-
摘要:目的
探讨Merkel细胞癌患者的临床特征、治疗方法及转归特点。
方法回顾性分析2017年以来中国医学科学院肿瘤医院收治的6例Merkel细胞癌患者的临床表现、辅助检查、诊治经过及随访资料。
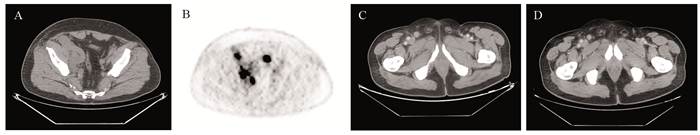
结果6例Merkel细胞癌患者中男4例、女2例,中位发病年龄66岁(57~76岁)。6例患者均以出现皮肤肿物起病,临床分期:Ⅰ期3例、Ⅲ期1例、Ⅳ期2例。单纯手术治疗2例、手术联合放疗和(或)化疗3例,免疫治疗联合化疗1例。截至随访时间,4例疾病无进展,1例因疾病进展死亡,1例仍规律治疗中。
结论局限期Merkel细胞癌以手术、放疗为主,转移性Merkel细胞癌多需应用全身治疗,一线选择靶向程序性死亡受体1(PD-1)/程序性死亡配体1(PD-L1)途径的免疫检查点抑制剂可取得较好的治疗效果。
Abstract:ObjectiveTo investigate the clinical features, treatment, and outcome characteristics of patients with Merkel cell carcinoma.
MethodsThe clinical manifestations, laboratory tests, diagnosis and treatment, and follow-up data of six patients with Merkel cell carcinoma were retrospectively analyzed.
ResultsAmong the six patients with Merkel cell carcinoma, four were males and two were females, with a median age of 66 years old (57-76 years old). All six patients presented with skin swelling, and the clinical stages were as follows: stageⅠ in three patients, stage Ⅲ in one patient, and stage IV in two patients. Two patients were treated with surgery alone, three patients with surgery combined with radiotherapy and/or chemotherapy, and one patient with immunotherapy combined with chemotherapy. Until the follow-up time, four patients had no disease progression, one patient died because of disease progression, and one patient remained under treatment.
ConclusionLimited-stage Merkel cell carcinoma is primarily treated with surgery and radiotherapy, meanwhile, metastatic Merkel cell carcinoma needs systemic therapy, and first-line immune checkpoint inhibitors targeting PD-1/ PD-L1 pathway can achieve better therapeutic results.
-
0 引言
多形性腺瘤(pleomorphic adenoma, PA)是腮腺中最常见的肿瘤,也被称为混合瘤,占所有腮腺肿瘤的60%~70%,世界卫生组织根据PA的组织病理学及生物学特性,将其界定为临界瘤[1]。据统计,每10万人就有1.4~2.3人发生该病,且PA发病率呈逐年递增趋势[2]。虽然PA生长比较缓慢,但其术后复发率可达到20%~45%,且多次复发可致恶变[3]。PA最常见的类型是癌在多形性腺瘤(carcinoma in pleomorphic adenoma, CA-EX-PA),约占涎腺恶性肿瘤的10%~20%,且90%以上都是由PA恶变所致,可危及生命,尽早发现两者的生物学行为、治疗方法及判断预后一直是医学界研究的热点[2]。
线粒体在细胞生存和死亡中发挥重要作用。为维持细胞的正常活动状态, 细胞要选择性地清除受损伤或不需要的线粒体, 这一过程被称为线粒体自噬(mitochondrial autophagy或mitophagy)[4]。线粒体自噬与肿瘤的关系成为医学研究的热点,作为诱导线粒体自噬的关键基因,PINK1/Parkin通路是主要分子调控机制之一,也是线粒体自噬的经典途径之一[5]。PINK1和Parkin具有协同作用,当它们任何一方表达水平异常或功能受到抑制时,清除受损线粒体的自噬能力会明显下降,使细胞正常生理功能进一步受到损害[6-7]。
目前,线粒体自噬活性的改变与恶性肿瘤的发生、发展密切相关, 且线粒体自噬在肿瘤发生发展的不同阶段所发挥的作用也不同。肿瘤发生早期,线粒体自噬维持细胞正常的新陈代谢,抑制肿瘤的发生;肿瘤发生后期,线粒体自噬的发生则会提高细胞的耐受,促进肿瘤的发展[8]。在卵巢癌、乳腺癌等多种肿瘤中也发现线粒体自噬基因出现等位缺失现象,但其在腮腺肿瘤的发生发展阶段,线粒体活性是否发生变化目前尚不清楚[9]。因此,本研究拟通过免疫组织化学的方法检测正常腮腺组织、腮腺多形性腺瘤及癌在多形性腺瘤组织中PINK1和Parkin的蛋白表达差异,探讨线粒体自噬在多形性腺瘤及其恶变中的作用,为进一步了解多形性腺瘤及其恶变提供理论依据。
1 资料与方法
1.1 资料来源
收集2016年4月—2018年4月间郑州大学附属肿瘤医院确诊的PA组织32例、CA-EX-PA组织42例。所有组织标本均未行术前放疗和化疗,并收集24例正常腮腺组织作为对照组。4%甲醛溶液常规固定,石蜡包埋,4 μm厚度连续切片,HE染色。所有患者事前均已签署《生物标本二次利用知情同意书》。
1.2 主要试剂
兔抗人PINK1和Parkin单克隆抗体购自武汉三鹰生物技术有限公司;免疫组织化学检测试剂盒、磷酸盐缓冲溶液、苏木素染色试剂购于上海碧云天生物技术有限公司,其余试剂购于中国医药上海化学试剂公司。
1.3 免疫组织化学检测PINK1和Parkin的蛋白表达差异
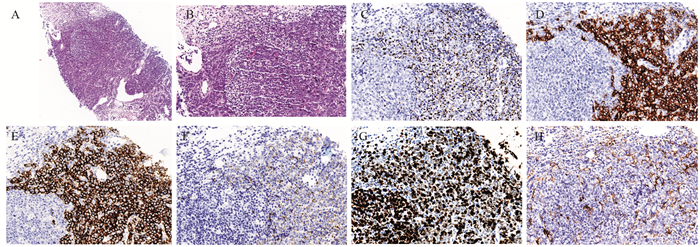
10%福尔马林固定标本,常规石蜡包埋,4 μm厚连续切片,脱蜡,依次经100%、95%、80%、70%及50%梯度浓度酒精水化,每个浓度5 min,PBS缓冲液冲洗,热抗原修复,滴加5%的山羊血清,室温下放置20~30 min,封闭非特异性抗原,滴加一抗PINK1(1:2 000)、Parkin(1:2 000)并置于4℃的冰箱过夜,取出切片,37℃孵育箱复温30 min后,PBS冲洗,滴加二抗37℃孵育30 min后PBS冲洗,加辣根过氧化物酶工作液反应15 min,DAB显色。苏木素对比染色,常规脱水、透明、干燥、封片,并拍照观察。
1.4 免疫组织化学结果评价标准
当细胞质染成棕褐色、棕黄色、黄色或淡黄色时,认为PINK1和Parkin两种蛋白阳性表达。利用二级计分法对两种蛋白的表达进行评定:随机选取每个切片10个200×的显微镜视野,通过阳性细胞百分率和染色深度进行评分。阳性率评分:阳性细胞数占总细胞数 < 5%计为0分,5%~20%计为1分,≥20%~50%计为2分,≥50%计为3分。染色深度评分:无染色计为0分,淡黄色计为1分,黄色/棕黄色计为2分,褐色/棕褐色计为3分。两项评分相乘即为总评分:0~2分计为阴性(-),3~4分计为弱阳性(+),5~6分计为中强阳性(++),6分以上计为强阳性(+++)。
1.5 统计学方法
应用Image-Pro Plus 6.0系统软件分析阳性细胞的吸光度(IOD),采用χ2检验和Spearman等级分析进行数据分析,实验结果采用统计学软件SPSS22.0进行分析,P < 0.05表示差异有统计学意义。
2 结果
2.1 PINK1和Parkin蛋白在三种组织中的表达
正常腮腺组织、PA组织和CA-EX-PA组织中PINK1蛋白阳性表达率依次为100%、91%和67%,PA和CA-EX-PA组织中PINK1的表达率显著低于正常组织(均P < 0.05)。Parkin蛋白在三种组织中阳性表达率依次为100%、88%和52%,PA和CA-EX-PA组织中Parkin的表达率同样显著低于正常组织(均P < 0.05),见表 1、图 1。
表 1 PINK1和Parkin在不同组织中的表达(n)Table 1 Expression of PINK1 and Parkin in different tissues (n)
2.2 CA-EX-PA组织中PINK1和Parkin蛋白表达的相关性
Spearman等级分析结果显示PINK1和Parkin蛋白在CA-EX-PA组织中的表达具有正相关性(r=0.877, P < 0.05),见表 2。
表 2 PINK1和Parkin在CA-EX-PA组织中表达的相关性Table 2 Correlation between PINK1 and Parkin expression in CA-EX-PA tissues
2.3 PINK1和Parkin蛋白在CA-EX-PA组织中表达与临床病理特征的关系
CA-EX-PA患者的年龄、性别和肿瘤直径与PINK1和Parkin蛋白表达不具有相关性(P > 0.05),CA-EX-PA患者的肿瘤侵袭性、TNM分期和淋巴结转移与PINK1和Parkin蛋白的表达显著相关(P < 0.05),见表 3。
表 3 CA-EX-PA患者中PINK1和Parkin表达与临床病理因素的关系Table 3 Correlation of PINK1 and Parkin expression with clinicopathological of CA-EX-PA patients
3 讨论
线粒体自噬能够选择性清除多余或受损线粒体,在维持细胞内线粒体数量以及线粒体的正常功能方面发挥重要的作用[10]。正常情况下,自噬体的生成和降解之间保持动态平衡,以维持细胞内环境的稳定;病理状态下,自噬体生成过多或清除过少,会引起细胞内受损线粒体大量堆积,导致细胞线粒体自噬异常,最终细胞功能产生紊乱甚至凋亡[11]。PINK1和Parkin是诱导线粒体自噬的重要因子,两者的表达缺失能够引起细胞线粒体自噬活性降低,可能导致肿瘤的发生[12-13]。研究显示在人类多种肿瘤中均有线粒体自噬活性改变的现象。例如,利用致癌物质诱导鼠的胰腺细胞后,线粒体自噬在癌变前期活性升高,但是当完成癌变以后,线粒体自噬活性能力反而减弱,提示线粒体自噬活性的降低可能促进一些肿瘤的恶化。腮腺肿瘤的发生是否与线粒体自噬相关因子的表达发生变化相关,是否参与了腮腺肿瘤的病理进程,目前相关方面报道甚少。
PINK1和Parkin蛋白可能具有抑制肿瘤功能,在乳腺癌、肺癌、直肠癌、卵巢癌等肿瘤中均表现为缺失表达,两者的表达水平显著低于正常组织[14]。Jeong等研究证实在小鼠胰腺癌癌变早期线粒体自噬水平明显升高,而晚期则显著降低[15]。另有实验研究显示,在敲除PINK1等位基因的小鼠中,乳腺癌、肺癌、腮腺肿瘤等癌症的发病率均明显高于正常小鼠[16]。本研究实验结果提示在PA和CA-EX-PA组织中PINK1和Parkin蛋白表达显著低于正常组织。PINK1基因在PA和CA-EX-PA组织中低表达可能是因为PA组织的迅速生长,细胞需要降低线粒体自噬水平来适应低氧和营养缺乏等环境。
Spearman相关系数检验结果显示,在癌组织中PINK1和Parkin在CA-EX-PA中表达呈正相关(r=0.691, P=0.010),说明两者的低表达可以降低CA-EX-PA线粒体自噬活性,与CA-EX-PA的发生、发展密切相关,两者的联合检测对CA-EX-PA的治疗效果及预后具有重要的参考价值。淋巴转移是判断腮腺恶性肿瘤预后的重要指标,本研究证实,在CA-EX-PA组织中,PINK1和Parkin蛋白的表达与淋巴结转移、TNM分期、肿瘤侵袭性显著相关。
在PA和CA-EX-PA组织中PINK1和Parkin蛋白表达明显降低,说明腮腺肿瘤的发生、演进和恶化可能和线粒体自噬活性降低相关,因此同时检测PINK1和Parkin蛋白表达水平可以更加客观准确地评估腮腺恶性肿瘤患者的预后,从而制定合理、有效的治疗方法,以提高患者的生存率。线粒体自噬的具体调控机制尚未完全明确,仍需进一步研究,为腮腺肿瘤的治疗提供新的靶点和新思路。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:任梦薇:查阅文献、论文撰写薛学敏:病例筛选、资料收集刘鹏:论文修改、研究指导 -
表 1 6例Merkel细胞癌患者基本资料
Table 1 Basic information of six patients with MCC

表 2 关于转移性MCC化疗的临床研究
Table 2 Previous clinical studies on chemotherapy for metastatic MCC

表 3 关于MCC治疗的Ⅱ期抗PD-1/PD-L1临床试验
Table 3 Summary of phaseⅡ anti-PD-1/PD-L1 clinical trials for the treatment of MCC

-
[1] Walsh NM, Cerroni L. Merkel cell carcinoma: A review[J]. J Cutan Pathol, 2021, 48(3): 411-421. doi: 10.1111/cup.13910
[2] Nghiem P, Kaufman HL, Bharmal M, et al. Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma[J]. Future Oncol, 2017, 13(14): 1263-1279. doi: 10.2217/fon-2017-0072
[3] Xue Y, Thakuria M. Merkel Cell Carcinoma Review[J]. Hematol Oncol Clin North Am, 2019, 33(1): 39-52. doi: 10.1016/j.hoc.2018.08.002
[4] Bobos M, Hytiroglou P, Kostopoulos I, et al. Immunohistochemical distinction between merkel cell carcinoma and small cell carcinoma of the lung[J]. Am J Dermatopathol, 2006, 28(2): 99-104. doi: 10.1097/01.dad.0000183701.67366.c7
[5] Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma[J]. Nat Rev Dis Primers, 2017, 3: 17077. doi: 10.1038/nrdp.2017.77
[6] Harms KL, Healy MA, Nghiem P, et al. Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System[J]. Ann Surg Oncol, 2016, 23(11): 3564-3571. doi: 10.1245/s10434-016-5266-4
[7] Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution[J]. J Clin Oncol, 2005, 23(10): 2300-2309. doi: 10.1200/JCO.2005.02.329
[8] Wong SQ, Waldeck K, Vergara IA, et al. UV-Associated Mutations Underlie the Etiology of MCV-Negative Merkel Cell Carcinomas[J]. Cancer Res, 2015, 75(24): 5228-5234. doi: 10.1158/0008-5472.CAN-15-1877
[9] Vandeven N, Lewis CW, Makarov V, et al. Merkel Cell Carcinoma Patients Presenting Without a Primary Lesion Have Elevated Markers of Immunity, Higher Tumor Mutation Burden, and Improved Survival[J]. Clin Cancer Res, 2018, 24(4): 963-971. doi: 10.1158/1078-0432.CCR-17-1678
[10] Suárez AL, Louis P, Kitts J, et al. Clinical and dermoscopic features of combined cutaneous squamous cell carcinoma (SCC)/neuroendocrine[Merkel cell] carcinoma (MCC)[J]. J Am Acad Dermatol, 2015, 73(6): 968-975. doi: 10.1016/j.jaad.2015.08.041
[11] Ansai SI, Noro S, Ogita A, et al. Case of Merkel cell carcinoma with squamous cell carcinoma possibly arising in chronic radiodermatitis of the hand[J]. J Dermatol, 2015, 42(2): 207-209. doi: 10.1111/1346-8138.12737
[12] National Comprehensive Cancer Network. Merkel Cell Carcinoma (Version 2.2022)[DB/OL]. https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf.
[13] Voog E, Biron P, Martin JP, et al. Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma[J]. Cancer, 1999, 85(12): 2589-2595. doi: 10.1002/(SICI)1097-0142(19990615)85:12<2589::AID-CNCR15>3.0.CO;2-F
[14] Iyer JG, Blom A, Doumani R, et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma[J]. Cancer Med, 2016, 5(9): 2294-2301. doi: 10.1002/cam4.815
[15] Cowey CL, Mahnke L, Espirito J, et al. Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA[J]. Future Oncol, 2017, 13(19): 1699-1710. doi: 10.2217/fon-2017-0187
[16] Nghiem P, Bhatia S, Lipson EJ, et al. Durable Tumor Regression and Overall Survival in Patients With Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy[J]. J Clin Oncol, 2019, 37(9): 693-702. doi: 10.1200/JCO.18.01896
[17] Topalian SL, Bhatia S, Hollebecque A, et al. Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): Efficacy and safety in Merkel cell carcinoma (MCC)[J]. Cancer Res, 2017, 77(13_suppl): CT074.
[18] D'Angelo SP, Russell J, Lebbé C, et al. Efficacy and Safety of First-line Avelumab Treatment in Patients With Stage ⅣMetastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial[J]. JAMA Oncol, 2018, 4(9): e180077. doi: 10.1001/jamaoncol.2018.0077
[19] D'Angelo SP, Bhatia S, Brohl AS, et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial[J]. J Immunother Cancer, 2020, 8(1): e000674. doi: 10.1136/jitc-2020-000674
[20] Harms PW, Harms KL, Moore PS, et al. The biology and treatment of Merkel cell carcinoma: current understanding and research priorities[J]. Nat Rev Clin Oncol, 2018, 15(12): 763-776. doi: 10.1038/s41571-018-0103-2
[21] Knepper TC, Montesion M, Russell JS, et al. The Genomic Landscape of Merkel Cell Carcinoma and Clinicogenomic Biomarkers of Response to Immune Checkpoint Inhibitor Therapy[J]. Clin Cancer Res, 2019, 25(19): 5961-5971. doi: 10.1158/1078-0432.CCR-18-4159
[22] Tarabadkar ES, Thomas H, Blom A, et al. Clinical Benefit from Tyrosine Kinase Inhibitors in Metastatic Merkel Cell Carcinoma: A Case Series of 5 Patients[J]. Am J Case Rep, 2018, 19: 505-511. doi: 10.12659/AJCR.908649
[23] Akaike T, Qazi J, Anderson A, et al. High somatostatin receptor expression and efficacy of somatostatin analogues in patients with metastatic Merkel cell carcinoma[J]. Br J Dermatol, 2021, 184(2): 319-327. doi: 10.1111/bjd.19150



 下载:
下载: