mutant Non-Small-Cell Lung Cancer: Results From a PhaseⅡ Trial[J]. J Clin Oncol, 2022, 40(7): 702-709. doi: 10.1200/JCO.21.01113
[35] Iwama E, Zenke Y, Sugawara S, et al. Trastuzumab emtansine for patients with non-small cell lung cancer positive for human epidermal growth factor receptor 2 exon-20 insertion mutations[J]. Eur J Cancer, 2022, 162: 99-106. doi: 10.1016/j.ejca.2021.11.021
[36] Li BT, Smit EF, Goto Y, et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer[J]. N Engl J Med, 2022, 386(3): 241-251. doi: 10.1056/NEJMoa2112431
[37] Han BH, Li K, Wang QM, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer The ALTER 0303 Phase 3 Randomized Clinical Trial[J]. JAMA Oncol, 2018, 4(11): 1569-1575. doi: 10.1001/jamaoncol.2018.3039
[38] Cheng Y, Wang QM, Li K, et al. Anlotinib vs. placebo as third- or further-line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled Phase 2 study[J]. Brit J Cancer, 2021, 125(3): 366-371. doi: 10.1038/s41416-021-01356-3
[39] Reck M, Rodriguez-Abreu D, Robinson AG, et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score≥50%[J]. J Clin Oncol, 2021, 39(21): 2339-2349. doi: 10.1200/JCO.21.00174
[40] Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial[J]. Lancet, 2019, 393(10183): 1819-1830. doi: 10.1016/S0140-6736(18)32409-7
[41] Jassem J, de Marinis F, Giaccone G, et al. Updated Overall Survival Analysis From IMpower110: Atezolizumab Versus Platinum-Based Chemotherapy in Treatment-Naive Programmed Death-Ligand 1-Selected NSCLC[J]. J Thorac Oncol, 2021, 16(11): 1872-1882. doi: 10.1016/j.jtho.2021.06.019
[42] Sezer A, Kilickap S, Gumus M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial[J]. Lancet, 2021, 397(10274): 592-604. doi: 10.1016/S0140-6736(21)00228-2
[43] Peters S, Creelan B, Hellmann MD, et al. Abstract CT082; Impact of tumor mutation burden on the efficacy of first-line nivolumab in stage iv or recurrent non-small cell lung cancer: An exploratory analysis of CheckMate 026[J]. Cancer Res, 2017, 77(13 Suppl): CT082-CT082. http://cancerres.aacrjournals.org/content/77/13_Supplement/CT082
[44] Judd J, Borghaei H. Combining Immunotherapy and Chemotherapy for Non-Small Cell Lung Cancer[J]. Thorac Surg Clin, 2020, 30(2): 199-206. doi: 10.1016/j.thorsurg.2020.01.006
[45] Cheng Y, Zhang L, Hu J, et al. Pembrolizumab Plus Chemotherapy for Chinese Patients With Metastatic Squamous NSCLC in KEYNOTE-407[J]. JTO Clin Res Rep, 2021, 2(10): 100225. http://www.sciencedirect.com/science/article/pii/S2666364321000849
[46] Wang J, Lu S, Yu XM, et al. Tislelizumab Plus Chemotherapy vs. Chemotherapy Alone as First-line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer A Phase 3 Randomized Clinical Trial[J]. JAMA Oncol, 2021, 7(5): 709-717. doi: 10.1001/jamaoncol.2021.0366
[47] Lu S, Wang J, Yu Y, et al. Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial[J]. J Thorac Oncol, 2021, 16(9): 1512-1522. doi: 10.1016/j.jtho.2021.05.005
[48] Yang Y, Wang Z, Fang J, et al. Final overall survival (OS) data of sintilimab plus pemetrexed (SPP) and platinum as first-line (1L) treatment for locally advanced or metastatic nonsquamous NSCLC (AMnsqNSCLC) in the phaseⅢ ORIENT-11 study[J]. Ann Oncol, 2022, 33: S28-S28.
[49] Zhou CC, Wu L, Fan Y, et al. Sintilimab Plus Platinum and Gemcitabine as First-Line Treatment for Advanced or Metastatic Squamous NSCLC: Results From a Randomized, Double-Blind, Phase 3 Trial (ORIENT-12)[J]. J Thorac Oncol, 2021, 16(9): 1501-1511. doi: 10.1016/j.jtho.2021.04.011
[50] Zhou CC, Chen GY, Huang YC, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial[J]. Lancet Respir Med, 2021, 9(3): 305-314. doi: 10.1016/S2213-2600(20)30365-9
[51] Zhou C, Cheng Y, Chen J, et al. First-line camrelizumab plus carboplatin and paclitaxel for advanced squamous non-small cell lung cancer: Updated overall survival results from the phase Ⅲ CameL-sq trial[J]. Ann Oncol, 2022, 33(suppl 2): S28.
[52] Wang J, Wang Z, Wu L, et al. CHOICE-01: A Phase 3 Study of Toripalimab Versus Placebo in Combination With First-Line Chemotherapy for Advanced NSCLC[J]. J Thorac Oncol, 2021, 16(10): S927-S928. doi: 10.1016/j.jtho.2021.08.181
[53] Lu S, Fang J, Wang ZP, et al. Results from the IMpower132 China cohort: Atezolizumab plus platinum-based chemotherapy in advanced non-small cell lung cancer[J]. Cancer Med, 2023, 12(3): 2666-2667. doi: 10.1002/cam4.5144
[54] Horn L, Mansfield AS, Szczesna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer[J]. N Engl J Med, 2018, 379(23): 2220-2229. doi: 10.1056/NEJMoa1809064
[55] Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab +/- tremelimumab plus platinum-etoposide in first-line extensive-stage SCLC (ES-SCLC): 3-year overall survival update from the phaseⅢ CASPIAN study[J]. Ann Oncol, 2021, 32(suppl 5): S1338-S1338.
[56] Paz-Ares LG, Ciuleanu TE, Lee JS, et al. Nivolumab (NIVO) plus ipilimumab (IPI) versus chemotherapy (chemo) as first-line (1L) treatment for advanced non-small cell lung cancer (NSCLC): 4-year update from CheckMate 227[J]. J Clin Oncol, 2021, 39(15 suppl): 9016. http://www.researchgate.net/publication/352077636_Nivolumab_NIVO_plus_ipilimumab_IPI_versus_chemotherapy_chemo_as_first-line_1L_treatment_for_advanced_non-small_cell_lung_cancer_NSCLC_4-year_update_from_CheckMate_227
[57] Reck M, Ciuleanu TE, Cobo M, et al. First-line nivolumab (NIVO) plus ipilimumab (IPI) plus two cycles of chemotherapy (chemo) versus chemo alone (4 cycles) in patients with advanced non-small cell lung cancer (NSCLC): Two-year update from CheckMate 9LA[J]. J Clin Oncol, 2021, 39(15_suppl): 9000. doi: 10.1200/JCO.2021.39.15_suppl.9000
[58] Reinmuth N, Johnson M, Cho BC, et al. Durvalumab +/- Tremelimumab plus Chemotherapy as First-line Treatment for mNSCLC: Results from the Phase 3 POSEIDON Study[J]. J Thorac Oncol, 2021, 16(10_supplement): S844. http://www.sciencedirect.com/science/article/pii/S1556086421024527
[59] Rizvi NA, Cho BC, Reinmuth N. Durvalumab With or Without Tremelimumab vs. Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial[J]. JAMA Oncol, 2020, 6(5): 661-674. doi: 10.1001/jamaoncol.2020.0237
[60] Boyer M, Sendur MAN, Rodriguez-Abreu D, et al. Pembrolizumab Plus Ipilimumab or Placebo for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score≥50%: Randomized, Double-Blind PhaseⅢ KEYNOTE-598 Study[J]. J Clin Oncol, 2021, 39(21): 2327-2338. doi: 10.1200/JCO.20.03579
[61] Ciciola P, Cascetta P, Bianco C, et al. Combining Immune Checkpoint Inhibitors with Anti-Angiogenic Agents[J]. J Clin Med, 2020, 9(3): 675. doi: 10.3390/jcm9030675
[62] Socinski MA, Nishio M, Jotte RM, et al. IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC[J]. J Thorac Oncol, 2021, 16(11): 1909-1924. doi: 10.1016/j.jtho.2021.07.009
[63] Lu S, Wu L, Jian H, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial[J]. Lancet Oncol, 2022, 23(9): 1167-1179. doi: 10.1016/S1470-2045(22)00382-5
[64] Fan Y, Zhao J, Wang QM, et al. Camrelizumab Plus Apatinib in Extensive-Stage SCLC (PASSION): A Multicenter, Two-Stage, Phase 2 Trial[J]. J Thorac Oncol, 2021, 16(2): 299-309. doi: 10.1016/j.jtho.2020.10.002
[65] Yang JCH, Luft A, Jimenez ED, et al. Pembrolizumab (Pembro) with or without lenvatinib (Lenva) in first-line metastatic NSCLC with PD-L1 TPS≥1% (LEAP-007): A phaseⅢ, randomized, double-blind study[J]. Ann Oncol, 2021, 32: S1429-S1430.
[66] Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in StageⅢ Non-Small-Cell Lung Cancer[J]. J Clin Oncol, 2022, 40(12): 1301-1311. doi: 10.1200/JCO.21.01308
[67] Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer[J]. N Engl J Med, 2022, 386(21): 1973-1985. doi: 10.1056/NEJMoa2202170
[68] Zhou N, Sepesi B, Leung CH, et al. Impact of genomic aberrations and additional therapies on survival outcomes of patients with operable non-small cell lung cancer (NSCLC) from the NEOSTAR study[J]. J Clin Oncol, 2021, 39(15_suppl): 8542. doi: 10.1200/JCO.2021.39.15_suppl.8542
[69] Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stageⅠB-ⅢA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial[J]. Lancet, 2021, 398(10308): 1344-1357. doi: 10.1016/S0140-6736(21)02098-5
[70] Wei J, Liu L, Chen X, et al. Improved sensitivity of integrative DNA and RNA NGS assay on fusion detection[J]. Ann Oncol, 2020, 31: S1105. http://www.sciencedirect.com/science/article/pii/S0923753420413547
[71] Cohen D, Hondelink LM, Solleveld-Westerink N, et al. Optimizing Mutation and Fusion Detection in NSCLC by Sequential DNA and RNA Sequencing[J]. J Thorac Oncol, 2020, 15(6): 1000-1014. doi: 10.1016/j.jtho.2020.01.019
[72] Kurata J, Price K, Banks K, et al. Multiomic, Plasma-Only ctDNA NGS Assay Developed for Minimal Residual Disease (MRD) Detection in Early-Stage NSCLC[J]. J Thorac Oncol, 2021, 16(10): S952-S953. http://www.sciencedirect.com/science/article/pii/S1556086421026435
[73] Liu SY, Wu YL. Biomarker for personalized immunotherapy[J]. Transl Lung Cancer Res, 2019, 8(Suppl 3): S308-S317.
[74] Wang MN, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer[J]. Nat Med, 2021, 27(8): 1345-1356. doi: 10.1038/s41591-021-01450-2
[75] Lim JU. Overcoming Osimertinib Resistance in Advanced Non-small Cell Lung Cancer[J]. Clin Oncol, 2021, 33(10): 619-626. doi: 10.1016/j.clon.2021.07.015
[76] Lim SM, Park CW, Zhang Z, et al. BLU-945, a fourth-generation, potent and highly selective epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) with intracranial activity, demonstrates robust in vivo antitumor activity in models of osimertinib-resistant non-small cell lung cancer (NSCLC)[J]. Cancer Res, 2021, 81(13_Supplement): 1467. doi: 10.1158/1538-7445.AM2021-1467
-
期刊类型引用(6)
1. 华华, 樊华, 邹思璐, 李洪晓. 吉西他滨或培美曲塞联合顺铂治疗老年晚期非鳞非小细胞肺癌的临床临床效果. 中国老年学杂志. 2025(14)  百度学术
百度学术
2. 李月华. 血清CA125、CEA和CA19-9检测对非小细胞肺癌患者骨转移的预测价值. 医药前沿. 2025(07): 12-15 .  百度学术
百度学术
3. 温晴. 无创呼吸机辅助治疗对肺癌根治术后并发呼吸衰竭患者氧代谢及并发症风险的影响. 中国医疗器械信息. 2024(04): 23-26 .  百度学术
百度学术
4. 杨卷红,吴博云,崔丝雨,童丽,张博阳,张斌,薛红强. 多模态影像技术对不同病理类型肺癌患者骨转移的诊断及转移灶分布特点分析. 中国临床实用医学. 2024(01): 1-6 .  百度学术
百度学术
5. 庞乐乐. 帕米膦酸二钠对肺癌骨转移性疼痛及骨纤维结构的影响研究. 药品评价. 2024(01): 56-59 .  百度学术
百度学术
6. 闫红江,李铁志,焦晓丹,高少林. 卡瑞利珠单抗用于Ⅲa期NSCLC患者术前新辅助化疗的临床研究. 中国临床药理学杂志. 2024(17): 2469-2473 .  百度学术
百度学术
其他类型引用(5)



 下载:
下载: