Recent Progress in Nano-biomaterials for Tumor Hypoxia Relief and Enhanced Cancer Radiotherapy
-
摘要:
放射治疗是临床肿瘤治疗常用的一种治疗策略,包括将放射性核素注射或植入体内的核素内放疗以及利用外界的射线(如X射线)对肿瘤部位进行照射的外照射放疗两大类。由于氧分子在电离辐射杀伤肿瘤细胞的过程中发挥着重要的作用,而实体肿瘤内部却普遍存在乏氧的微环境,肿瘤乏氧是导致其对放疗耐受的重要原因之一。近年来,随着生物材料和纳米技术的发展,一系列有效调控和逆转肿瘤乏氧微环境的技术被报道。本文将介绍近年来发展的基于纳米生物材料的肿瘤乏氧调控策略及其在放疗增敏中的应用,并对该类技术未来的临床应用进行展望和讨论。
Abstract:Radiation therapy is an extensively applied cancer treatment methods in clinic. Radiation therapy can be conducted by either administrating/implanting radioactive isotope agents into the patients to irradiate tumors from inside, or using external beams (e.g. X-ray beams) to irradiate the tumor from the outside. It is well known that oxygen molecules play an important role in enhancing radiation-induced cancer cell killing. However, as most of solid tumors possess hypoxic tumor microenvironment, the tumor hypoxia is well recognized as one of important reasons that lead to the resistance of tumors to radiation therapies. In recent years, owing to the rapid development of biomaterials and nanotechnology, many different strategies have been reported to effectively modulate and reverse the hypoxic tumor microenvironment. In this article, we will summarize various types of nano-biomaterials for tumor hypoxia relief and their application in radiotherapy sensibilization, as well as the future clinical translation.
-
Key words:
- Nanotechnology /
- Biomaterials /
- Tumor hypoxia /
- Cancer radiotherapye
-
0 引言
结直肠癌(colorectal cancer, CRC)是一种常见的消化道恶性肿瘤,在我国患病率和死亡率分别居于第三位和第五位[1],尽管近年来在诊断和治疗技术上取得了较好的进展,但进展期结直肠癌尤其伴发远端转移的患者预后仍然较差[2]。中心体相关激酶2(NIMA-related kinase 2, NEK2)是一种周期性表达的丝氨酸/苏氨酸蛋白激酶,调控多种细胞周期相关蛋白活性,也是中心体的重要组成部分,对维持有丝分裂进展和双极纺锤体的正确形成发挥重要的调控作用,它还与细胞中心体上的微管相结合促进G2/M期细胞中心体分离。研究发现NEK2异常活化将导致中心体的复制失败和不成熟的中心粒分离,出现染色体不稳定和非整倍体,从而参与了癌症的起始过程。目前已经在胃癌[3]、小细胞肺癌[4]、前列腺癌[5]等多种肿瘤中发现NEK2表达上调并促进肿瘤细胞增殖及侵袭,然而NEK2的表达水平与结直肠癌的关系目前还鲜有报道,本研究旨在探明NEK2在结直肠癌组织中的表达水平及其与临床病理特征的关系,研究沉默NEK2对结直肠癌细胞HCT116增殖及侵袭迁移能力的影响及可能的作用机制。
1 资料与方法
1.1 资料
1.1.1 临床资料
收集2016年1月—12月在河南省人民医院行结直肠癌根治术的100例患者的癌组织及癌旁组织(距癌灶边缘 > 5 cm取材),其中男68例、女32例,中位年龄59岁(24~76岁)。所有患者术前均未接受放化疗,术后病理结果均经2名以上病理医师确认,且都有完整的临床病例资料,本研究遵循医学伦理学要求。
1.1.2 细胞株及主要试剂
人结直肠癌细胞株HCT116、SW480、SW620和人正常结直肠黏膜细胞FHC均保存于河南省人民医院中心实验室。胎牛血清(FBS)、RPMI1640培养基、胰蛋白酶及流式细胞术单染试剂碘化丙锭(PI)购自美国Gibco公司;RNA提取液、qRT-PCR试剂盒及反转录试剂盒均购自武汉赛维尔生物科技有限公司;引物由武汉擎科创新生物科技有限公司合成;免疫组织化学试剂盒及DAB显色液、兔抗人多克隆抗体NEK2(PAH562)、E-cadherin(PAB290)、N-cadherin(PAE582)、CDK4(PAC045)及兔抗人单克隆抗体cyclin D1(PAB021)均购自武汉云克隆科技股份有限公司;CCK8试剂盒购自美国Sigma-Aldrich公司;Transwell小室(孔径3.0 μm)购自美国Corning公司;脂质体转染试剂LipofectamineTM2000购自美国Invitrogen公司。
1.2 实验方法
1.2.1 免疫组织化学染色法检测结直肠癌组织中NEK2表达
结直肠癌组织石蜡块以4 μm连续切片,S-ABC法染色;二甲苯脱蜡,乙醇水化后行抗原修复,4%H2O2阻断10 min,5%BSA于37℃封闭1 h,滴加兔抗人多克隆抗体(1:100)4℃过夜。生物素化二抗(1:500)孵育1 h后加入新鲜配置的DAB液显色,苏木精对比染色后树胶封片,观察染色情况。结果判定参照文献报道[6],采用5点评分系统评价阳性细胞数:无阳性细胞数为0分,1%~25%为1分,26%~50%为2分,51%~75%为3分,≥76%为4分;采用4点评分系统评价细胞染色强度:无染色(-)为0分,染色弱为(+)为1分,染色呈棕黄色(++)为2分,呈深棕色(+++)为3分。两项计分的乘积为免疫反应性分数(immunoreactivity scores, IRSs),IRSs≤4为NEK2蛋白低表达, > 4为NEK2蛋白高表达。
1.2.2 实时荧光定量PCR(qRT-PCR)法检测NEK2基因在不同结直肠癌细胞系中的表达水平
以细胞生长融合度达80%~90%时收集细胞,根据TRIzol操作说明书提取细胞总RNA。根据反转录试剂盒和荧光定量试剂盒操作说明书进行PCR定量反应。引物序列分别为:NEK2正义链为5'-TGCTTCGTGAACTGAAACATCC-3',反义链为5'-CCAGAGTCAACTGAGTCATCACT-3';GAPDH正义链为5'-GGAGCGAGATCCCTCCAAAAT-3',反义链为5'-GGCTGTTGTCATAC TTCTCATGG-3'。PCR反应条件如下,反转录反应为42℃ 60 min,70℃ 10 min,冰上降温,PCR预变性95℃ 10 min,然后95℃ 15 s、60℃ 60 s、每20 s升温1℃,共40次循环。根据目的基因NEK2和内参基因GAPDH测得CT值差异,运用2-ΔΔCt(RQ)法计算NEK2的相对表达量,实验重复3次。
1.2.3 细胞分组与转染
取对数生长期的HCT116细胞,以2×105个/孔接种至6孔培养板,培养24 h。待细胞达到70%~80%融合时,按照LipofectamineTM 2000脂质体试剂盒说明书转染细胞,实验细胞分为阳性转染组(si-NEK2)、阴性对照组(si-CTRL)和空白组。NEK2 siRNA干扰正义链:5'-GCUUGUUUCUGAAGUGAAUTT-3';CTRL siRNA干扰正义链:5'-AAGTAGCCGAGCTTCGATTGC-3',均由吉玛基因合成。
1.2.4 免疫印迹法检测敲低NEK2对HCT116细胞NEK2、E-cadherin、N-cadherin、CDK4及cyclin D1蛋白表达的影响
收集细胞并用细胞裂解液裂解提取细胞总蛋白,取50 μg蛋白进行SDS聚丙烯酰胺凝胶电泳1.5 h,然后将蛋白转移到PVDF膜上,1%BSA封闭过夜,分别加入NEK2(1:500)、N-cadherin(1:500)、E-cadherin(1:500)、CDK4(1:200)、cyclin D1(1:400)及GAPDH(1:1 000)一抗37℃孵育2 h,TBST漂洗后,加入1:1 000稀释的羊抗兔二抗37℃孵育1 h,将PVDF膜浸于1 ml显色液中避光约10 min后观察结果,用凝胶图像处理系统分析目标条带的相对分子质量和净吸光度值。实验重复3次。
1.2.5 CCK8法检测敲低NEK2对HCT116细胞增殖能力的影响
将100 μl密度为每毫升5×104个细胞的悬液加入96孔板中,细胞贴壁后加入CCK8试剂,分别在24、48、72 h后应用酶标仪测定450 nm处的光密度(OD)值,代表细胞增殖水平。实验重复3次。
1.2.6 流式细胞学检测敲低NEK2对HCT116细胞周期分布的影响
细胞按照每毫升5×105个接种于培养瓶中常规培养,细胞融合度达到80%时转染细胞并继续培养48 h,胰酶消化并收集细胞,2 500 r/min离心5 min取细胞沉淀,PBS洗涤两次,细胞经预冷的70%乙醇4℃固定18 h,离心弃固定液,PBS洗涤3次,调整细胞浓度为每毫升1×106,取1 ml细胞悬液,与含20 μg/ml RNA酶的Tris-HCL缓冲液37 ℃孵育30 min,用50 μg/ml碘化丙锭(PI)进行细胞DNA染色。样品用Beckman counter公司FC500MCL/MPL流式细胞仪测细胞周期,采用Multicycle for windows数据分析系统进行数据分析,实验重复3次。
1.2.7 Transwell小室法检测敲低NEK2对HCT116细胞侵袭能力的影响
紫外线照射消毒8 μm膜孔Transwell板,下室加入含趋化因子的细胞培养液,各实验组细胞以5×104/ml接种于含人工基底膜成份的Transwell小室并套入下室,37℃培养箱孵育24 h。取出上室,吸取残液。在下室中添加70%甲醛固定30 min,常规结晶紫染色,在高倍镜视野下计数小室面细胞数即为穿透细胞数。
1.2.8 细胞划痕实验检测敲低NEK2对HCT116细胞迁移能力的影响
各实验组细胞胰酶消化后以5×105个/毫升接种于6孔板中,待细胞融合度达到100%时用10 µl移液器枪头在培养孔底部划痕,空白组作为对照,继续培养48 h后显微镜下观察细胞迁移距离并拍照,测量多个点划痕宽度,计算平均划痕愈合率。划痕愈合率(%)=(0 h划痕宽度-48 h划痕宽度)/0 h划痕宽度×100%。实验重复3次。
1.3 统计学方法
SPSS17.0软件进行统计学分析,计量资料以均数±标准差(x±s)表示,多组间均数差异的比较采用单因素方差分析,两两比较采用t检验。采用秩和检验分析NEK2表达水平与临床病理特征的关系。P < 0.05为差异有统计学意义。
2 结果
2.1 NEK2蛋白在CRC组织中的表达及其与CRC临床病理特征的关系
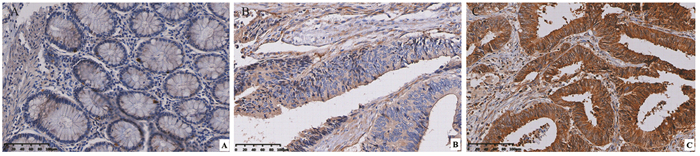
免疫组织化学结果显示NEK2蛋白主要定位于肿瘤细胞胞质,少部分定位于胞核内,染色呈棕黄色和深黄色,而在癌旁组织中不表达或弱阳性表达于正常黏膜细胞胞质内,见图 1;根据免疫组织化学评分标准,NEK2蛋白在癌组织中阳性表达率为65%(65/100),明显高于癌旁组织38%(38/100),差异有统计学意义(χ2=14.593, P < 0.01);结果发现NEK2表达与结直肠癌TNM分期、淋巴结转移和远处转移显著相关(均P < 0.05),而与性别、年龄、肿瘤直径大小及肿瘤分化程度无显著相关(均P > 0.05),见表 1。
表 1 NEK2表达水平与结直肠癌患者临床病理特征的关系Table 1 Relation between NEK2 expression and clinicopathological characteristics of colorectal cancer (CRC) patients
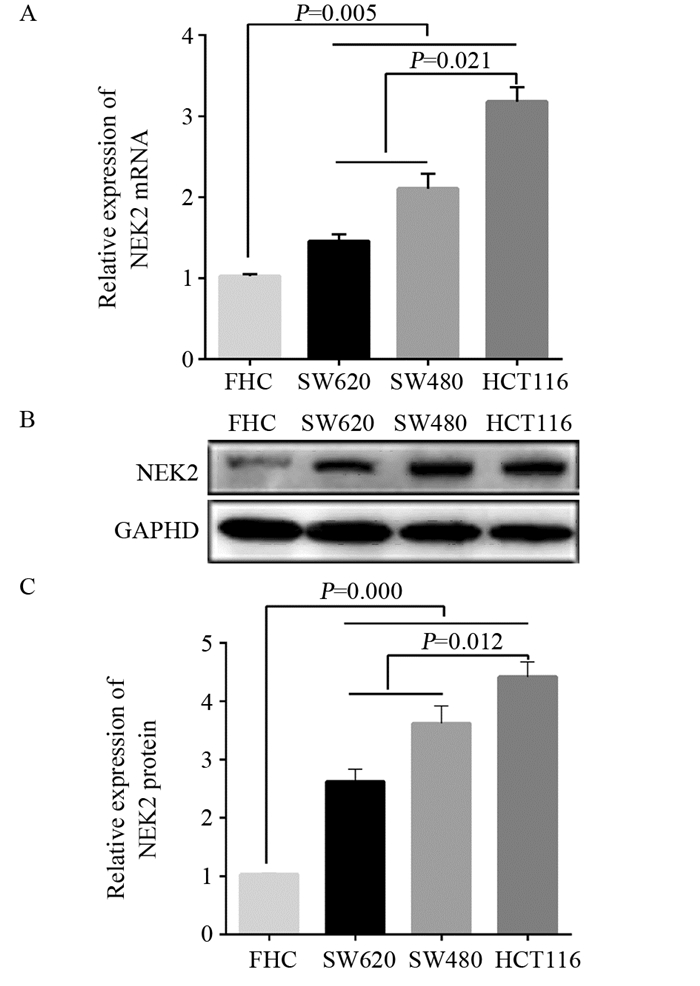
2.2 NEK2 mRNA和蛋白在结直肠癌细胞中表达高于正常对照细胞
qRT-PCR结果显示NEK2 mRNA在结直肠癌细胞SW480、SW620和HCT116中的表达含量明显高于正常结直肠黏膜细胞FHC(均P < 0.01),以低分化HCT116细胞表达含量最高,见图 2A;Western blot结果显示,NEK2蛋白在结直肠癌细胞SW480(3.78±0.17)、SW620(2.62±0.36)和HCT116(4.35±0.18)中的表达含量明显高于正常结直肠黏膜细胞FHC(均P < 0.01),以低分化HCT116细胞表达含量最高,见图 2B。
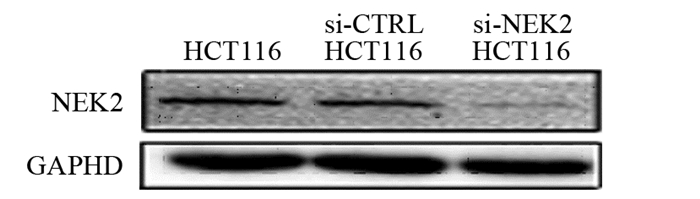
2.3 NEK2 siRNA可显著降低HCT116细胞中NEK2 mRNA及蛋白表达量
si-NEK2及si-CTRL转染细胞后,阳性转染(si-NEK2 HCT116)组的NEK2 mRNA表达水平(0.28±0.07)显著低于阴性对照(si-CTRL HCT116)组(1.03±0.14)及空白(HCT116)组(均P < 0.01),阳性干扰组NEK2蛋白相对表达含量(0.18±0.07)显著低于阴性对照组(1.03±0.14)及空白组(均P < 0.01),见图 3,阴性对照组与空白组间NEK2 mRNA及蛋白表达水平差异无统计学意义(P > 0.05)。
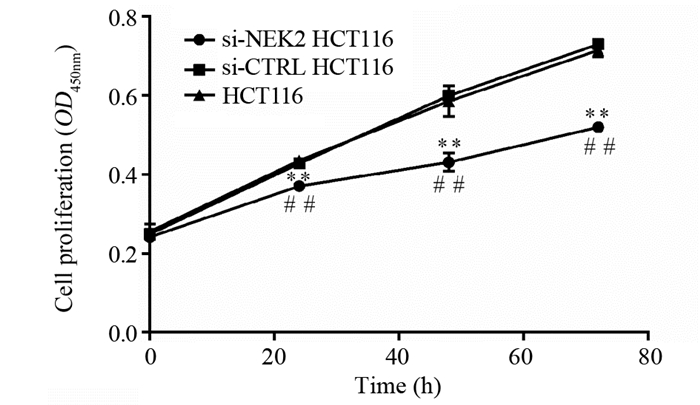
2.4 敲低NEK2后结直肠癌细胞增殖能力显著下降
CCK8检测结果显示细胞转染24~72 h后,阳性转染组细胞HCT116的增殖能力较阴性对照组和空白组明显下降(均P < 0.01),而空白组与阴性对照组间差异无统计学意义(均P > 0.05),表明干扰NEK2表达可显著抑制结直肠癌细胞的增殖能力,见图 4。
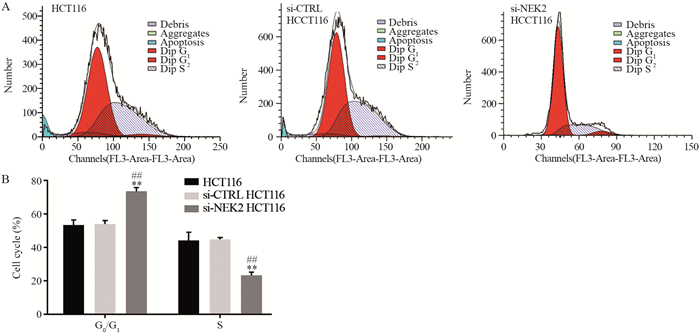
2.5 敲低NEK2后结直肠癌细胞发生G0/G1期周期阻滞
流式细胞学检测结果显示,与阴性对照组和空白组相比,阳性对照组G0/G1期细胞比例升高,S期比例下降,差异均有统计学意义(均P < 0.01),而阴性对照组和空白组间的细胞周期比例差异无统计学意义(P > 0.05),见图 5。
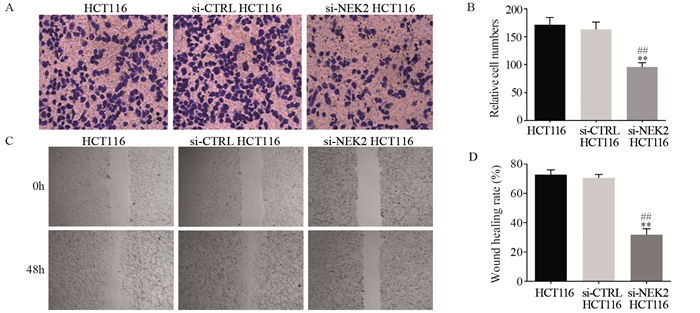
2.6 敲低NEK2后结直肠癌细胞的侵袭和迁移能力显著下降
Transwell小室法检测结果显示,阳性转染组的穿膜细胞数明显低于阴性对照组和空白组((95.67±7.77)vs.(163.00±13.08)、(171.33±13.05),均P < 0.01),阴性对照组和空白组间穿膜细胞数差异无统计学意义(P > 0.05),见图 6A、B;划痕实验结果显示,阳性转染组的划痕愈合率明显低于阴性对照组和空白组((31.80±4.10)% vs.(70.57±2.32)%、(72.77±3.25)%,均P < 0.01),阴性对照组和空白组间划痕愈合率差异无统计学意义(P > 0.05),图 6C、D。
![]() 图 6 敲低NEK2对结直肠癌细胞HCT116侵袭(A, B)和迁移(C, D)能力的影响**: P < 0.01, compared between si-NEK2 HCT116 cells and si-CTRL HCT116 cells; ##: P < 0.01, compared between si-NEK2 HCT116 cells and HCT116 cells.Figure 6 Effects of NEK knockdown on invasion(A, B) and migration(C, D) abilities of colorectal cancer HCT116 cells
图 6 敲低NEK2对结直肠癌细胞HCT116侵袭(A, B)和迁移(C, D)能力的影响**: P < 0.01, compared between si-NEK2 HCT116 cells and si-CTRL HCT116 cells; ##: P < 0.01, compared between si-NEK2 HCT116 cells and HCT116 cells.Figure 6 Effects of NEK knockdown on invasion(A, B) and migration(C, D) abilities of colorectal cancer HCT116 cells2.7 敲低NEK2后结直肠癌细胞E-cadherin、N-cadherin、CDK4和cyclin D1的表达情况
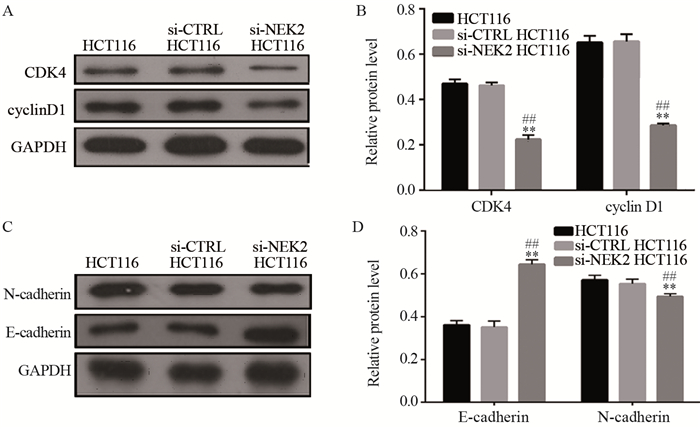
Western blot检测结果显示,对比阴性对照组和空白组,细胞周期相关蛋白CDK4和cyclin D1,EMT相关分子标志物N-cadherin在阳性对照组的表达量显著下降(P < 0.01),EMT相关分子标志物E-cadherin在阳性对照组的表达量显著升高(P < 0.01)。上述蛋白在阴性对照组和空白组间的表达差异无统计学意义(P > 0.05),见图 7。
![]() 图 7 细胞周期蛋白CDK4和cyclin D1(A, B),EMT相关分子标志物E-cadherin和N-cadherin(C, D)在结直肠癌细胞HCT116中的表达**: P < 0.01, compared between si-NEK2 HCT116 cells and si-CTRL HCT116 cells; ##: P < 0.01, compared between si-NEK2 HCT116 cells and HCT116 cells.Figure 7 Expression of CDK4 and cyclin D1(A, B), E-cadherin and N-cadherin(C, D) in colorectal cancer HCT116 cells
图 7 细胞周期蛋白CDK4和cyclin D1(A, B),EMT相关分子标志物E-cadherin和N-cadherin(C, D)在结直肠癌细胞HCT116中的表达**: P < 0.01, compared between si-NEK2 HCT116 cells and si-CTRL HCT116 cells; ##: P < 0.01, compared between si-NEK2 HCT116 cells and HCT116 cells.Figure 7 Expression of CDK4 and cyclin D1(A, B), E-cadherin and N-cadherin(C, D) in colorectal cancer HCT116 cells3 讨论
NEK2基因定位于染色体1q32.2~1q41,其表达蛋白是一种与中心体(never in mitosis A,NIMA)有丝分裂调节器结构相似的丝氨酸/苏氨酸激酶,它包含一个N端的催化激酶结构域,C端结构域包含亮氨酸拉链、卷曲螺旋、中心体结合位点、核仁定位和微管结合位点、PP1结合位点和APC结合位点等多种结构基团[7],NEK2通过结合并磷酸化有丝分裂相关蛋白参与染色体的复制和分离[8]、微管稳定[9]、着丝点附着和纺锤体组装等细胞周期过程[10-11]。近年来研究发现,NEK2异常活化会导致染色体不稳定和染色体包含异常内容,最终导致肿瘤发生[12]。Zhang等[13]研究发现NEK2在肝细胞癌中的表达水平与患者的预后呈负相关,NEK2通过激活Wnt、NF-κB、VEGF及P53信号通路介导EMT机制促进了肝癌细胞的侵袭和转移,另有研究表明NEK2可通过活化蛋白激酶磷酸酶(mitogen-activated protein kinase, MAPK)信号通路调控HepG2细胞的增殖、凋亡及其他生物学行为[14]。在前列腺癌的研究中,Zeng等[15]发现NEK2在人前列腺癌细胞和原发性前列腺癌组织中表达升高并与患者的Gleason评分及病理分期呈正相关,通过siRNA技术沉默NEK2表达可抑制前列腺癌细胞的体外增殖和异种移植体的体内生长。此外,Kokuryo等[16]也发现NEK2在胰腺癌细胞株中高表达,NEK2 siRNA可抑制胰腺癌皮下移植瘤小鼠模型的肿瘤生长,延长腹腔内移植瘤小鼠模型的生存时间,并利用门静脉导管系统有效地阻止肝转移的进展。以上结果表明,NEK2的异常表达促进了肿瘤的增殖、侵袭和转移,而针对NEK2的siRNA治疗有可能作为肿瘤治疗的一个有效手段。
本研究采用免疫组织化学技术、qRT-PCR和Western blot检测NEK2在结直肠癌组织及细胞中的表达,结果显示NEK2蛋白及mRNA在结直肠癌组织及细胞中高表达,并与结直肠癌TNM分期、淋巴结转移和远处转移相关,提示NEK2可能与结直肠癌的发生发展有关。为探究NEK2在结直肠癌中的作用机制,我们选取结直肠癌细胞HCT116作为研究对象,通过体外瞬时转染NEK2 siRNA抑制HCT116中NEK2 mRNA表达。CCK8实验及流式细胞学检测结果显示,NEK2干扰后,HCT116细胞G1期比例增加,S期比例减低,提示出现G0/G1期阻滞,并且细胞增殖率较对照组显著下降。CDK4是细胞G1/S期转换的关键调控因子,它与细胞cyclin D1形成复合物后可使视网膜母细胞瘤蛋白发生磷酸化失活,驱动细胞周期进展[17],目前在乳腺癌研究中已发现NEK2与CDK4的表达存在分子连接性[18]。NEK2是否通过与CDK4和cyclin D1的相互作用参与结直肠癌细胞的周期调控,本研究结果显示在NEK2干扰阳性转染组中CDK4和cyclin D1的表达量显著降低,表明NEK2下调可通过改变CDK4和cyclin D1表达量使结直肠癌细胞发生周期阻滞,抑制细胞生长。此外Transwell小室法和划痕实验的检测结果显示,NEK2干扰后HCT116细胞穿膜细胞数及划痕愈合率均显著下降,表明NEK2下调能显著降低HCT116细胞的侵袭转移能力。高度保守的Wnt/β-catenin信号通路在调控胚胎发育、器官形成、组织再生及决定细胞命运方面发挥着重要作用,而多种肿瘤癌基因则通过调控Wnt/β-catenin信号通路使其异常活化促进结直肠癌细胞的增殖、侵袭和转移[19-21]。在肝细胞癌的研究中发现NEK2的异常活化可改变β-catenin在细胞内定位,进而促进肿瘤细胞EMT进程并获得侵袭性表型[22]。为研究结直肠癌中NEK2表达与EMT机制的关系,本研究通过Western blot检测EMT关键组件E-cadherin和N-cadherin在HCT116细胞中的变化,结果显示敲低NEK2后HCT116细胞E-cadherin蛋白高表达,N-cadherin低表达,表明NEK2在EMT过程中及结直肠癌侵袭转移的肿瘤促进功能上发挥着关键作用,然而NEK2具体通过何种信号通路参与调控EMT进程仍有待进一步的研究。
本研究结果表明,NEK2在结直肠癌组织中高表达,下调NEK2表达可抑制人结直肠癌细胞的增殖及侵袭转移能力,提示NEK2在结直肠癌的发生发展过程中发挥着重要的作用,可作为结直肠癌基因治疗的有效靶点。
Competing interests: The authors declare that they have no competing interests.作者贡献:赵琪:资料收集与分析,论文撰写刘庄:论文审核 -
[1] Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs[J]. Nat Rev Cancer, 2011, 11(4): 239-253. doi: 10.1038/nrc3007
[2] Horsman MR, Mortensen LS, Petersen JB, et al. Imaging hypoxia to improve radiotherapy outcome[J]. Nat Rev Clin Oncol, 2012, 9(12): 674-687. doi: 10.1038/nrclinonc.2012.171
[3] Moeller BJ, Richardson RA, Dewhirst MW. Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment[J]. Cancer Metastasis Rev, 2007, 26(2): 241-248. doi: 10.1007/s10555-007-9056-0
[4] Zhang C, Yan L, Gu Z, et al. Strategies based on metal-based nanoparticles for hypoxic-tumor radiotherapy[J]. Chem Sci, 2019, 10(29): 6932-6943. doi: 10.1039/C9SC02107H
[5] Song G, Liang C, Yi X, et al. Perfluorocarbon-loaded hollow Bi2Se3 nanoparticles for timely supply of oxygen under near-infrared light to enhance the radiotherapy of cancer[J]. Adv Mater, 2016, 28(14): 2716-2723. doi: 10.1002/adma.201504617
[6] Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy[J]. Nat Med, 2001, 7(9): 987-989. doi: 10.1038/nm0901-987
[7] Li W, Zhao X, Du B, et al. Gold nanoparticle-mediated targeted delivery of recombinant human endostatin normalizes tumour vasculature and improves cancer therapy[J]. Sci Rep, 2016, 6: 30619. doi: 10.1038/srep30619
[8] Gao M, Liang C, Song X, et al. Erythrocyte-Membrane-Enveloped Perfluorocarbon as Nanoscale Artificial Red Blood Cells to Relieve Tumor Hypoxia and Enhance Cancer Radiotherapy[J]. Adv Mater, 2017, 29(35). Epub 2017 Jul 19.
[9] Zhang R, Song X, Liang C, et al. Catalase-loaded cisplatin-prodrug-constructed liposomes to overcome tumor hypoxia for enhanced chemo-radiotherapy of cancer[J]. Biomaterials, 2017, 138: 13-21. doi: 10.1016/j.biomaterials.2017.05.025
[10] Chen Y, Zhong H, Wang J, et al. Catalase-like metal-organic framework nanoparticles to enhance radiotherapy in hypoxic cancer and prevent cancer recurrence[J]. Chem Sci, 2019, 10(22): 5773-5778. doi: 10.1039/C9SC00747D
[11] Zannella VE, Dal Pra A, Muaddi H, et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response[J]. Clin Cancer Res, 2013, 19(24): 6741-6750. doi: 10.1158/1078-0432.CCR-13-1787
[12] Lomax ME, Folkes LK, O'neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy[J]. Clin Oncol (R Coll Radiol), 2013, 25(10): 578-585. doi: 10.1016/j.clon.2013.06.007
[13] Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response[J]. Nat Rev Cancer, 2008, 8(6): 425-437. doi: 10.1038/nrc2397
[14] Moen I, Stuhr LE. Hyperbaric oxygen therapy and cancer—a review[J]. Target Oncol, 2012, 7(4): 233-242. doi: 10.1007/s11523-012-0233-x
[15] Xin Y, Yin M, Zhao L, et al. Recent progress on nanoparticle-based drug delivery systems for cancer therapy[J]. Cancer Biol Med, 2017, 14(3): 228-241. doi: 10.20892/j.issn.2095-3941.2017.0052
[16] Aghebati-Maleki A, Dolati S, Ahmadi M, et al. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers[J]. J Cell Physiol, 2020, 235(3): 1962-1972. doi: 10.1002/jcp.29126
[17] Cheng L, Wang C, Feng L, et al. Functional nanomaterials for phototherapies of cancer[J]. Chem Rev, 2014, 114(21): 10869-10939. doi: 10.1021/cr400532z
[18] Song G, Liang C, Gong H, et al. Core-shell MnSe@ Bi2Se3 fabricated via a cation exchange method as novel nanotheranostics for multimodal imaging and synergistic thermoradiotherapy[J]. Adv Mater, 2015, 27(40): 6110-6117. doi: 10.1002/adma.201503006
[19] Wang Y, Wu Y, Liu Y, et al. BSA-mediated synthesis of bismuth sulfide nanotheranostic agents for tumor multimodal imaging and thermoradiotherapy[J]. Adv Funct Mater, 2016, 26(29): 5335-5344. doi: 10.1002/adfm.201601341
[20] Wang Y, Song S, Lu T, et al. Oxygen-supplementing mesoporous polydopamine nanosponges with WS2 QDs-embedded for CT/MSOT/MR imaging and thermoradiotherapy of hypoxic cancer[J]. Biomaterials, 2019, 220: 119405. doi: 10.1016/j.biomaterials.2019.119405
[21] Chen Q, Xu L, Chen J, et al. Tumor vasculature normalization by orally fed erlotinib to modulate the tumor microenvironment for enhanced cancer nanomedicine and immunotherapy[J]. Biomaterials, 2017, 148: 69-80. doi: 10.1016/j.biomaterials.2017.09.021
[22] Gong H, Chao Y, Xiang J, et al. Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy[J]. Nano Lett, 2016, 16(4): 2512-2521. doi: 10.1021/acs.nanolett.6b00068
[23] Liu J, Tian L, Zhang R, et al. Collagenase-encapsulated pH-responsive nanoscale coordination polymers for tumor microenvironment modulation and enhanced photodynamic nanomedicine[J]. ACS Appl Mater Interfaces, 2018, 10(50): 43493-43502. doi: 10.1021/acsami.8b17684
[24] Fokas E, Im JH, Hill S, et al. Dual inhibition of the PI3K/mTOR pathway increases tumor radiosensitivity by normalizing tumor vasculature[J]. Cancer Res, 2012, 72(1): 239-248. doi: 10.1158/0008-5472.CAN-11-2263
[25] Graham K, Unger E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment[J]. Int J Nanomedicine, 2018, 13: 6049-6058. doi: 10.2147/IJN.S140462
[26] Feng L, Betzer O, Tao D, et al. Oxygen nanoshuttles for tumor oxygenation and enhanced cancer treatment [J]. CCS Chem, 2019, 1(3): 239-250.
[27] Murayama C, Kawaguchi AT, Ishikawa K, et al. Liposome-encapsulated hemoglobin ameliorates tumor hypoxia and enhances radiation therapy to suppress tumor growth in mice[J]. Artif Organs, 2012, 36(2): 170-177. doi: 10.1111/j.1525-1594.2011.01418.x
[28] Wu W, Yang Q, Tao Li, et al. Hemoglobin-based oxygen carriers combined with anticancer drugs may enhance sensitivity of radiotherapy and chemotherapy to solid tumors[J]. Artif Cells Blood Substit Immobil Biotechnol, 2009, 37(4): 163-165. doi: 10.1080/10731190903043218
[29] Castro CI, Briceno JC. Perfluorocarbon-based oxygen carriers: review of products and trials[J]. Artif Organs, 2010, 34(8): 622-634.
[30] Lowe KC. Perfluorinated blood substitutes and artificial oxygen carriers[J]. Blood Rev, 1999, 13(3): 171-184. doi: 10.1054/blre.1999.0113
[31] Kuznetsova IN. Perfluorocarbon emulsions: stability in vitro and in vivo (a review) [J]. Pharma Chem J, 2003, 37(8): 415-420. doi: 10.1023/A:1027355913348
[32] Song X, Feng L, Liang C, et al. Ultrasound triggered tumor oxygenation with oxygen-shuttle nanoperfluorocarbon to overcome hypoxia-associated resistance in cancer therapies[J]. Nano Lett, 2016, 16(10): 6145-6153. doi: 10.1021/acs.nanolett.6b02365
[33] Chen Q, Liang C, Sun X, et al. H2O2-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay[J]. Proc Natl Acad Sci U S A, 2017, 114(21): 5343-5348. doi: 10.1073/pnas.1701976114
[34] Chao Y, Xu L, Liang C, et al. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses[J]. Nat Biomed Eng, 2018, 2(8): 611-621. doi: 10.1038/s41551-018-0262-6
[35] Song G, Chen Y, Liang C, et al. Catalase-Loaded TaOx Nanoshells as Bio-Nanoreactors Combining High-Z Element and Enzyme Delivery for Enhancing Radiotherapy[J]. Adv Mater, 2016, 28(33): 7143-7148. doi: 10.1002/adma.201602111
[36] Chen Q, Chen J, Yang Z, et al. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy[J]. Adv Mat, 2019, 31(10): e1802228. doi: 10.1002/adma.201802228
[37] Cho MH, Choi ES, Kim S, et al. Redox-responsive manganese dioxide nanoparticles for enhanced MR imaging and radiotherapy of lung cancer[J]. Front Chem, 2017, 5: 109. doi: 10.3389/fchem.2017.00109
[38] Gong F, Chen J, Han X, et al. Core–shell TaOx@ MnO 2 nanoparticles as a nano-radiosensitizer for effective cancer radiotherapy[J]. J Mater Chem B, 2018, 6(15): 2250-2257. doi: 10.1039/C8TB00070K
[39] Liu J, Chen Q, Feng L, et al. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement [J]. Nano Today, 2018, 21: 55-73. doi: 10.1016/j.nantod.2018.06.008
[40] Song X, Feng L, Liang C, et al. Liposomes co-loaded with metformin and chlorin e6 modulate tumor hypoxia during enhanced photodynamic therapy[J]. Nano Res, 2017, 10(4): 1200-1212. doi: 10.1007/s12274-016-1274-8
[41] De Bruycker S, Vangestel C, Staelens S, et al. Effects of metformin on tumor hypoxia and radiotherapy efficacy: a [18F] HX4 PET imaging study in colorectal cancer xenografts[J]. EJNMMI Res, 2019, 9(1): 74. doi: 10.1186/s13550-019-0543-4
[42] Yang Z, Chen Q, Chen J, et al. Tumor-pH-responsive dissociable albumin–tamoxifen nanocomplexes enabling efficient tumor penetration and hypoxia relief for enhanced cancer photodynamic therapy[J]. Small, 2018, 14(49): e1803262. doi: 10.1002/smll.201803262
[43] Yang X, Gao L, Guo Q, et al. Nanomaterials for radiotherapeutics-based multimodal synergistic cancer therapy[J]. Nano Res, 2020, 13: 2579-2594. doi: 10.1007/s12274-020-2722-z
[44] 王松, 李兴川, 刘兆辰. 放疗联合PD1/PD-L1抑制剂抗肿瘤治疗[J]. 中国临床研究, 2020, 33(2): 261-264. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGCK202002033.htm Wang S, Li XC, Liu ZC. Radiotherapy in combination with PD1/PD-L1 blockade for cancer treatment[J]. Zhongguo Lin Chuang Yan Jiu, 2020, 33(2): 261-264. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGCK202002033.htm
[45] 于金明, 滕菲菲. 放疗与免疫治疗联合应用的相关机制及研究进展[J]. 中国肿瘤临床, 2014, 41(9): 547-550. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGZL201409001.htm Yu JM, Teng FF. Mechanisms and challenges in joint application of radiotherapy and cancer immunotherapy[J]. Zhongguo Zhong Liu Lin Chuang, 2014, 41(9): 547-550. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGZL201409001.htm
[46] Zeng X, Yan S, Chen P, et al. Modulation of tumor microenvironment by metal-organic-framework-derived nanoenzyme for enhancing nucleus-targeted photodynamic therapy[J]. Nano Res, 2020, 13(6): 1527-1535. doi: 10.1007/s12274-020-2746-4
[47] 肖俊娟, 李岩, 梁婧. 乏氧微环境与肿瘤免疫应答[J]. 国际肿瘤学杂志, 2017, 44(1): 31-33. Xiao JJ, Li Y, Liang J. Hypoxic tumor microenvironment and immune response[J]. Guo Ji Zhong Liu Xue Za Zhi, 2017, 44(1): 31-33.
[48] Park JE, Dutta B, Tse SW, et al. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift[J]. Oncogene, 2019, 38(26): 5158-5173. doi: 10.1038/s41388-019-0782-x
计量
- 文章访问数: 2800
- HTML全文浏览量: 536
- PDF下载量: 2433



 刘庄 苏州大学教授,2004年获北京大学学士学位,2008年获美国斯坦福大学博士学位,2009年6月加入苏州大学功能纳米与软物质研究院。近年来在纳米生物医学材料与肿瘤纳米技术领域从事研究,共发表学术论文300余篇,论文总引用超过55 000次,SCI H指标=127。2012年获基金委优秀青年基金资助;2013年入选百千万人才工程国家级人选;2013年获江苏省杰出青年基金资助;2015年获国家杰出青年基金资助;2015年入选中组部万人计划“青年拔尖人才”;2016年获江苏省科学技术一等奖(第一完成人);2017年入选中组部万人计划“领军人才”;2017年入选教育部“长江学者特聘教授”;2017年获Biomaterials Science Lectureship;2015年受邀成为英国皇家化学会Fellow(RSC Fellow);2019年入选美国生物与医学工程学院Fellow(AIMBE Fellow);2015—2018年连续入选Clarivate Analytics(原Thomson Reuters)发布的“全球高引用科学家名录”(Highly Cited Researchers)(化学、材料领域);担任生物材料领域国际著名期刊Biomaterials杂志副主编和多家国际主流期刊编委
。
刘庄 苏州大学教授,2004年获北京大学学士学位,2008年获美国斯坦福大学博士学位,2009年6月加入苏州大学功能纳米与软物质研究院。近年来在纳米生物医学材料与肿瘤纳米技术领域从事研究,共发表学术论文300余篇,论文总引用超过55 000次,SCI H指标=127。2012年获基金委优秀青年基金资助;2013年入选百千万人才工程国家级人选;2013年获江苏省杰出青年基金资助;2015年获国家杰出青年基金资助;2015年入选中组部万人计划“青年拔尖人才”;2016年获江苏省科学技术一等奖(第一完成人);2017年入选中组部万人计划“领军人才”;2017年入选教育部“长江学者特聘教授”;2017年获Biomaterials Science Lectureship;2015年受邀成为英国皇家化学会Fellow(RSC Fellow);2019年入选美国生物与医学工程学院Fellow(AIMBE Fellow);2015—2018年连续入选Clarivate Analytics(原Thomson Reuters)发布的“全球高引用科学家名录”(Highly Cited Researchers)(化学、材料领域);担任生物材料领域国际著名期刊Biomaterials杂志副主编和多家国际主流期刊编委
。
 下载:
下载: