Effect of Lymph Node Metastasis on Prognosis of Small Cell Lung Cancer with M1a Disease: A Study Based on SEER database
-
摘要:目的
探讨淋巴结转移对M1a期小细胞肺癌(SCLC)患者生存的影响。
方法回顾性分析SEER数据库中2004—2015年7027例M1a期SCLC患者病例资料,采用Kaplan-Meier法及Log rank检验比较不同N分期亚组患者总体生存率(OS),Cox比例风险模型评估N分期是否为影响预后的独立危险因素。
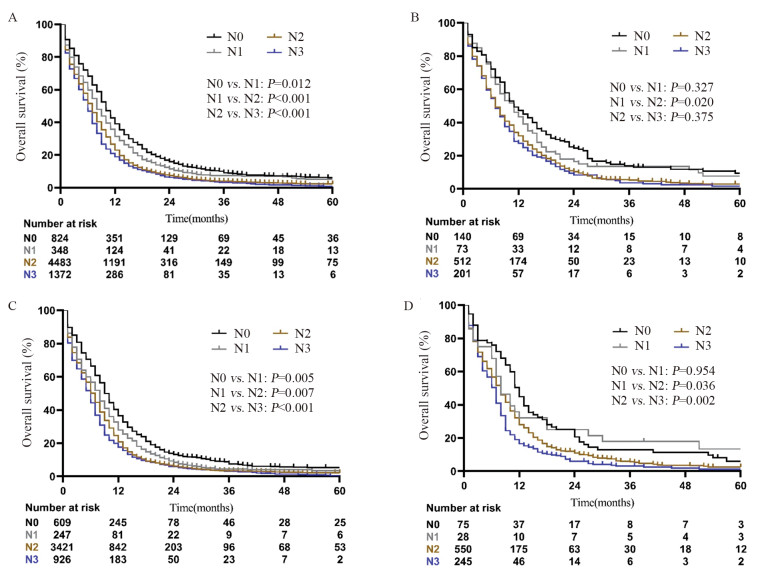
结果全组中位OS为7月。在所有M1a患者中,无淋巴结转移(N0)患者的OS最好,其次是N1患者,而N2和N3患者的预后最差(P < 0.001)。亚组分析也显示对侧肺结节组、恶性胸腔积液组和恶性心包积液组也存在随N分期升高OS递减的趋势。多因素分析显示淋巴结转移是M1a期SCLC患者的独立预后因素,在M1a不同亚群中也观察到相同的结果。
结论淋巴结转移能够影响M1a期SCLC患者生存,增加额外的预后信息,建议在下一版本的TNM分期系统中进一步完善N描述符。
Abstract:ObjectiveTo investigate the impact of lymph node metastasis on the survival of SCLC patients with M1a disease.
MethodsWe retrospectively analyzed the medical records of 7027 SCLC patients with M1a disease from 2004 to 2015 in SEER database. The Kaplan-Meier method and log-rank test were used to estimate the OS in all N stage subgroups. Cox proportional hazard model was used to assess whether N stage was an independent risk factor for prognosis.
ResultsThe median OS of all patients was 7 months. Among all M1a patients, the patients without lymph node involvement (N0) had the best OS, followed by N1 stage patients; N2 and N3 stage patients had the worst OS (P < 0.001). Similarly, this trend was observed when M1a disease was subdivided into contralateral pulmonary nodules, malignant pleural effusion and malignant pericardial effusion. Multivariate analysis showed that lymph node metastasis was an independent prognostic factor for SCLC patients with M1a disease, and this result was also noticed in all subgroups of M1a disease.
ConclusionLymph node metastasis may affect the survival of SCLC patients with M1a disease, adding prognostic information. And it is recommended to further improve the N descriptor in the next version of TNM staging system.
-
Key words:
- Small cell lung cancer /
- N stage /
- M1a stage /
- SEER database /
- Prognosis
-
0 引言
胆道恶性梗阻多由胆管癌、胆管周围肿瘤所致,多数患者就诊时已失去手术根治机会[1-2]。光动力治疗(photodynamic therapy, PDT)是胆道恶性梗阻患者的重要姑息性治疗方式之一,联合胆道支架植入能够延长胆道通畅时间、降低支架再植入率[3-6]、提高患者生活质量并得到生存获益[7]。但对于复杂肝门胆道梗阻,如Bismuth Ⅲ~Ⅳ型,则资料较少。本文回顾性分析我中心接受经皮光动力治疗的胆管恶性梗阻患者治疗过程,观察肝功能变化、胆道通畅时间、术后1月内并发症,总结术后并发症处置措施,为其更广泛的临床应用提供参考。
1 资料与方法
1.1 一般资料
1.1.1 研究对象
收集2020年9月—2022年3月在我中心接受经皮光动力治疗的胆道恶性梗阻患者(胆道细胞刷检、穿刺病理或手术病理确诊,包括胆管癌及胆管周围恶性肿瘤侵犯)临床资料。入组标准:无法外科手术根治的晚期胆管癌患者、合并胆道局限性狭窄、通过术前引流可有效减黄、肝功能Child-Pugh A-B级、ECOG评分0~1分。排除标准:合并急性心脑血管疾病;肝肾功能衰竭;重症感染;生命体征不稳定;对光敏剂过敏;无法保证术后避光者。本研究经本院伦理委员会批准。
本研究共入组胆道恶性梗阻患者19例,其中男12例,女7例,年龄34~70岁,平均年龄64.0岁。Bismuth Ⅰ型梗阻1例,Bismuth Ⅲ型12例,Bismuth Ⅳ型6例。术前肝功能Child-Pugh A级7例,Child-Pugh B级12例。术前总胆红素水平为(74.4±62.2)mol/L,直接胆红素(40.3±38.8)mol/L。其中13例患者在光动力治疗前已行胆道支架植入,6例患者未放置支架。入组患者中15例术后联合化疗、靶向或免疫药物治疗(联合组),4例术后未联合全身药物治疗(光动力组),两组基线水平大致相同,联合组术前总胆红素水平较单纯光动力组高((88.4±72.2)vs.(43.4±25.9)mol/L)。入组患者术后存活时间均超过3月,术后随访资料完整。随访截至2022年7月,其中9例患者死亡。
1.1.2 材料与器械
石英光纤、光纤保护鞘及半导体激光光动力治疗仪(中国,国医华科医疗科技集团有限公司),塑料光纤(中国,桂林市兴达光电医疗器械有限公司),5Fr长血管鞘(日本,泰尔茂株式会社),造影导管、胆道引流管(美国,库克医疗贸易有限公司),数字减影机(DSA)Innova 4100-IQ(美国,通用电气公司)。
1.2 方法
1.2.1 术前准备
入组患者均术前完善血常规、尿便常规、肝肾功能、腹部增强CT和(或)核磁共振胰胆管造影(MRCP)。并已经皮肝胆道穿刺引流治疗,且胆道引流通畅。术前均已签署知情同意书。皮试阴性后,术前40~48 h静脉输注血卟啉注射液(2.5 mg/kg),病房暗光处理。
1.2.2 手术操作
患者取平卧位,术区常规消毒铺巾。DSA下经胆道引流管造影,观察引流区域,定位胆道狭窄位置:置入交换导丝拔除胆道引流管,导丝配合5Fr造影导管经狭窄段插管至远端,造影并测量胆道狭窄段长度,选取相应长度柱状光纤。拔除造影导管后沿导丝置入光纤保护鞘或血管鞘,透视下经鞘管插入光纤,使光纤工作段完全覆盖胆道狭窄段。应用630 nm激光局部照射,能量250~300 J/cm,功率600~1500 mV,过程中每10分钟停止1分钟。局部照射结束后,拔除光纤观察光纤完整后,沿原穿刺道置入胆道外引流管,造影确认外引流通畅。
1.2.3 术后随访
嘱患者避光1个月,术后1~2个月复查胆道造影,见图 1,视情况决定是否拔除胆道引流管。通过门诊定期监测肝功能,通过生化检查、胆道造影或腹部影像学检查(CT/MRCP)观察胆红素水平、胆道通畅情况。夹闭外引流管或拔除引流管后,胆红素未见进行性升高判定为胆道通畅。记录术后1、3、6和12月胆道通畅情况。
![]() 图 1 患者老年女性,胆道恶性肿瘤合并胆道狭窄,支架后再次出现皮肤巩膜黄染,PDT术前胆道造影见支架内充盈缺损(箭头处),对比剂无法顺利通过(左图);PDT治疗1月后再次造影见支架恢复通畅,对比剂可顺利通过并进入肠道内(右图)Figure 1 An elderly female patient with biliary malignancy complicated with biliary stricture, presented with yellow staining of skin and sclera after stenting. Biliary angiography before photodynamic therapy (PDT) showed a filling defect in the stent (arrow), and the contrast agent could not pass smoothly (left). One month after PDT, the stent was unobstructed again and the contrast agent could pass through and enter the intestine smoothly (right)
图 1 患者老年女性,胆道恶性肿瘤合并胆道狭窄,支架后再次出现皮肤巩膜黄染,PDT术前胆道造影见支架内充盈缺损(箭头处),对比剂无法顺利通过(左图);PDT治疗1月后再次造影见支架恢复通畅,对比剂可顺利通过并进入肠道内(右图)Figure 1 An elderly female patient with biliary malignancy complicated with biliary stricture, presented with yellow staining of skin and sclera after stenting. Biliary angiography before photodynamic therapy (PDT) showed a filling defect in the stent (arrow), and the contrast agent could not pass smoothly (left). One month after PDT, the stent was unobstructed again and the contrast agent could pass through and enter the intestine smoothly (right)1.3 统计学方法
应用SPSS20.0进行统计学分析。连续变量采用均值±标准差(x±s)表示,分类变量采用率表示,采用Kaplan-Meier统计学方法分析胆道通畅时间,P < 0.05为差异有统计学意义。
2 结果
2.1 手术情况及术后1月内并发症
19例患者均成功进行了光动力治疗,技术成功率100.0%。右侧入路光动力治疗13例,双侧入路光动力治疗6例。胆道狭窄治疗段长度平均(4.3±0.7)cm(3~5 cm)。术后19例患者(100%)均出现一过性发热,经胆道冲洗、通畅引流及抗感染治疗后症状缓解,平均发热时间6.1天(2~14天)。10例患者(52.6%)因避光不当出现光敏性皮炎,口服抗过敏药物或联合激素软膏后均得到缓解。6例患者(31.6%)出现术后腹部疼痛,均在48小时内缓解。入组患者均未出现气胸、腹腔大出血、胆道穿孔、胆漏、肝脓肿、急性胰腺炎等严重并发症。
2.2 术后1月胆红素及肝功能变化
术后肝功能Child-Pugh A级7例,Child-Pugh B级12例,同术前。总胆红素水平为(42.0±37.9)mol/L,较术前下降约(32.3±46.5)mol/L;直接胆红素(27.2±30.1)mol/L,较术前下降约(13.2±27.0)mol/L。
2.3 胆道通畅时间
本研究中胆道外引流管顺利拔除或长期夹闭,无胆红素进行性升高认定为胆道通畅。入组患者最长随访时间17.7月。术后1、3、6和12月胆道通畅率分别为100%、89.5%、72%和64%。胆道通畅时间约(6.9±0.8)月(95%CI: 5.2~8.7月),见图 2;Bismuth Ⅲ型胆道通畅时间(7.5±1.1)月,Bismuth Ⅳ型胆道通畅时间(6.1±1.3)月。单纯光动力治疗组胆道通畅时间约(3.3±0.7)月,联合治疗组患者胆道通畅时间约(7.9±0.9)月,两组差异有统计学意义(P=0.017),见图 3。
3 讨论
胆道恶性梗阻术后复发较为常见[8]。胆道狭窄、梗阻是晚期胆管恶性肿瘤常见情况,通过医疗手段恢复胆道通畅可有效保护肝功能并减低胆管炎发生风险。目前,支架植入是胆道恶性梗阻的常见治疗手段,但肿瘤生长、胆汁淤积和肉芽组织增生多可导致胆道再狭窄[2]。
光动力治疗作为一种局部姑息性治疗方法已广泛应用于临床中,可对口腔癌、膀胱癌、胃肠道肿瘤、食管癌、宫颈癌等多种肿瘤起到治疗作用[9-11]。其抗肿瘤机制主要包括产生活性氧直接导致肿瘤细胞的凋亡和坏死、破坏肿瘤相关血管系统、释放多种促炎因子激活免疫系统[12]。联合金属支架植入能够延长胆道恶性梗阻患者支架通畅时间、降低支架再植入率[3-6]、提高患者生活质量,并达到生存获益[7, 13-14]。
本研究回顾性分析我中心接受光动力治疗的胆管癌患者,观察肝功能变化、胆道通畅时间、术后1个月内并发症及生存时间,发现光动力治疗是安全、有效的。对于Bismuth-Corlette Ⅲ~Ⅳ型胆道梗阻患者,尽管光动力治疗围手术期出现发热等表现,经过胆道冲洗、通畅引流、静脉应用抗生素等治疗后,均得到了有效控制,未出现肝脓肿、肝功能衰竭等严重并发症。同时部分患者治疗后肿瘤出现坏死,原胆道二级分支梗阻情况得到明显改善。胆道通畅时间与既往研究报道基本一致[13, 15]。
进展期胆管癌患者的姑息性治疗主要包括化疗、靶向治疗、免疫治疗、光动力治疗及射频消融治疗等[16-20]。有研究表明,光动力治疗总体生存时间长于全身化疗,两者联合疗效更为显著[21]。同时,PDT可调动机体免疫反应,联合免疫治疗可有效抑制肿瘤生长[22-24]。本文对比单纯光动力治疗组及联合化疗、靶向和(或)免疫治疗组两组患者生存时间,发现联合组患者肿瘤控制更为满意,胆道通畅时间显著延长。这提示我们对无法手术切除的晚期胆道梗阻患者,积极采取光动力治疗联合全身治疗可进一步延长胆道通畅时间,降低支架再植入率。
本研究是一项单中心小样本回顾性研究,仅在入组的患者中发现光动力治疗联合全身治疗较单纯光动力治疗能够延长胆道通畅时间,结论尚需进一步扩大样本量来研究证实。同时联合治疗是否会带来更大的生存获益也尚需进一步研究验证。
综上所述,光动力治疗Bismuth Ⅲ~Ⅳ型胆道恶性梗阻是安全有效的,联合全身治疗可使胆道通畅时间显著延长。
Competing interests: The authors declare that they have no competing interests.作者贡献:阳昊:查阅文献、提取数据、统计分析、论文撰写梅同华:把握研究方向、论文指导及修改 -
表 1 7 027例M1a期小细胞肺癌患者临床特征(n(%))
Table 1 Clinical characteristics of 7027 SCLC patients with M1a disease (n(%))

表 2 影响M1a期SCLC患者OS的单因素和多因素分析
Table 2 Univariate and multivariate survival analyses of OS in SCLC patients with M1a Disease

表 3 Cox比例风险回归模型分析对侧肺结节、恶性胸腔积液和恶性心包积液患者的OS
Table 3 Cox proportional hazards regression model analysis of OS in patients with contralateral lung nodules, malignant pleural effusion and malignant pericardial effusion

-
[1] Rudin CM, Brambilla E, Faivre-Finn C, et al. Small-cell lung cancer[J]. Nat Rev Dis Primers, 2021, 7(1): 3. doi: 10.1038/s41572-020-00235-0
[2] Hiddinga BI, Raskin J, Janssens A, et al. Recent developments in the treatment of small cell lung cancer[J]. Eur Respir Rev, 2021, 30(161): 210079. doi: 10.1183/16000617.0079-2021
[3] Poirier JT, George J, Owonikoko TK, et al. New Approaches to SCLC Therapy: From the Laboratory to the Clinic[J]. J Thorac Oncol, 2020, 15(4): 520-540. doi: 10.1016/j.jtho.2020.01.016
[4] Nicholson AG, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer[J]. J Thorac Oncol, 2016, 11(3): 300-311. doi: 10.1016/j.jtho.2015.10.008
[5] Lin X, Xiao Z, Hu Y, et al. Combining 18F-FDG PET/CT and Serum Lactate Dehydrogenase for Prognostic Evaluation of Small Cell Lung Cancer[J]. Front Pharmacol, 2020, 11: 592768. doi: 10.3389/fphar.2020.592768
[6] Dingemans AC, Früh M, Ardizzoni A, et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2021, 32(7): 839-853. doi: 10.1016/j.annonc.2021.03.207
[7] 张文珏, 朱慧, 周宗玫, 等. TNM分期在局限期小细胞肺癌预后评估中的价值[J]. 中华肿瘤杂志, 2015, 37(12): 917-922. doi: 10.3760/cma.j.issn.0253-3766.2015.12.008 Zhang WJ, Zhu H, Zhou ZM, et al. Prognostic value of AJCC TNM Staging 7th edition in limited-stage small cell lung cancer: validation in 437 patients[J]. Zhonghua Zhong Liu Za Zhi, 2015, 37(12): 917-922. doi: 10.3760/cma.j.issn.0253-3766.2015.12.008
[8] Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer[J]. J Thorac Oncol, 2016, 11(1): 39-51. doi: 10.1016/j.jtho.2015.09.009
[9] Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer[J]. J Thorac Oncol, 2009, 4(9): 1049-1059. doi: 10.1097/JTO.0b013e3181b27799
[10] Dai C, Ren Y, Xie D, et al. Does Lymph Node Metastasis Have a Negative Prognostic Impact in Patients with NSCLC and M1a Disease?[J]. J Thorac Oncol, 2016, 11(10): 1745-1754. doi: 10.1016/j.jtho.2016.06.030
[11] Rami-Porta R, Asamura H, Travis WD, et al. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual[J]. CA Cancer J Clin, 2017, 67(2): 138-155. doi: 10.3322/caac.21390
[12] Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer[J]. J Thorac Oncol, 2015, 10(12): 1675-1684. doi: 10.1097/JTO.0000000000000678
[13] Iida T, Shiba M, Yoshino I, et al. Surgical Intervention for Non-Small-Cell Lung Cancer Patients with Pleural Carcinomatosis: Results From the Japanese Lung Cancer Registry in 2004[J]. J Thorac Oncol, 2015, 10(7): 1076-1082. doi: 10.1097/JTO.0000000000000554
[14] Ryu JS, Lim JH, Lee JM, et al. Minimal Pleural Effusion in Small Cell Lung Cancer: Proportion, Mechanisms, and Prognostic Effect[J]. Radiology, 2016, 278(2): 593-600. doi: 10.1148/radiol.2015142388
[15] Kato R, Hayashi H, Chiba Y, et al. Prognostic Impact of Minimal Pericardial Effusion in Patients With Advanced Non-small-cell Lung Cancer[J]. Clin Lung Cancer, 2017, 18(6): e449-e455. doi: 10.1016/j.cllc.2017.05.011
[16] Morris ZS, Cannon DM, Morris BA, et al. Impact of a Contralateral Tumor Nodule on Survival in Non-Small-Cell Lung Cancer[J]. J Thorac Oncol, 2015, 10(11): 1608-1615. doi: 10.1097/JTO.0000000000000655
[17] Ignatius Ou SH, Zell JA. The applicability of the proposed IASLC staging revisions to small cell lung cancer (SCLC) with comparison to the current UICC 6th TNM Edition[J]. J Thorac Oncol, 2009, 4(3): 300-310. doi: 10.1097/JTO.0b013e318194a355
[18] Girard N, Ostrovnaya I, Lau C, et al. Genomic and mutational profiling to assess clonal relationships between multiple non-small cell lung cancers[J]. Clin Cancer Res, 2009, 15(16): 5184-5190. doi: 10.1158/1078-0432.CCR-09-0594
[19] 王继凡, 张特, 丁翰林, 等. 同时性多原发肺癌与肺内转移鉴别方法研究进展[J]. 中国肺癌杂志, 2021, 24(5): 365-371. https://www.cnki.com.cn/Article/CJFDTOTAL-FAIZ202105009.htm Wang JF, Zhang T, Ding HL, et al. Research Progress in Distinguishing Methods of Simultaneous Multiple Primary Lung Cancer and Intrapulmonary Metastasis[J]. Zhongguo Fei Ai Za Zhi, 2021, 24(5): 365-371. https://www.cnki.com.cn/Article/CJFDTOTAL-FAIZ202105009.htm
[20] Zou J, Guo S, Xiong MT, et al. Ageing as key factor for distant metastasis patterns and prognosis in patients with extensive-stage Small Cell Lung Cancer[J]. J Cancer, 2021, 12(6): 1575-1582. doi: 10.7150/jca.49681
[21] Huang LL, Hu XS, Wang Y, et al. Survival and pretreatment prognostic factors for extensive-stage small cell lung cancer: A comprehensive analysis of 358 patients[J]. Thorac Cancer, 2021, 12(13): 1943-1951. doi: 10.1111/1759-7714.13977
[22] Lim JH, Ryu JS, Kim JH, et al. Gender as an independent prognostic factor in small-cell lung cancer: Inha Lung Cancer Cohort study using propensity score matching[J]. PLoS One, 2018, 13(12): e0208492. doi: 10.1371/journal.pone.0208492
[23] Zhou K, Shi H, Chen R, et al. Association of Race, Socioeconomic Factors, and Treatment Characteristics With Overall Survival in Patients With Limited-Stage Small Cell Lung Cancer[J]. JAMA Netw Open, 2021, 4(1): e2032276. doi: 10.1001/jamanetworkopen.2020.32276
[24] Shan Q, Shi J, Wang X, et al. A new nomogram and risk classification system for predicting survival in small cell lung cancer patients diagnosed with brain metastasis: a large population-based study[J]. BMC Cancer, 2021, 21(1): 640. doi: 10.1186/s12885-021-08384-5
[25] Daly ME, Ismaila N, Decker RH, et al. Radiation Therapy for Small-Cell Lung Cancer: ASCO Guideline Endorsement of an ASTRO Guideline[J]. J Clin Oncol, 2021, 39(8): 931-939. doi: 10.1200/JCO.20.03364
[26] Sheikh S, Dey A, Datta S, et al. Role of radiation in extensive stage small cell lung cancer: a National Cancer Database registry analysis[J]. Future Oncol, 2021, 17(21): 2713-2724. doi: 10.2217/fon-2020-1095
[27] Lee JS, Kim S, Sung SY, et al. Treatment Outcomes of 9, 994 Patients With Extensive-Disease Small-Cell Lung Cancer From a Retrospective Nationwide Population-Based Cohort in the Korean HIRA Database[J]. Front Oncol, 2021, 11: 546672. doi: 10.3389/fonc.2021.546672
[28] Noronha V, Ravind R, Patil VM, et al. The role of chemotherapy in patients with small cell lung cancer and poor performance status[J]. Acta Oncol, 2020, 59(12): 1520-1527. doi: 10.1080/0284186X.2020.1819562
-
期刊类型引用(1)
1. 蒋遥,温伟红,杨发,聂迪森,张武合,秦卫军. 多靶点CAR-T细胞治疗肿瘤的研究进展. 肿瘤防治研究. 2022(07): 709-714 .  本站查看
本站查看
其他类型引用(3)




 下载:
下载: