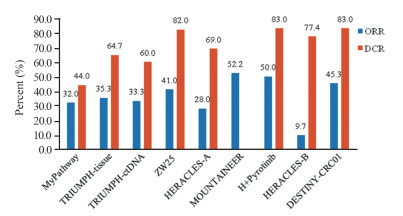
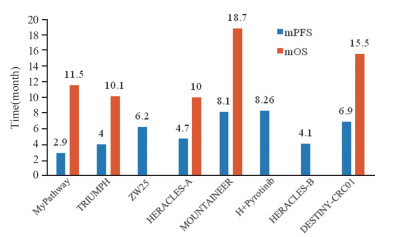
-
摘要:
表皮生长因子受体2(HER2)是肿瘤发生、发展过程中的癌基因,在7%的结直肠癌患者中表达,与表皮生长因子受体单克隆抗体的耐药相关。随着CRC治疗困境的出现,以及靶向HER2为乳腺癌、胃癌患者带来生存获益,HER2在CRC中的意义及抗HER2治疗的预后价值被广泛关注,围绕HER2阳性CRC的临床研究亦不断开展。目前,CRC中HER2阳性的诊断标准已逐渐统一,HER2靶向治疗如单克隆抗体、酪氨酸激酶抑制剂、抗体-药物耦联物及HER2相关免疫治疗的单独或联合治疗策略在HER2阳性CRC中显示出较好的疗效,能为患者带来生存获益,本文就此方面的研究进展作一综述。
Abstract:Epidermal growth factor receptor 2 (HER2) is an oncogene involved in tumour genesis and progression. It is expressed in 7% of patients with colorectal cancer (CRC) and is associated with drug resistance of epidermal growth factor receptor monoclonal antibodies. With the emergence of the therapeutic dilemma of CRC and the survival benefits of targeting HER2 for patients with breast cancer and gastric cancer, the significance of HER2 in CRC and the prognostic value of anti-HER2 therapy have been widely concerned, clinical researches on HER2-positive CRC have been continuously carried out. Currently, the diagnostic criteria for HER2 positive CRC have gradually been unified. HER2-targeting therapies such as monoclonal antibodies, tyrosine kinase inhibitors, antibody-drug coupling and HER2-related immunotherapy alone or in combination have shown good efficacy and brought significant survival benefits for HER2 positive CRC. This paper reviews the research progress of HER2 in CRC.
-
Key words:
- Colorectalcancer /
- HER2/neu /
- Targettherapy /
- Immunotherapy
-
Competing interests: The authors declare that they have no competing interests.作者贡献:祁雅丽:研究选题、论文构思、文献检索、数据解析及论文撰写苟亚妮:协助文献检索及数据整理达丽隽:文献检索交叉核验及协助数据解析李恩喜、刘雅婷、裴霞霞:协助论文撰写宋飞雪:论文设计及指导
-
表 1 HER2阳性结直肠癌患者中正在开展的临床研究
Table 1 Ongoing studies in patients with HER2-positive metastatic CRC

-
[1] Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network[J]. Nat Rev Mol Cell Biol, 2001, 2(2): 127-137. doi: 10.1038/35052073
[2] Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer[J]. Nature, 2012, 487(7407): 330-337. doi: 10.1038/nature11252
[3] Richman SD, Southward K, Chambers P, et al. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials[J]. J Pathol, 2016, 238(4): 562-570. doi: 10.1002/path.4679
[4] Sartore-Bianchi A, Amatu A, Porcu L, et al. HER2 Positivity Predicts Unresponsiveness to EGFR-Targeted Treatment in Metastatic Colorectal Cancer[J]. Oncologist, 2019, 24(10): 1395-1402. doi: 10.1634/theoncologist.2018-0785
[5] Yonesaka K, Zejnullahu K, Okamoto I, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab[J]. Sci Transl Med, 2011, 3(99): 99ra86.
[6] Ross J, Fakih M, Ali S, et al. Targeting HER2 in colorectal cancer: The landscape of amplification and short variant mutations in ERBB2 and ERBB3[J]. Cancer, 2018, 124(7): 1358-1373. doi: 10.1002/cncr.31125
[7] Seo AN, Kwak Y, Kim DW, et al. HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression[J]. PLoS One, 2014, 9(5): e98528. doi: 10.1371/journal.pone.0098528
[8] Cenaj O, Ligon AH, Hornick JL, et al. Detection of ERBB2 Amplification by Next-Generation Sequencing Predicts HER2 Expression in Colorectal Carcinoma[J]. Am J Clin Pathol, 2019, 152(1): 97-108. doi: 10.1093/ajcp/aqz031
[9] Siravegna G, Sartore-Bianchi A, Nagy RJ, et al. Plasma HER2(ERBB2) Copy Number Predicts Response to HER2-targeted Therapy in Metastatic Colorectal Cancer[J]. Clin Cancer Res, 2019, 25(10): 3046-3053. doi: 10.1158/1078-0432.CCR-18-3389
[10] Pan G, Li D, Li X, et al. SPECT/CT imaging of HER2 expression in colon cancer-bearing nude mice using (125) I-Herceptin[J]. Biochem Biophys Res Commun, 2018, 504(4): 765-770. doi: 10.1016/j.bbrc.2018.08.201
[11] Valtorta E, Martino C, Sartore-Bianchi A, et al. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study[J]. Mod Pathol, 2015, 28(11): 1481-1491. doi: 10.1038/modpathol.2015.98
[12] Fujii S, Magliocco AM, Kim J, et al. International Harmonization of Provisional Diagnostic Criteria for ERBB2-Amplified Metastatic Colorectal Cancer Allowing for Screening by Next-Generation Sequencing Panel[J]. JCO Precis Oncol, 2020, 4(11): 6-19.
[13] Wang G, He Y, Sun Y, et al. Prevalence, prognosis and predictive status of HER2 amplification in anti-EGFR-resistant metastatic colorectal cancer[J]. Clin Transl Oncol, 2020, 22(6): 813-822. doi: 10.1007/s12094-019-02213-9
[14] Siravegna G, Mussolin B, Buscarino M, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients[J]. Nat Med, 2015, 21(7): 795-801. doi: 10.1038/nm.3870
[15] Shan L, Lv Y, Bai B, et al. Variability in HER2 expression between primary colorectal cancer and corresponding metastases[J]. J Cancer Res Clin Oncol, 2018, 144(11): 2275-2281. doi: 10.1007/s00432-018-2744-z
[16] Tan RYC, Camat MD, Ng M, et al. HER2 positive rates are enriched amongst colorectal cancer brain metastases: a study amongst 1920 consecutive patients[J]. Ann Oncol, 2018, 29(7): 1598-1599. doi: 10.1093/annonc/mdy156
[17] Lee WS, Park YH, Lee JN, et al. Comparison of HER2 expression between primary colorectal cancer and their corresponding metastases[J]. Cancer Med, 2014, 3(3): 674-680. doi: 10.1002/cam4.228
[18] Ramanathan RK, Hwang JJ, Zamboni WC, et al. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phaseⅡ trial[J]. Cancer Invest, 2004, 22(6): 858-865. doi: 10.1081/CNV-200039645
[19] Kavuri SM, Jain N, Galimi F, et al. HER2 activating mutations are targets for colorectal cancer treatment[J]. Cancer Discov, 2015, 5(8): 832-841. doi: 10.1158/2159-8290.CD-14-1211
[20] Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer[J]. Cancer Discov, 2011, 1(6): 508-523. doi: 10.1158/2159-8290.CD-11-0109
[21] Hainsworth J D, Meric-Bernstam F, Swanton C, et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From My Pathway, an Open-Label, Phase IIa Multiple Basket Study[J]. J Clin Oncol, 2018, 36(6): 536-542. doi: 10.1200/JCO.2017.75.3780
[22] Meric-Bernstam F, Hurwitz H, Raghav KPS, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study[J]. Lancet Oncol, 2019, 20(4): 518-530. doi: 10.1016/S1470-2045(18)30904-5
[23] Nakamura Y, Okamoto W, Kato T, et al. TRIUMPH: Primary efficacy of a phase II trial of trastuzumab (T) and pertuzumab (P) in patients (pts) with metastatic colorectal cancer (mCRC) with HER2(ERBB2) amplification (amp) in tumour tissue or circulating tumour DNA (ctDNA): A GOZILA sub-study[J]. Ann Oncol, 2019, 30: v199-v200.
[24] ZW25 Effective in HER2-Positive Cancers[J]. Cancer Discov, 2019, 9(1): 8.
[25] Tosi F, Sartore-Bianchi A, Lonardi S, et al. Long-term Clinical Outcome of Trastuzumab and Lapatinib for HER2-positive Metastatic Colorectal Cancer[J]. Clin Colorectal Cancer, 2020, 19(4): 256-62.e2. doi: 10.1016/j.clcc.2020.06.009
[26] Taskar KS, Rudraraju VR, Mittapalli RK, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer[J]. Pharm Res, 2012, 29(3): 770-781. doi: 10.1007/s11095-011-0601-8
[27] Sartore-Bianchi A, Trusolino L, Martino C, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial[J]. Lancet Oncol, 2016, 17(6): 738-746. doi: 10.1016/S1470-2045(16)00150-9
[28] Fakih MG. Trastuzumab Plus Pertuzumab Resistance Does Not Preclude Response to Lapatinib Plus Trastuzumab in HER2-Amplified Colorectal Cancer[J]. Oncologist, 2018, 23(4): 474-477. doi: 10.1634/theoncologist.2017-0436
[29] Lee A. Tucatinib: First Approval[J]. Drugs, 2020, 80(10): 1033-1038. doi: 10.1007/s40265-020-01340-w
[30] 仲茜, 靳水玲, 宗红. 曲妥珠单抗联合吡咯替尼治疗人表皮生长因子-2阳性晚期结直肠癌临床观察[J]. 肿瘤基础与临床, 2021, 34(2): 114-117. doi: 10.3969/j.issn.1673-5412.2021.02.006 Zhong X, Jin SL, Zong H. Clinical observation of trastuzumab combined with pyrrotinib in the treatment of hu-man epidermal growth factor-2 positive advanced intestinal cancer[J]. Zhong Liu Ji Chu Yu Lin Chuang, 2021, 34(2): 114-117. doi: 10.3969/j.issn.1673-5412.2021.02.006
[31] Li HS, Yang LL, Zhang MY, et al. Remarkable Response of EGFR-and HER2-Amplified Metastatic Colon Cancer to Pyrotinib After Failed Multiline Treatments: A Case Report and Literature Review[J]. Front Oncol, 2020, 10: 548867. doi: 10.3389/fonc.2020.548867
[32] Yang M, Fang X, Li J, et al. Afatinib treatment for her-2 amplified metastatic colorectal cancer based on patient-derived xenograft models and next generation sequencing[J]. Cancer Biol Ther, 2019, 20(4): 391-396. doi: 10.1080/15384047.2018.1529120
[33] Sartore-Bianchi A, Lonardi S, Martino C, et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phaseⅡ HERACLES-B trial[J]. ESMO Open, 2020, 5(5): e000911. doi: 10.1136/esmoopen-2020-000911
[34] Sandhu J, Wang C, Fakih M. Clinical Response to T-DM1 in HER2-Amplified, KRAS-Mutated Metastatic Colorectal Cancer[J]. J Natl Compr Canc Netw, 2020, 18(2): 116-119.
[35] Siena S, Di Bartolomeo M, Raghav K, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial[J]. Lancet Oncol, 2021, 22(6): 779-789. doi: 10.1016/S1470-2045(21)00086-3
[36] Takegawa N, Tsurutani J, Kawakami H, et al. [fam-] trastuzumab deruxtecan, antitumor activity is dependent on HER2 expression level rather than on HER2 amplification[J]. Inter J Cancer, 2019, 145(12): 3414-3424. doi: 10.1002/ijc.32408
[37] Goodman A, Sokol E, Frampton G, et al. Microsatellite-Stable Tumors with High Mutational Burden Benefit from Immunotherapy[J]. Cancer Immunol Res, 2019, 7(10): 1570-1573. doi: 10.1158/2326-6066.CIR-19-0149
[38] Qiu MZ, He CY, Yang XH, et al. Relationship of HER2 Alteration and Microsatellite Instability Status in Colorectal Adenocarcinoma[J]. Oncologist, 2021, 26(7): e1161-e1170. doi: 10.1002/onco.13786
[39] Disis M, Knutson K, Schiffman K, et al. Pre-existent immunity to the HER-2/neu oncogenic protein in patients with HER-2/neu overexpressing breast and ovarian cancer[J]. Breast Cancer Res Treat, 2000, 62(3): 245-252. doi: 10.1023/A:1006438507898
[40] Ward R, Hawkins N, Coomber D, et al. Antibody immunity to the HER-2/neu oncogenic protein in patients with colorectal cancer[J]. Human Immunol, 1999, 60(6): 510-515. doi: 10.1016/S0198-8859(99)00003-8
[41] Shahabi V, Seavey MM, Maciag PC, et al. Development of a live and highly attenuated Listeria monocytogenes-based vaccine for the treatment of Her2/neu-overexpressing cancers in human[J]. Cancer Gene Ther, 2011, 18(1): 53-62. doi: 10.1038/cgt.2010.48
[42] Feng Q, Manabe Y, Kabayama K, et al. Syntheses and Functional Studies of Self-Adjuvanting Anti-HER2 Cancer Vaccines[J]. Chem Asian J, 2019, 14(23): 4268-4273. doi: 10.1002/asia.201901002
[43] Zhang W, Wang S, Gu J, et al. Synergistic tumoricidal effect of combined hPD-L1 vaccine and HER2 gene vaccine[J]. Biochem Biophys Res Commun, 2018, 497(1): 394-400. doi: 10.1016/j.bbrc.2018.02.092
[44] Lynch KT, Squeo GC, Kane WJ, et al. A pilot trial of vaccination with carcinoembryonic antigenand Her2/neu peptides in advanced colorectal cancer[J]. Int J Cancer, 2021, 150(1): 164-173.
[45] Kaumaya PT, Foy KC, Garrett J. Phase I Active Immunotherapy With Combination ofTwo Chimeric, Human Epidermal Growth Factor Receptor 2, B-Cell Epitopes Fused to a Promiscuous T-Cell Epitope in Patients With Metastatic and/or Recurrent Solid Tumors[J]. J Clin Oncol, 2009, 27(31): 5270-5277. doi: 10.1200/JCO.2009.22.3883
[46] Schönfeld K, Sahm C, Zhang C, et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor[J]. Mol Ther, 2015, 23(2): 330-338. doi: 10.1038/mt.2014.219
[47] Zhang C, Burger MC, Jennewein L, et al. ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma[J]. J Nat Cancer Inst, 2016, 108(5): 375-386.
[48] Rosewell Shaw A, Porter CE, Yip T, et al. Oncolytic adeno-immunotherapy modulates the immune system enabling CAR T-cells to cure pancreatic tumors[J]. Commun Biol, 2021, 4(1): 368-380. doi: 10.1038/s42003-021-01914-8



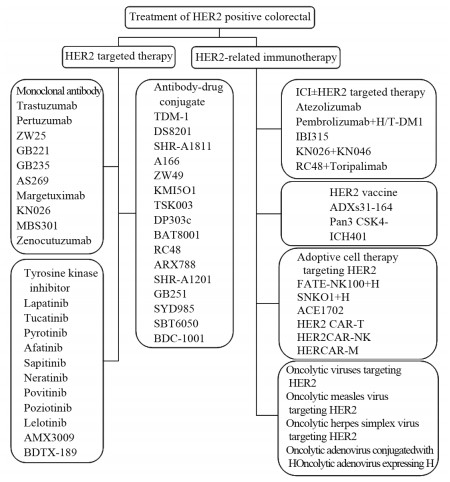
 下载:
下载: