Safety and Efficacy of Irreversible Electroporation Combined with Neoadjuvant Chemotherapy for Locally Advanced Pancreatic Cancer: A Meta-analysis
-
摘要:目的
评估不可逆电穿孔术(IRE)联合新辅助化疗治疗局部进展期胰腺癌患者的安全性和有效性。
方法检索PubMed、Embase、Cochrane Library、Web of Science、中国生物医学文献数据库、知网、万方和维普等数据库从建库至2022年3月发表的相关文献。采用RevMan5.4软件进行Meta分析。
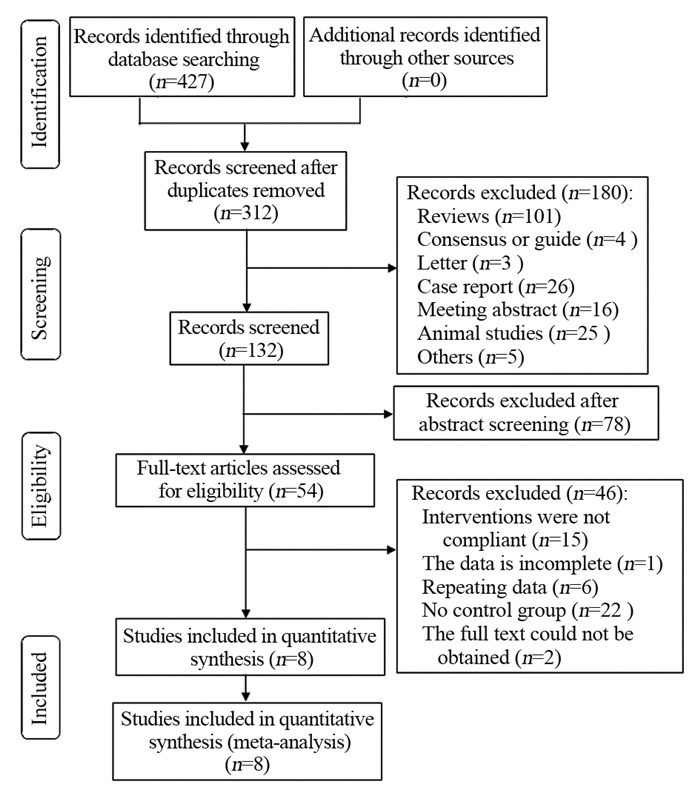
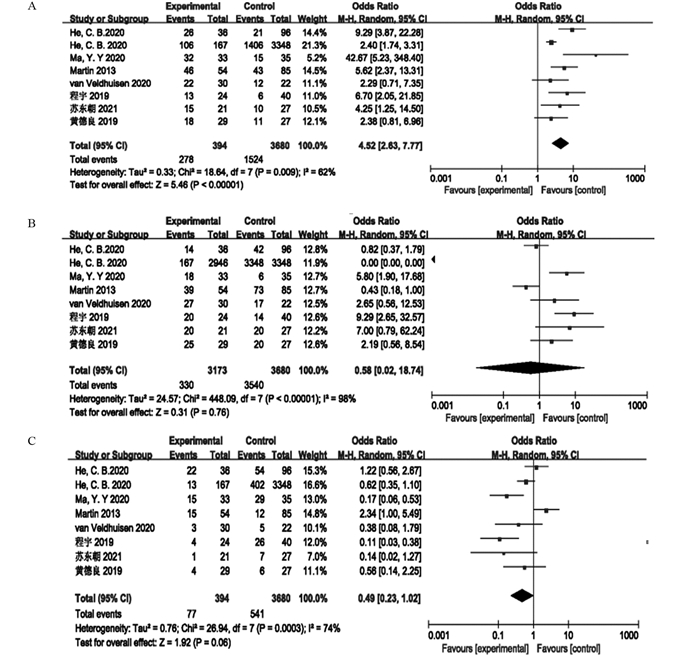
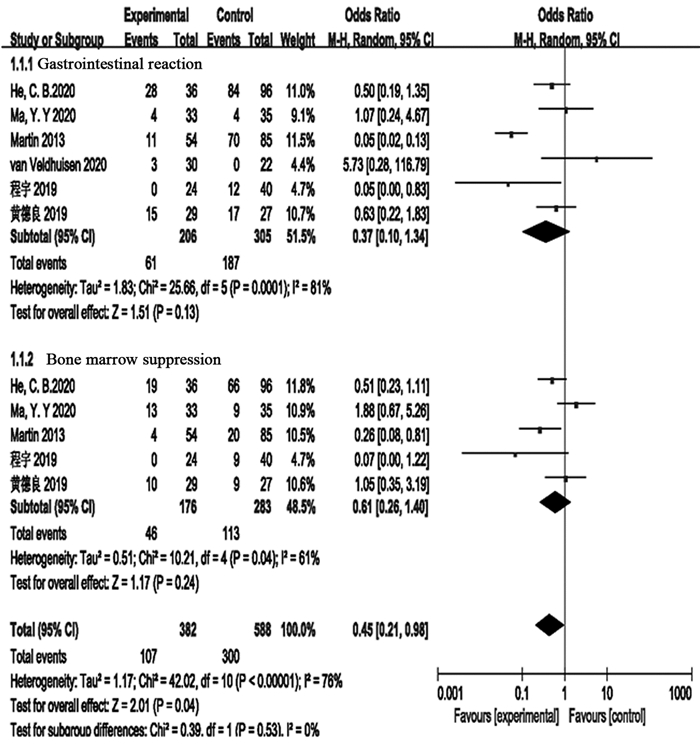
结果最终纳入8项研究,其中随机对照试验1项、回顾性研究4项和前瞻性研究3项。共3 970例局部进展期胰腺癌患者,其中联合组344例,单纯化疗组3 626例。Meta分析显示联合组患者的总生存期明显高于单纯化疗组(OR=4.52, 95%CI: 2.63~7.77, P < 0.00001)。但是联合组和单纯化疗组患者的疾病控制率差异无统计学意义(OR=0.58, 95%CI: 0.02~18.74, P=0.76),疾病进展两组差异无统计学意义(OR=0.49, 95%CI: 0.23~1.02, P=0.06)。新辅助化疗联合IRE对患者治疗期间胃肠道反应(OR=0.37, 95%CI: 0.10~1.34, P=0.13)和骨髓抑制(OR=0.61, 95%CI: 0.26~1.40, P=0.24)等不良反应的发生率并无明显影响。
结论不可逆电穿孔术联合新辅助化疗可以显著改善局部进展期胰腺癌患者的预后,明显提高患者的总生存时间。
Abstract:ObjectiveTo evaluate the safety and efficacy of irreversible electroporation (IRE) combined with neoadjuvant chemotherapy in patients with locally advanced pancreatic cancer.
MethodsWe searched PubMed, Embase, Cochrane Library, Web of Science, China Biomedical Literature Database, CNKI, Wanfang, and VIP databases for articles dated from the establishment of each database to March 2022. Meta-analysis was performed using RevMan5.4 software.
ResultsA total of 3970 patients with locally advanced pancreatic cancer were enrolled in eight studies, including one randomized controlled trial, four retrospective studies, and three prospective studies. The patients were divided into the combined therapy group with 344 patients and the chemotherapy-only group with 3626 patients. Meta-analysis showed that the overall survival of patients in the combined therapy group was significantly higher than that in the chemotherapy-only group (OR=4.52; 95%CI: 2.63-7.77; P < 0.00001). However, no significant difference existed in the disease control rate between the combined therapy group and the chemotherapy-only group (OR=0.58; 95%CI: 0.02-18.74; P=0.76). Moreover, no significant difference existed in the disease progression between the two groups (OR=0.49; 95%CI: 0.23-1.02; P=0.06). The combination of neoadjuvant chemotherapy and IRE had no significant effect on the incidence of adverse reactions of gastrointestinal reaction (OR=0.37; 95%CI: 0.10-1.34; P=0.13) and bone marrow suppression (OR=0.61; 95%CI: 0.26-1.40; P=0.24).
ConclusionIRE combined with neoadjuvant chemotherapy can remarkably improve the prognosis of patients with locally advanced pancreatic cancer, and significantly prolong the overall survival.
-
Key words:
- Irreversible electroporation /
- Neoadjuvant chemotherapy /
- Pancreatic cancer /
- Safety /
- Efficacy /
- Meta-analysis
-
0 引言
前列腺癌(prostate adenocarcinoma, PCa)是老年男性最常见的恶性肿瘤之一[1],位居全球恶性肿瘤死亡率第8位。2019年国家癌症中心发布的数据显示我国PCa发病率、死亡率分别居男性恶性肿瘤的第6和第10位,严重威胁老年男性健康[2-4]。目前,对于PCa的治疗方法有根治性切除、放射治疗、冷冻治疗及内分泌治疗等。PCa内分泌治疗通过减少或去除体内雄激素的产生或抑制体内雄激素活性,减轻肿瘤负荷、消灭可能存在的微转移。近年来,随着新型靶向雄激素受体途径药物的出现,已证实内分泌治疗不仅可以实现肿瘤降级、降期的目的,为局部肿瘤晚期患者争取到手术根治的机会,而且可以降低转移率、手术切缘阳性率及复发率,并可改善患者的生活质量、延长生存期[5]。
目前,监测PCa内分泌治疗疗效的最常用指标是前列腺特异性抗原(prostate specific antigen, PSA),但因PSA缺乏特异性而无法全面评估。前列腺影像报告和数据系统(prostate imaging reporting and data system version, PI-RADS)指导委员会于2019年发布了最新版即2.1版。自发布以来,PI-RADS v2.1运用于临床工作中,其临床价值得到了充分证实[6-7],提高了多参数磁共振成像(multi-parametric MRI, mpMRI)对有临床意义前列腺癌(clinically significant PCa, csPCa)的检出率,但基于PI-RADS v2.1利用磁共振的扩散加权成像(diffusion weighted imaging, DWI)技术监测PCa内分泌治疗疗效的研究较少。
本研究旨在基于PI-RADS v2.1探讨DWI定量参数即表观弥散系数(apparent diffusion coefficient, ADC)值在内分泌治疗前后的变化及其与血清PSA的相关性,评估ADC值在预测PCa内分泌治疗疗效中的价值。
1 资料与方法
1.1 临床资料
回顾性分析2020年5月—2022年4月在华中科技大学同济医学院附属同济医院行前列腺mpMRI检查、经穿刺病理证实为PCa并行内分泌治疗患者的临床资料(包括穿刺病理结果、治疗前后睾酮及PSA水平等)。
纳入标准:(1)所有病例均行前列腺mpMRI检查,经过穿刺活检后病理证实为PCa。mpMRI检查序列完整,符合PI-RADS v2.1技术规范;(2)采用内分泌药物治疗方案不间断,且未行其他方案治疗。(3)治疗后6个月复查了mpMRI、PSA。排除标准:(1)图像存在伪影,图像质量不符合要求,各序列图像欠完整,无法行PI-RADS v2.1评分;(2)患者有前列腺手术史或接受过放疗、冷冻消融等其他治疗;(3)有内分泌治疗禁忌证。
1.2 MRI扫描方法
取仰卧位,扫描中心位于耻骨联合上方约2.0 cm,前列腺mpMRI检查采用德国西门子公司3.0T磁共振(Siemens Skyra)扫描仪,使用18通道腹部相控阵线圈。mpMRI扫描序列包括轴位/矢状位/冠状位T2WI、轴位T1WI、DWI和DCE-MRI以及可变翻转角三维快速自旋回波(3D-SPACE)序列,层间距为0 mm。扫描序列包括:(1)T2WI扫描序列为TSE,其扫描参数为:TR=6 750 ms,TE=104 ms,FOV 180 mm×l80 mm,激励次数2次。(2)DWI扫描序列为SS-EPI,扫描参数为:TR=4 500 ms,TE=85 ms,FOV 215 mm×172 mm,矩阵72×90。扩散敏感系数(b)值分别为0、50、200、400、800、1 000、1 500、2 000 s/mm2。扫描完成后, 该MRI扫描仪自带后处理软件自动生成ADC图;(3)DCE-MRI采用梯度回波容积内插法(volumetric interpolated breath-hold examination, VIBE)序列TR=3.40 ms,TE=1.23 ms,FOV 260 mm×260 mm。对比剂为马根维显(Gd-DTPA),注射速率2 ml/s,剂量0.2 mmol/kg;(4)可变翻转角三维快速自旋回波(3D-SPACE)序列,采用三维横断位扫描。TR=1 500 ms,TE=100 ms,矩阵233×256,激励次数为1次。
1.3 MRI图像处理及数据测量
将所有扫描图像传至西门子磁共振自带工作站进行分析。由前列腺MRI诊断经验丰富的影像科医师根据病理阳性穿刺部位,在不知晓Gleason评分的情况下,参照T2WI低信号区、DWI高信号区域,在ADC图上绘制感兴趣区(region of interest, ROI)并测量ADC值。癌区的ROI放置原则以ADC图上含病灶层面信号相对最低的区域为准,尽量避开尿道、钙化、囊边及坏死区,每个病灶测量3次,取平均值。对于内分泌治疗后癌灶不明显的病变,则以治疗前mpMRI图像的相应位置为参照进行测量。在癌灶对侧测量正常的前列腺外周带(peripheral zone, PZ)和移行带(transition zone, TZ)ADC值,当癌灶范围过大并且不存在正常前列腺组织时不予测量。另将所采集mpMRI图像传入图像存储与传输系统(picture archiving and communication system, PACS)。由两名有前列腺MRI诊断工作经验的高年资医师采用双盲法独立对患者的mpMRI图像进行观察分析并参照PI-RADS v2.1评分标准进行评分,意见不一致由双方共同协商达成一致意见。将PI-RADS v2.1评分4~5分定义为前列腺癌灶。分别于T2WI序列测量前列腺的左右径、前后径和上下径的最大值,并计算前列腺体积(prostatic volume, PV),PV=π/6×左右径×前后径×上下径。
1.4 观察指标
根据修订版的实体瘤疗效评价标准(modified response evaluation criteria in solid tumors, mRECIST),结合T2WI、DWI及DCE-MRI图像,定位并测量癌灶的体积,基于3D-SPACE序列采用鼠标人工绘出癌灶的轮廓由计算机自动算出癌灶体积。对于内分泌治疗后的病灶则测量“存活肿瘤”的体积。计算治疗前和内分泌治疗6个月后的肿瘤消退率(regression rate, RR),RR=(肿瘤治疗前最大体积-肿瘤治疗后最大体积)/肿瘤治疗前最大体积×100%。由两名有前列腺MRI诊断工作经验的高年资医师采用盲法对两次MRI图像进行RR评估,意见不一致时由双方共同协商达成一致意见。本研究中,依据mpMRI表现和PSA结果联合判定疗效。治疗有效定义为肿瘤体积显著缩小(RR≥30%)、PSA值处于正常值范围(0~4 ng/ml)及睾酮值降至50 ng/dl(1.7 mmol)以下,余则视为无效。
患者内分泌治疗后的ADC变化值=治疗后ADC值-治疗前ADC值,ADC变化率=(治疗后ADC值-治疗前ADC值)/治疗前ADC值。比较两组间ADC变化值、ADC变化率。
1.5 统计学方法
采用SPSS21.0统计软件进行统计学分析。计量资料以均数±标准差(x±s)表示,计数资料以率或百分比表示。两位观察者PI-RADS v2.1评分的一致性分析采用Kappa检验。内分泌治疗前后癌灶、PZ、TZ区域ADC值变化采用配对样本t检验。治疗前后癌灶区域ADC值与PSA值的相关性采用Spearman相关性检验。绘制受试者工作特征(ROC)曲线分析ADC变化值及变化率预测PCa内分泌治疗反应程度的敏感度、特异性、阳性预测值(positive predictive value, PPV)、阴性预测值(negative predict value, NPV)及曲线下面积(area under curve, AUC)。P<0.05为差异有统计学意义。
2 结果
本研究共纳入患者57例,有效组45例、无效组12例。其中PCa位于PZ有22例,TZ有16例,PZ和TZ均受累19例。所有癌灶于内分泌治疗前T2WI呈低信号、DWI呈高信号、ADC图呈低信号,见图 1。
![]() 图 1 前列腺腺癌患者内分泌治疗前后MRI结果(箭头所示为癌灶)Figure 1 MRI results of prostate adenocarcinoma patients before and after endocrine therapy (the arrow shows the cancer lesion)A 72-year-old male patient with pathologically proven prostate cancer. A-C: transverse T2WI, DWI, ADC images before endocrine therapy show hyperintensity on DWI and hypointensity on ADC; D-F: The corresponding axial T2WI, DWI, and ADC images six months after endocrine therapy show a reduced lesion range, and a decreased signal intensity on the DWI. ADC: apparent diffusion coefficient.
图 1 前列腺腺癌患者内分泌治疗前后MRI结果(箭头所示为癌灶)Figure 1 MRI results of prostate adenocarcinoma patients before and after endocrine therapy (the arrow shows the cancer lesion)A 72-year-old male patient with pathologically proven prostate cancer. A-C: transverse T2WI, DWI, ADC images before endocrine therapy show hyperintensity on DWI and hypointensity on ADC; D-F: The corresponding axial T2WI, DWI, and ADC images six months after endocrine therapy show a reduced lesion range, and a decreased signal intensity on the DWI. ADC: apparent diffusion coefficient.两名医师使用PI-RADS v2.1评分时,二者评分一致性为优秀,Kappa值为0.81。57例患者内分泌治疗前后平均PV分别为(65.56±32.31)cm3和(30.35±13.58)cm3,差异有统计学意义(P<0.001)。治疗前后肿瘤平均体积分别为(3705±3153)mm3和(1128±1136)mm3,差异有统计学意义(P<0.001)。内分泌治疗前后ADC值比较差异均有统计学意义(P<0.05),见表 1。
表 1 癌灶、非癌灶区域内分泌治疗前后ADC值Table 1 ADC values before and after endocrine therapy in cancerous and noncancerous areas
有效组治疗后ADC值显著升高(P<0.001),无效组治疗后ADC值无显著改变(P=0.714),见表 2;有效组、无效组ADC变化值差异和ADC变化率差异均有统计学意义(均P<0.001),见表 3。
表 2 有效组和无效组癌灶区域内分泌治疗前后ADC值Table 2 ADC values before and after endocrine therapy in the responder and non-responder groups 表 3 有效组和无效组ADC变化值和变化率比较Table 3 Comparison of ADC change and ADC ratio in responder and non-responder groups
表 3 有效组和无效组ADC变化值和变化率比较Table 3 Comparison of ADC change and ADC ratio in responder and non-responder groups
ROC曲线分析结果显示:ADC变化值取0.165作为最佳阈值时,对应的敏感度、特异性、PPV、NPV、AUC分别为88.89%、100%、100%、70.59%、0.974,见图 2A。ADC变化率取16.827%作为最佳阈值时,对应的敏感度、特异性、PPV、NPV、AUC分别为91.11%、100%、100%、75.00%、0.980,见图 2B。
治疗前后睾酮值分别为(428.96±76.3)ng/dl和(5.29±2.56)ng/dl。治疗前后PSA值分别为(156.50±558.90)ng/ml和(9.21±35.52)ng/ml,差异有统计学意义(P<0.001)。PCa治疗前后PSA与ADC值呈负相关(r=-0.787, P<0.001)。
3 结论
PCa是一种雄激素依赖性肿瘤,80%~90%的患者对激素治疗敏感,故目前内分泌治疗作为一种常用手段应用于PCa根治性切除术前或术后、根治性放疗后及放疗前的辅助性治疗[8]。PCa内分泌治疗通过抑制雄激素分泌和阻断雄激素受体,抑制PCa细胞生长,从而使癌细胞凋亡达到控制PCa进展的目的[9]。内分泌治疗根据作用机制和靶点的不同分为去势治疗和抗雄治疗。抗雄治疗也就是应用抗雄激素药物竞争性地阻断雄激素与前列腺细胞上雄激素受体的结合,本研究中应用抗雄激素内分泌治疗药物即为抗雄治疗。对于PCa患者,内分泌治疗可以达到减瘤、减期的效果,降低其生化复发率及外科切缘阳性率,同时有利于控制残余病灶、微小转移灶、残余阳性淋巴结,从而提高患者生存率[10]。
目前,PCa内分泌治疗后疗效的评估指标主要是PSA,但单用PSA具有局限性和片面性,约20%的PCa患者内分泌治疗后虽PSA<4.0 ng/ml,但仍出现疾病进展[11]。mpMRI既在PCa的检测、定位和分期中起着重要的作用,亦可用于PCa疗效监测,评估PCa的肿瘤形态学改变[12]。随着MRI功能成像的迅速发展,其逐渐运用于临床反映PCa的功能学改变,如功能成像序列:DWI[13]、IVIM[14]、DCE-MRI[15]。DWI不仅能够反映组织中水分子布朗运动特征,而且是目前唯一在活体状态下能无创定量评估水分子扩散运动的成像方法。ADC值反映水分子在组织中扩散的程度, 扩散快的组织ADC值高,反之,ADC值则较低。Kim等[16]利用mpMRI对48例PCa患者内分泌治疗前后癌灶区域和非癌灶区域的ADC值进行测定,结果显示癌灶区域内分泌治疗后ADC值升高,非癌灶区域的ADC值则明显降低。既往研究利用DWI监测PCa内分泌治疗疗效,大多数研究结果显示PCa癌灶减小,前列腺萎缩,癌灶区域ADC值明显升高,PSA值则明显降低,本研究结果与其相近。
本研究显示内分泌治疗后前列腺非癌灶区域PZ、TZ的ADC值减低。既往研究报道PCa内分泌治疗后行前列腺根治术获得的手术标本中的组织病理显示有退化的前列腺良性组织和残留癌灶[17-18]。内分泌治疗后非癌灶区域ADC值降低[19],究其原因有两个:(1)内分泌治疗后非癌灶区域前列腺组织的变化包括腺体萎缩、纤维化、基底细胞增生和基质细胞过度增生,导致腺体基质组织和腺体体积均减少[11, 20-21];(2)细胞外液减少,自由水运动受限[22]。
本研究结果显示PCa内分泌治疗后ADC值的变化与PSA呈负相关;治疗有效组PCa癌灶的ADC变化值、变化率均明显大于无效组,以ADC变化值、变化率预测PCa内分泌治疗反应程度的AUC分别为0.974、0.980,提示ADC变化值、变化率可作为PCa内分泌治疗疗效的有效监测指标。
自PI-RADS v2.1发布以来,基于mpMRI的PI-RADS v2.1在csPCa的定位、定性诊断中得到日益广泛的应用,其可行性、有效性及实用性在临床实践中得到了放射学界和泌尿外科领域的肯定[23]。本研究将PI-RADS v2.1评分为4~5分定义为PCa癌灶,在ADC图中测量其ADC值。国内外学者[24-26]对PI-RADS v2.1和PI-RADS v2.0在前列腺移行带PCa中的诊断价值进行了对比,发现PI-RADS v2.1一致性更佳,诊断效能不低于甚至略高于PI-RADS v2.0。本研究基于PI-RADS v2.1对感兴趣区癌灶定位和测量的一致性更高,可避免漏诊。PI-RADS v2.1版本[27]首次详细说明了PV的测量方法,在T2 WI序列图像上测量PV,于轴位测量前列腺最大横径,于正中矢状位测量最大前后径、最大长径[28]。本研究癌灶体积是基于3D-SPACE进行轮廓勾画而通过计算机自动算出,其提供了快速高分辨率的三维容积快速自旋回波对比度成像,相较于仅测量肿瘤最大径更加准确[29]。
本研究的局限性:(1)样本量较少,为单中心回顾性分析,且内分泌治疗术后MRI评估时间节点较单一(术后6个月),可能会对统计结果产生偏倚;(2)本研究对PCa内分泌治疗术后疗效评估主要是根据mRECIST标准及PSA,而未与根治术后的病理结果对照,在未来的研究中尚需进一步前瞻性研究ADC值的改变,并与切除术后的病理学反应进行对照。
综上所述,DWI可作为PCa患者内分泌治疗疗效的定量评估手段。内分泌治疗后癌灶区域ADC值升高, 其与PSA水平均呈负相关。ADC变化值及ADC变化率可有效监测PCa内分泌治疗疗效,以便于更好指导临床决策及治疗。
Competing interests: The authors declare that they have no competing interests.作者贡献:赵宝银:数据分析、文章撰写梁昭君、张丽霞:文献检索陈顺、贾栋、仵朝晖:核对数据李斌、王俊科、马俊:提取数据、资料整理于晓辉:课题指导、数据核对、指导文章 -
图 4 不可逆电穿孔术联合新辅助化疗组对比单纯新辅助化疗组患者发生胃肠道反应和骨髓抑制等不良反应的Meta分析
Figure 4 Meta-analysis results of adverse reactions, such as gastrointestinal reactions and bone marrow suppression, in patients who received irreversible electroporation combined with neoadjuvant chemotherapy compared with patients who received neoadjuvant chemotherapy alone
表 1 纳入Meta分析的8篇文献基本特征
Table 1 Basic characteristics of the eight articles included in the meta-analysis

-
[1] Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022[J]. CA Cancer J Clin, 2022, 72(1): 7-33. doi: 10.3322/caac.21708
[2] 蔡洁, 陈宏达, 卢明, 等. 2005—2015年中国胰腺癌发病与死亡趋势分析[J]. 中华流行病学杂志, 2021, 42(5): 794-800. doi: 10.3760/cma.j.cn112338-20201115-01328 Cai J, Chen HD, Lu M, et al. Trend analysis on morbidity and mortality of pancreatic cancer in China, 2005-2015[J]. Zhonghua Liu Xing Bing Xue Za Zhi, 2021, 42(5): 794-800. doi: 10.3760/cma.j.cn112338-20201115-01328
[3] Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants[J]. Chin Med J (Engl), 2022, 135(5): 584-590. doi: 10.1097/CM9.0000000000002108
[4] Karunakaran M, Barreto SG. Surgery for pancreatic cancer: current controversies and challenges[J]. Future Oncol, 2021, 17(36): 5135-5162. doi: 10.2217/fon-2021-0533
[5] Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology[J]. J Natl Compr Canc Netw, 2021, 19(4): 439-457. doi: 10.6004/jnccn.2021.0017
[6] Granata V, Grassi R, Fusco R, et al. Local ablation of pancreatic tumors: State of the art and future perspectives[J]. World J Gastroenterol, 2021, 27(23): 3413-3428. doi: 10.3748/wjg.v27.i23.3413
[7] Zhang N, Li Z, Han X, et al. Irreversible Electroporation: An Emerging Immunomodulatory Therapy on Solid Tumors[J]. Front Immunol, 2022, 12: 811726. doi: 10.3389/fimmu.2021.811726
[8] Geboers B, Scheffer HJ, Graybill PM, et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy[J]. Radiology, 2020, 295(2): 254-272. doi: 10.1148/radiol.2020192190
[9] Savović J, Weeks L, Sterne JA, et al. Evaluation of the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation[J]. Syst Rev, 2014, 3(4): 37.
[10] He C, Wang J, Zhang Y, et al. Irreversible electroporation after induction chemotherapy versus chemotherapy alone for patients with locally advanced pancreatic cancer: A propensity score matching analysis[J]. Pancreatology, 2020, 20(3): 477-484. doi: 10.1016/j.pan.2020.02.009
[11] 程宇, 王国经, 闫涛, 等. 纳米刀消融术联合吉西他滨治疗局部进展期胰腺癌的疗效分析[J]. 中华损伤与修复杂志, 2019, 14(3): 174-181. doi: 10.3877/cma.j.issn.1673-9450.2019.03.003 Cheng Y, Wang GJ, Yan T, et al. Treatment of advanced pancreatic cancer by nanoknife ablation combined with gemcitabine[J]. Zhonghua Sun Shang Yu Xiu Fu Za Zhi, 2019, 14(3): 174-181. doi: 10.3877/cma.j.issn.1673-9450.2019.03.003
[12] 黄德良, 胡莹, 万友华, 等. 纳米刀联合吉西他滨与吉西他滨同步放化疗治疗局部晚期胰腺癌对比评价[J]. 实用医学杂志, 2019, 35(12): 1948-1952. doi: 10.3969/j.issn.1006-5725.2019.12.019 Huang DL, Hu Y, Wan YH, et al. Comparative evaluation of irreversible electroporation combined with gemcitabine and gemcitabine in con-current radiotherapy and chemoradiotherapy for locally advanced pancreatic cancer[J]. Shi Yong Yi Xue Za Zhi, 2019, 35(12): 1948-1952. doi: 10.3969/j.issn.1006-5725.2019.12.019
[13] 苏东朝. 纳米刀消融联合化疗治疗局部进展期胰腺癌的应用研究[D]. 郑州: 郑州大学, 2021. Su DC. Application of nano-knife ablation combined with chemotherapy in the treatment of locally advanced pancreatic cancer[D]. Zhengzhou: Zhengzhou University, 2021.
[14] Ma YY, Leng Y, Xing YL, et al. Gemcitabine plus concurrent irreversible electroporation vs. gemcitabine alone for locally advanced pancreatic cancer[J]. World J Clin Cases, 2020, 8(22): 5564-5575. doi: 10.12998/wjcc.v8.i22.5564
[15] He C, Huang X, Zhang Y, et al. Comparison of Survival Between Irreversible Electroporation Followed by Chemotherapy and Chemotherapy Alone for Locally Advanced Pancreatic Cancer[J]. Front Oncol, 2020, 10(1): 6.
[16] van Veldhuisen E, Vroomen LG, Ruarus AH, et al. Value of CT-Guided Percutaneous Irreversible Electroporation Added to FOLFIRINOX Chemotherapy in Locally Advanced Pancreatic Cancer: A Post Hoc Comparison[J]. J Vasc Interv Radiol, 2020, 31(10): 1600-1608. doi: 10.1016/j.jvir.2020.02.024
[17] Martin RC 2nd, McFarland K, Ellis S, et al. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival[J]. Ann Surg Oncol, 2013, 20(3): S443-S449.
[18] Dalgleish AG, Stebbing J, Adamson DJ, et al. Randomised, open-label, phase Ⅱ study of gemcitabine with and without IMM-101 for advanced pancreatic cancer[J]. Br J Cancer, 2016, 115(7): 789-796. doi: 10.1038/bjc.2016.271
[19] Wang M, Zhu P, Chen Z, et al. Conversion therapy, palliative chemotherapy and surgery, which of these is the best treatment for locally advanced and advanced pancreatic cancer?[J]. Anticancer Drugs, 2022, 33(1): e686-e691. doi: 10.1097/CAD.0000000000001235
[20] Yoo C, Shin SH, Kim KP, et al. Clinical Outcomes of Conversion Surgery after Neoadjuvant Chemotherapy in Patients with Borderline Resectable and Locally Advanced Unresectable Pancreatic Cancer: A Single-Center, Retrospective Analysis[J]. Cancers (Basel), 2019, 11(3): 278. doi: 10.3390/cancers11030278
[21] 中华医学会外科学分会胰腺外科学组. 中国胰腺癌诊治指南(2021)[J]. 中华外科杂志, 2021, 59(7): 561-577. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWK202107002.htm Pancreatic Surgery Group, Surgical Society of Chinese Medical Association. Guidelines for diagnosis and treatment of pancreatic cancer in China(2021)[J]. Zhonghua Wai Ke Za Zhi, 2021, 59(7): 561-577. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWK202107002.htm
[22] Huang Y, Yan X, Ren T, et al. The safety and efficacy of chemotherapy combined with immunotherapy for pancreatic cancer: A meta-analysis[J]. Medicine (Baltimore), 2021, 100(29): e26673. doi: 10.1097/MD.0000000000026673
[23] O Kane GM, Knox JJ. Locally advanced pancreatic cancer: An emerging entity[J]. Curr Probl Cancer, 2018, 42(1): 12-25.
[24] Timmer FEF, Geboers B, Nieuwenhuizen S, et al. Locally Advanced Pancreatic Cancer: Percutaneous Management Using Ablation, Brachytherapy, Intra-arterial Chemotherapy, and Intra-tumoral Immunotherapy[J]. Curr Oncol Rep, 2021, 23(6): 68.
[25] 中国医药教育协会介入微创治疗专业委员会. 影像学引导胰腺癌不可逆电穿孔消融治疗专家共识2018版[J]. 中华医学杂志, 2018, 98(39): 3148-3152. https://www.cnki.com.cn/Article/CJFDTOTAL-LCGD201902014.htm Interventional Minimally Invasive Therapy Professional Committee of Chinese Medical Education Association. Treatment of pancreatic cancer by radio-guided irreversible electroporation[J]. Zhonghua Yi Xue Za Zhi, 2018, 98(39): 3148-3152. https://www.cnki.com.cn/Article/CJFDTOTAL-LCGD201902014.htm
[26] Oikonomou D, Karamouzis MV, Moris D, et al. Irreversible Electroporation (IRE) Combined With Chemotherapy Increases Survival in Locally Advanced Pancreatic Cancer (LAPC)[J]. Am J Clin Oncol, 2021, 44(7): 325-330.
[27] He C, Sun S, Huang X, et al. Survival Comparison of Neoadjuvant Chemotherapy Followed by Irreversible Electroporation Versus Conversional Resection for Locally Advanced Pancreatic Cancer[J]. Front Oncol, 2021, 10(2): 622318.
[28] Zhang N, Li Z, Han X, et al. Irreversible Electroporation: An Emerging Immunomodulatory Therapy on Solid Tumors[J]. Front Immunol, 2022, 12: 811726.
-
期刊类型引用(1)
1. 朱翩, 杨凡, 周浪. 多模态MRI在前列腺癌疗效评价中的应用. 中国CT和MRI杂志. 2025(07)  百度学术
百度学术
其他类型引用(1)




 下载:
下载: