-
摘要:
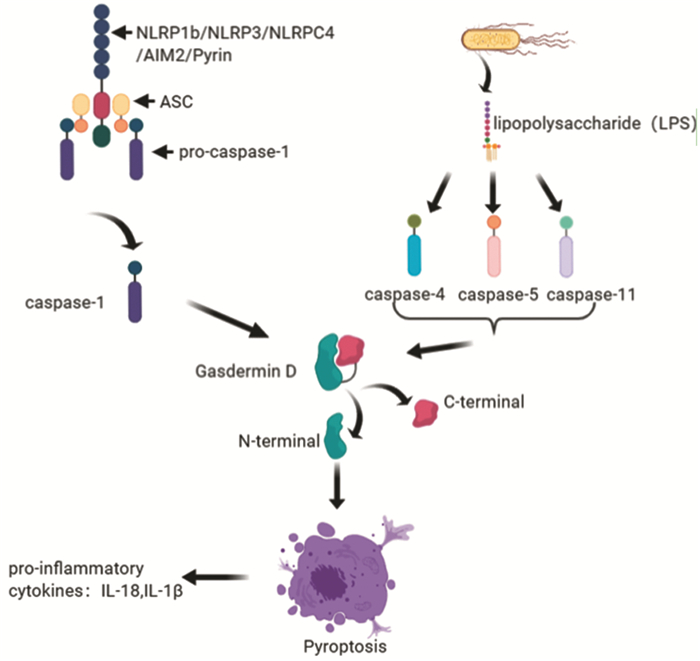
细胞焦亡是一种非典型的细胞程序性死亡方式,其主要表现为各种外界刺激后细胞膜产生孔隙,随后细胞膨胀、胞液外流、分泌炎性因子引起炎性反应,最终导致细胞崩解。焦亡起初发现于感染性疾病,但后来在神经系统、心血管系统中均有所发现,近期研究也发现了其在肿瘤中也存在,探究其对肿瘤的作用成为研究热点之一。目前已有研究提出抗肿瘤药物、中药单体等可以诱导肿瘤细胞焦亡,故本文从此入手,探究肿瘤细胞焦亡相关影响分子,并总结归纳其作用方向,提出阻止化疗耐药与调节代谢分子两条可能的抗肿瘤新策略。
Abstract:Pyroptosis is an atypical mode of cell programmed death, which is mainly manifested by pore formation, cell expansion, cell fluid outflow, secretion of inflammatory factors causing inflammatory response, and ultimately leading to cell disintegration after various external stimuli. Pyroptosis was first found in infectious diseases, but later found in the nervous system and cardiovascular system. Recent studies have also found that pyroptosis exists in tumors. It has become one of the hot research topics to explore its role in tumors. At present, studies have proposed that anti-cancer drugs, Chinese medicine monomers and so on can induce the pyroptosis of cancer cells. Therefore, this paper explores the impact molecules related to tumor cell pyroptosis, and summarizes its effect, then proposes two possible new strategies of preventing chemoresistance and regulating metabolic molecules.
-
Key words:
- Pyroptosis /
- Malignant tumor /
- Chemotherapy /
- Metabolism /
- Strategy
-
0 引言
原发性肝细胞癌(hepatocellular carcinoma, HCC)死亡率高居癌症第三位[1],是长期慢性炎性反应转化为肿瘤的典型案例。目前,除手术和肝移植外,放化疗是主要的治疗手段,但放化疗药物的不良反应及耐受性限制了其治疗效果,使患者五年生存率低于30%[1]。因此,制定新的治疗策略,对于肝癌治疗及降低死亡率具有重要意义。
中药有效组分配伍是中药配伍的新模式。课题组前期研究已确定了丹参素、丹酚酸A、丹酚酸B和原儿茶醛四种水溶性成分作用于动物的浓度和比例,并将此最佳组合命名为SABP[2]。另外也有研究表明,丹参多酚酸盐(depsides salts)可以降低Ki-67和VEGF的表达,抑制肝癌细胞SMMC-7721裸鼠皮下移植瘤的生长[3]。本研究利用H22细胞肝癌原位移植瘤小鼠模型探讨丹参水溶性组分SABP对H22细胞肝癌原位移植瘤的作用以及对肝癌免疫微环境的影响。
1 材料与方法
1.1 实验材料及试剂
小鼠腹水型肝癌细胞株H22由河北医科大学免疫学教研室曾瑞红教授惠赠。接种于含10%胎牛血清的高糖DMEM完全培养基,于37℃、CO2培养箱中培养。35只雄性BALB/c小鼠,4~6周龄,18~20 g,SPF级购于北京维通利华实验动物技术有限公司。动物的饲养及操作通过河北中医学院伦理委员会批准(编号:YXLL2018002)。丹参素、丹参酚酸A、丹参酚酸B及原儿茶醛(纯度≥98%)均购自上海将来实业股份有限公司,批次分别为20180109、20180110、20180110及20170606;0.9%氯化钠溶液(批号:20160129)购自石家庄四药有限公司。
1.2 实验方法
1.2.1 H22细胞肝癌原位移植瘤小鼠模型
将浓度为1×107个/毫升的H22肝癌细胞注射入5只BALB/c小鼠腹腔进行肿瘤细胞复壮,每只小鼠的注射剂量为0.2 ml。7天后,用异氟烷麻醉小鼠,抽取腹腔腹水,并用0.9%氯化钠溶液调整H22细胞浓度为2×106个/毫升。然后再取30只BALB/c小鼠进行肝脏H22肿瘤细胞移植手术,具体手术步骤参考文献[4]进行。每只小鼠肝脏注射H22肝癌细胞的剂量为0.05 ml。术后15天随机抽取10只,取肝脏进行HE染色,检测造模成功率。随后将剩余小鼠随机分为对照组(NC组)和SABP组,每组10只。SABP组:腹腔注射SABP的剂量为0.1 ml/d,SABP作用小鼠的浓度及比例按本课题组前期发表文献进行[2]。对照组:腹腔注射0.9%氯化钠溶液0.1 ml/d作为溶剂对照组。共注射15 d。
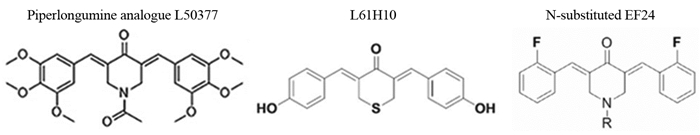
1.2.2 体重及肝、脾、肾系数的计算
每5天称量体重一次。末次干预后,禁食不禁水6~12 h,异氟烷吸入麻醉后,摘除小鼠眼球取血,并处死动物。立即摘取肝、脾、肾,用滤纸吸干残血后分别称重,计算肝、脾和肾系数。脏器系数=脏器重量/体重×100%。
1.2.3 酶联免疫吸附试验
肝脏组织匀浆后,收集上清液,标准品绘制标准曲线,然后按照试剂盒说明书步骤进行操作,用酶标仪测量OD450值,根据标准曲线求出相应的PD-L1、TGF-β、IL-1β、IL-10、IL-4、IFN-γ、IL-18、IL-7、IL-2、CCL-2和CCL-21的含量,试剂盒购自上海酶联生物科技有限公司。
1.3 统计学方法
用SPSS16.0统计软件进行数据分析。符合正态分布的计量资料采用均数±标准差(x±s)的方式表示,组间两两比较用独立样本t检验。P < 0.05为差异有统计学意义。
2 结果
2.1 丹参水溶性组分SABP抑制H22细胞肝癌原位移植瘤的生长
H22细胞进行复壮后的状态见图 1A。对照组小鼠的左肝叶可见两处突出肝脏表面的白色硬质瘤灶,正常肝组织颜色淡红。SABP组小鼠的左肝叶可见一处明显灰白色癌灶,而正常肝组织淡红,见图 1B。此结果说明SABP可以抑制肝原位移植瘤的生长。
![]() 图 1 SABP抑制H22细胞肝癌原位移植瘤的生长Figure 1 SABP inhibited growth of orthotopic transplantation of H22 cell liver cancerA: the picture on the left showed the pre-rehabilitation state of H22 cells and the picture on the right showed the post-rehabilitation state of H22 cells; B: the tumors were shown by white arrows.
图 1 SABP抑制H22细胞肝癌原位移植瘤的生长Figure 1 SABP inhibited growth of orthotopic transplantation of H22 cell liver cancerA: the picture on the left showed the pre-rehabilitation state of H22 cells and the picture on the right showed the post-rehabilitation state of H22 cells; B: the tumors were shown by white arrows.2.2 SABP提高H22细胞肝癌原位移植瘤小鼠脾重及肝、脾、肾系数
药物处理第5、10和15天,SABP组与对照组体质量差异无统计学意义(P=0.8342、0.4937和0.0782),见图 2A。SABP组小鼠脾重量是对照组重量的1.29倍,差异有统计学意义(P=0.0156),见图 2B。SABP组小鼠肝、脾、肾系数分别是对照组1.19、1.34和1.23倍,差异有统计学意义(P=0.0327、0.0083和0.0381),见图 2C~E。
2.3 SABP提高H22细胞肝癌原位移植瘤PD-L1、TGF-β、IL-1β、IL-10和IL-4的表达水平
ELISA检测结果显示,SABP组PD-L1表达量是对照组的1.4倍(P=0.0433),见图 3A。SABP组TGF-β表达量是对照组的1.3倍(P=0.0228),见图 3B。SABP组小鼠的IL-1β表达量是对照组1.5倍(P=0.0153),见图 3C。SABP组小鼠的IL-10指数是对照组1.3倍(P=0.0156),见图 3D。ELISA检测发现SABP组IL-4表达量是对照组的1.19倍,差异无统计学意义(P=0.2131),见图 3E。上述结果表明SABP可以通过上调PD-L1、TGF-β、IL-1β和IL-10的表达促进免疫抑制微环境的形成。
2.4 SABP不改变H22细胞肝癌原位移植瘤中IFN-γ、IL-18、IL-7和IL-2的表达水平
SABP组小鼠IFN-γ、IL-18、IL-7和IL-2的表达水平分别为(5703.33±471.70)pg/g、(892.45±62.39)pg/g、(1288.74±105.65)pg/g和(3379.23±374.67)pg/g。对照组的表达水平分别是(5923.99±362.61)pg/g、(927.10±91.26)pg/g、(1496.07±55.41)pg/g和(2736.23±111.94)pg/g。SABP组和对照组IFN-γ、IL-18、IL-7和IL-2的表达水平差异均无统计学意义(P=0.5556、0.6160、0.0901和0.1163)。结果表明SABP不改变抗肿瘤免疫因子的表达水平。
2.5 SABP不影响H22细胞肝癌原位移植瘤中CCL-2和CCL-21的表达水平
SABP组的CCL-2和CCL-21表达水平分别是(386.72±23.21)ng/g和(419.34±71.1)ng/g。对照组的表达水平分别是(495.09±67.66)ng/g和(388.72±34.87)ng/g。SABP组和对照组CCL-2和CCL-21表达水平差异均无统计学意义(P=0.0707和0.5395)。结果表明SABP不影响细胞趋化因子的表达水平。
3 讨论
肝癌的主要临床表现属于中医学“积聚”、“癥瘕”以及“肝积”等范畴。气滞血瘀湿热痰浊是肝癌发生的病理基础。丹参(Salvia miltiorrhiza)味苦微寒,归心肝经,是一味活血化淤药,同时还具有磨坚破滞、消瘿除瘤的作用,具有“一味丹参,功同四物”之说。丹参素(danshensu, DSS)、丹酚酸A(salvianolic acid A, Sal-A)、丹酚酸B(salvianolic acid B, Sal-B)以及原儿茶醛(protocatechuic aldehyde, PAL)是丹参主要的水溶性成分。丹参素能降低蛋白激酶B(protein kinase B, PKB)活性,从而抑制肝癌SMMC-7721细胞生长[5]。丹酚酸B通过激活线粒体途径,抑制AKT/mTOR通路使肝癌细胞降解,促进肝癌细胞的凋亡[6]。原儿茶醛靶向抑制肝癌细胞Wilms肿瘤蛋白1(Wilms tumor1, WT1)的表达,进而发挥抗肝癌作用[7]。上述研究均表明,丹参中主要水溶性成分均具有抑制肝癌细胞生长的作用。
SABP是丹参的四种水溶性成分的组合,本课题组前期研究也已证实SABP有一定的抗氧化作用。有研究发现,丹参注射液治疗能有效提高原发性肝癌患者免疫球蛋白水平,刺激CD3+T细胞和CD4+T细胞的增殖,改善肝癌患者的免疫功能[8]。本研究发现,SABP能抑制H22细胞肝癌原位移植瘤的生长。有研究报道,丹参能使小鼠脾脏树突状细胞增加[9]。本研究发现,SABP处理15天荷瘤小鼠的脾指数显著升高。有研究表明丹酚酸B通过抗氧化,发挥对肾缺血再灌注损伤的保护作用,恢复肾脏的功能[10]。本研究显示,SABP处理提高了H22细胞肝癌原位移植瘤模型的肾系数,说明SABP无肾毒性。SABP不改变荷瘤小鼠的体重,因此SABP可能是通过调节免疫发挥抗肿瘤的作用。
高表达程序性死亡配体1(programmed death ligand-1, PD-L1)的肿瘤细胞通过与T细胞表面的程序性死亡配体1(programmed death ligand-1, PD-1)相结合导致肿瘤浸润性淋巴细胞(tumor Infiltrating Lymphocytes, TILs)功能的耗竭,从而导致效应T细胞的功能被抑制。PD-L1基因的敲除有助于肝脏中小鼠CD8+T细胞的积累,表明PD-L1是调节肝脏CD8+T细胞聚集和清除的关键蛋白。因此SABP通过促进PD-L1的表达,促进免疫抑制微环境的形成。骨髓来源的抑制细胞产生的TGF-β促进CD8+T细胞上PD-1的表达,从而导致对肿瘤微环境(tumor microenvironment, TME)中PD-1/PD-L1阻断的抵抗[11]。另外升高的TGF-β还可以阻断未成熟的T细胞向Th1细胞分化,促进其向调节性T细胞(regulatory cell, Treg)亚群转化,并抑制树突细胞的抗原呈递功能,抑制CD8+T细胞产生IFN-γ的产生,从而造成肿瘤细胞的免疫逃逸[12]。IL-1β通过调节M2型巨噬细胞促进HCC细胞的迁移[13],M2型巨噬细胞到肿瘤组织的浸润与预后不良有关[14]。另外,由于巨噬细胞表达CCL-2受体,因此CCL-2对巨噬细胞具有趋化性,这种信号分子在巨噬细胞的募集过程中发挥作用[15]。但是在本研究中发现SABP可以促进IL-1β的表达,但并不改变CCL-2的表达量,所以我们推断SABP可能通过促进IL-1β表达,促进M2型巨噬细胞到肿瘤组织的浸润。
血清中高水平的IL-10与HCC患者的不良预后有关。IL-10可通过NF-κB和STAT3信号通路促进肿瘤细胞PD-L1的表达[16]。IL-10抑制T细胞产生IL-2和IFN-γ,并直接影响T细胞的分化和增殖。IL-10也可诱导肝癌中Treg的分化,Treg扩张与HCC的侵袭性和患者较差的生存期密切相关[17]。我们的研究发现SABP促进IL-10的表达,类似研究发现,应用丹酚酸B和原儿茶醛后IL-10的表达水平升高[18-19]。IL-10促进辅助性T细胞分化为Th2。这为癌细胞提供了逃避免疫系统的机会[20]。IL-7作为T细胞发育和成熟T细胞稳态的关键细胞因子,促进T细胞增殖[21]。IL-7刺激增加了外周和肝驻留CD8+T细胞的细胞毒性,这个过程伴随着CD8+T细胞上PD-1表达的下调[22]。前期的研究发现,SABP不改变IL-7、IL-2和IFN-γ的表达水平,所以SABP发挥抗肿瘤作用不是基于正向调控抗肿瘤免疫应答。
以上研究表明,SABP可以促进肿瘤免疫抑制微环境的形成,主要是通过提高免疫抑制因子PD-L1、TGF-β、IL-1β和IL-10的水平发挥作用,而没有通过改善抗肿瘤免疫的IFN-γ、IL-7、IL-18和IL-2来发挥抗肿瘤作用,也没有改变肿瘤相关的趋化因子CCL-2和CCL-21。丹参水溶性成分SABP可以发挥抗肿瘤的作用,但是并没有通过正向调节抗肿瘤免疫发挥抗肿瘤的效果,反而提高免疫抑制信号分子的表达水平促进免疫抑制微环境形成,影响SABP的抗肿瘤作用。后期本课题组将进一步探讨SABP发挥抗肿瘤作用机制,联合相关免疫治疗药物PD-1单抗或PD-L1单抗,观察SABP的抗肿瘤作用。
Competing interests: The authors declare that they have no competing interests.作者贡献:朱潇雨: 文献查找、论文构思与撰写李杰: 参与论文整体设计与审校 -
表 1 已知肿瘤细胞焦亡相关诱发分子
Table 1 Known molecules that induce tumor cell pyroptosis

-
[1] Cookson BT, Brennan MA. Pro-inflammatory programmed cell death[J]. Trends Microbiol, 2001, 9(3): 113-114. http://www.cell.com/trends/microbiology/comments/S0966-842X(00)01936-3
[2] Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages[J]. Cell Microbiol, 2006, 8(11): 1812-1825. doi: 10.1111/j.1462-5822.2006.00751.x
[3] Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling[J]. Nature, 2015, 526(7575): 666-671. doi: 10.1038/nature15541
[4] Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS[J]. Nature, 2014, 514(7521): 187-192. doi: 10.1038/nature13683
[5] Yang D, He Y, Muñoz-Planillo R, et al. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock[J]. Immunity, 2015, 43(5): 923-932. doi: 10.1016/j.immuni.2015.10.009
[6] Chen LC, Wang LJ, Tsang NM, et al. Tumour inflammasome-derived IL-1β recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma[J]. EMBO Mol Med, 2012, 4(12): 1276-1293. doi: 10.1002/emmm.201201569
[7] Dinarello CA. Why not treat human cancer with interleukin-1 blockade?[J]. Cancer Metastasis Rev, 2010, 29(2): 317-329. doi: 10.1007/s10555-010-9229-0
[8] Zhang H, Li L, Liu L. FcγRI (CD64) contributes to the severity of immune inflammation through regulating NF-κB/NLRP3 inflammasome pathway[J]. Life Sci, 2018, 207: 296-303. doi: 10.1016/j.lfs.2018.06.015
[9] Ungerbäck J, Belenki D, Jawad ul-Hassan A, et al. Genetic variation and alterations of genes involved in NF-κB/TNFAIP3- and NLRP3-inflammasome signaling affect susceptibility and outcome of colorectal cancer[J]. Carcinogenesis, 2012, 33(11): 2126-2134. doi: 10.1093/carcin/bgs256
[10] Wang Y, Kong H, Zeng X, et al. Activation of NLRP3 inflammasome enhances the proliferation and migration of A549 lung cancer cells[J]. Oncol Rep, 2016, 35(4): 2053-2064. doi: 10.3892/or.2016.4569
[11] Ma X. Loss of AIM2 expression promotes hepatocarcinoma progression through activation of m TOR-S6K1 pathway[J]. Oncotarget, 2016, 7(24): 36185-36197. doi: 10.18632/oncotarget.9154
[12] Xia X, Wang X, Cheng Z, et al. The role of pyroptosis in cancer: pro-cancer or pro-"host"?[J]. Cell Death Dis, 2019, 10(9): 650. doi: 10.1038/s41419-019-1883-8
[13] Zhang CC, Li CG, Wang YF, et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation[J]. Apoptosis, 2019, 24(3-4): 312-325. doi: 10.1007/s10495-019-01515-1
[14] Wang F, Liu W, Ning J, et al. Simvastatin Suppresses Proliferation and Migration in Non-small Cell Lung Cancer via Pyroptosis[J]. Int J Biol Sci, 2018, 14(4): 406-417. doi: 10.7150/ijbs.23542
[15] Teng JF, Mei QB, Zhou XG, et al. Polyphyllin VI Induces Caspase-1-Mediated Pyroptosis via the Induction of ROS/NF-κB/NLRP3/GSDMD Signal Axis in Non-Small Cell Lung Cancer[J]. Cancers, 2020, 12(1): 193. doi: 10.3390/cancers12010193
[16] Xie J, Zhuan B, Wang H, et al. Huaier extract suppresses non-small cell lung cancer progression through activating NLRP3-dependent pyroptosis[J]. Anat Rec(Hoboken), 2021, 304(2): 291-301. doi: 10.1002/ar.24307
[17] Zhu M, Wang J, Xie J, et al. Design, synthesis, and evaluation of chalcone analogues incorporateα, β-Unsaturated ketone functionality as anti-lung cancer agents via evoking ROS to induce pyroptosis[J]. Eur J Med Chem, 2018, 157: 1395-1405. doi: 10.1016/j.ejmech.2018.08.072
[18] Chen L, Weng B, Li H, et al. A thiopyran derivative with low murine toxicity with therapeutic potential on lung cancer acting through a NF-κB mediated apoptosis-to-pyroptosis switch[J]. Apoptosis, 2019, 24(1-2): 74-82. doi: 10.1007/s10495-018-1499-y
[19] Sannino F, Sansone C, Galasso C, et al. Pseudoalteromonas haloplanktis TAC125 produces 4-hydroxybenzoic acid that induces pyroptosis in human A459 lung adenocarcinoma cells[J]. Sci Rep, 2018, 8(1): 1190. doi: 10.1038/s41598-018-19536-2
[20] Chen L, Li Q, Zheng Z, et al. Design and optimize N-substituted EF24 as effective and low toxicity NF-κB inhibitor for lung cancer therapy via apoptosis-to-pyroptosis switch[J]. Chem Biol Drug Des, 2019, 94(1): 1368-1377. doi: 10.1111/cbdd.13514
[21] Li Q, Chen L, Dong Z, et al. Piperlongumine analogue L50377 induces pyroptosis via ROS mediated NF-κB suppression in non-small-cell lung cancer[J]. Chem Biol Interact, 2019, 313: 108820. doi: 10.1016/j.cbi.2019.108820
[22] Pizato N, Luzete BC, Kiffer LFMV, et al. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells[J]. Sci Rep, 2018, 8(1): 1952. doi: 10.1038/s41598-018-20422-0
[23] Wang Y, Yin B, Li D, et al. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer[J]. Biochem Biophys Res Commun, 2018, 495(1): 1418-1425. doi: 10.1016/j.bbrc.2017.11.156
[24] Yu J, Li S, Qi J, et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells[J]. Cell Death Dis, 2019, 10(3): 193. doi: 10.1038/s41419-019-1441-4
[25] Wei Q, Zhu R, Zhu J, et al. E2-Induced Activation of the NLRP3 Inflammasome Triggers Pyroptosis and Inhibits Autophagy in HCC Cells[J]. Oncol Res, 2019, 27(7): 827-834. doi: 10.3727/096504018X15462920753012
[26] Chu Q, Jiang Y, Zhang W, et al. Pyroptosis is involved in the pathogenesis of human hepatocellular carcinoma[J]. Oncotarget, 2016, 7(51): 84658-84665. doi: 10.18632/oncotarget.12384
[27] Wu M, Wang Y, Yang D, et al. A PLK1 kinase inhibitor enhances the chemosensitivity of cisplatin by inducing pyroptosis in oesophageal squamous cell carcinoma[J]. EBioMedicine, 2019, 41: 244-255. doi: 10.1016/j.ebiom.2019.02.012
[28] Wang L, Li K, Lin X, et al. Metformin induces human esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1 axis[J]. Cancer Lett, 2019, 450: 22-31. doi: 10.1016/j.canlet.2019.02.014
[29] Zhou B, Zhang JY, Liu XS, et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis[J]. Cell Res, 2018, 28(12): 1171-1185. doi: 10.1038/s41422-018-0090-y
[30] Okondo MC, Johnson DC, Sridharan R, et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis[J]. Nat Chem Biol, 2017, 13(1): 46-53. doi: 10.1038/nchembio.2229
[31] Yue E, Tuguzbaeva G, Chen X, et al. Anthocyanin is involved in the activation of pyroptosis in oral squamous cell carcinoma[J]. Phytomedicine, 2019, 56: 286-294. doi: 10.1016/j.phymed.2018.09.223
[32] Zhang R, Chen J, Mao L, et al. Nobiletin Triggers Reactive Oxygen Species-Mediated Pyroptosis through Regulating Autophagy in Ovarian Cancer Cells[J]. J Agric Food Chem, 2020, 68(5): 1326-1336. doi: 10.1021/acs.jafc.9b07908
[33] Wang X, Li H, Li W, et al. The role of Caspase-1/GSDMD-mediated pyroptosis in Taxol-induced cell death and a Taxol-resistant phenotype in nasopharyngeal carcinoma regulated by autophagy[J]. Cell Biol Toxicol, 2020, 36(5): 437-457. doi: 10.1007/s10565-020-09514-8
[34] Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin[J]. Nature, 2017, 547(7661): 99-103. doi: 10.1038/nature22393
[35] Pezuk JA. Pyroptosis in combinatorial treatment to improve cancer patients' outcome, is that what we want?[J]. EBioMedicine, 2019, 41: 17-18. doi: 10.1016/j.ebiom.2019.03.007
[36] Yu P, Wang HY, Tian M, et al. Eukaryotic elongation factor-2 kinase regulates the cross-talk between autophagy and pyroptosis in doxorubicin-treated human melanoma cells in vitro[J]. Acta Pharmacol Sin, 2019, 40(9): 1237-1244. doi: 10.1038/s41401-019-0222-z
[37] Meng L, Lin H, Zhang J, et al. Doxorubicin induces cardiomyocyte pyroptosis via the TINCR-mediated posttranscriptional stabilization of NLR family pyrin domain containing 3[J]. J Mol Cell Cardiol, 2019, 136: 15-26. doi: 10.1016/j.yjmcc.2019.08.009
[38] Zhou CB, Fang JY. The role of pyroptosis in gastrointestinal cancer and immune responses to intestinal microbial infection[J]. Biochim Biophys Acta Rev Cancer, 2019, 1872(1): 1-10. doi: 10.1016/j.bbcan.2019.05.001
[39] Wu D, Han R, Deng S, et al. Protective Effects of Flagellin A N/C Against Radiation-Induced NLR Pyrin Domain Containing 3 Inflammasome-Dependent Pyroptosis in Intestinal Cells[J]. Int J Radiat Oncol Biol Phys, 2018, 101(1): 107-117. doi: 10.1016/j.ijrobp.2018.01.035
[40] Lee S, Hirohama M, Noguchi M, et al. Influenza A Virus Infection Triggers Pyroptosis and Apoptosis of Respiratory Epithelial Cells through the Type I Interferon Signaling Pathway in a Mutually Exclusive Manner[J]. J Virol, 2018, 92(14): e00396-18. http://jvi.asm.org/content/92/14/e00396-18.full.pdf
[41] Rébé C, Derangère V, Ghiringhelli F. Induction of pyroptosis in colon cancer cells by LXRbeta[J]. Mol Cell Oncol, 2015, 2(1): e970094. doi: 10.4161/23723548.2014.970094
[42] Derangère V, Chevriaux A, Courtaut F, et al. Liver X receptorβ activation induces pyroptosis of human and murine colon cancer cells[J]. Cell Death Differ, 2014, 21(12): 1914-1924. doi: 10.1038/cdd.2014.117
[43] Jiao Y, Zhao H, Chen G, et al. Pyroptosis of MCF7 Cells Induced by the Secreted Factors of hUCMSCs[J]. Stem Cells Int, 2018, 2018: 5912194. http://www.researchgate.net/publication/328883475_Pyroptosis_of_MCF7_cells_induced_by_the_secreted_factors_of_HuCMSCs
[44] Chen YF, Qi HY, Wu FL. Euxanthone exhibits anti-proliferative and anti-invasive activities in hepatocellular carcinoma by inducing pyroptosis: preliminary results[J]. Eur Rev Med Pharmacol Sci, 2018, 22(23): 8186-8196. http://www.ncbi.nlm.nih.gov/pubmed/30556857
[45] Li G, Zhu L, Cao Z, et al. A New Participant in the Pathogenesis of Alcoholic Gastritis: Pyroptosis[J]. Cell Physiol Biochem, 2018, 49(1): 406-418. doi: 10.1159/000492902
[46] Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages[J]. Cell Chem Biol, 2017, 24(4): 507-514. doi: 10.1016/j.chembiol.2017.03.009
[47] Westbom C, Thompson JK, Leggett A, et al. Inflammasome Modulation by Chemotherapeutics in Malignant Mesothelioma[J]. PloS One, 2015, 10(12): e0145404. doi: 10.1371/journal.pone.0145404
[48] Akino K, Toyota M, Suzuki H, et al. Identification of DFNA5 as a target of epigenetic inactivation in gastric cancer[J]. Cancer Sci, 2007, 98(1): 88-95. doi: 10.1111/j.1349-7006.2006.00351.x
[49] Kim MS, Chang X, Yamashita K, et al. Aberrant promoter methylation and tumor suppressive activity of the DFNA5 gene in colorectal carcinoma[J]. Oncogene, 2008, 27(25): 3624-3634. doi: 10.1038/sj.onc.1211021
[50] Hu L, Chen M, Chen X, et al. Chemotherapy-induced pyroptosis is mediated by BAK/BAX-caspase-3-GSDME pathway and inhibited by 2-bromopalmitate[J]. Cell Death Dis, 2020, 11(4): 281. doi: 10.1038/s41419-020-2476-2
[51] Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores[J]. Nature, 2016, 535 (7610): 153-158. doi: 10.1038/nature18629
[52] Lage H, Helmbach H, Grottke C, et al. DFNA5 (ICERE-1) contributes to acquired etoposide resistance in melanoma cells[J]. FEBS Lett, 2001, 494(1-2): 54-59. doi: 10.1016/S0014-5793(01)02304-3
[53] Katheder N S, Khezri R, O'farrell F, et al. Microenvironmental autophagy promotes tumour growth[J]. Nature, 2017, 541(7637): 417-420. doi: 10.1038/nature20815
[54] 石汉平. 肿瘤是一种代谢性疾病[J]. 肿瘤代谢与营养电子杂志, 2018, 5(2): 111-116. Shi HP. Cancer is a metabolic disease[J]. Zhong Liu Dai Xie Yu Ying Yang Dian Zi Za Zhi, 2018, 5(2): 111-116.
[55] Gao P, He FF, Tang H, et al. NADPH oxidase-induced NALP3 inflammasome activation is driven by thioredoxin-interacting protein which contributes to podocyte injury in hyperglycemia[J]. J Diabetes Res, 2015, 2015: 504761. http://www.ncbi.nlm.nih.gov/pubmed/25834832
[56] Rheinheimer J, de Souza BM, Cardoso NS, et al. Current role of the NLRP3 inflammasome on obesity and insulin resistance: A systematic review[J]. Metabolism, 2017, 74: 1-9. doi: 10.1016/j.metabol.2017.06.002
[57] Leone RD, Powell JD. Metabolism of immune cells in cancer[J]. Nat Rev, Cancer, 2020, 20(9): 516-531. doi: 10.1038/s41568-020-0273-y
-
期刊类型引用(7)
1. 陈雅芳,许瑞旭,邱美云,高恒,张丽文. 基于耳揿针埋针联合中药穴位贴敷的针对性护理在胃癌病人临床护理中的应用效果. 全科护理. 2025(01): 153-156 .  百度学术
百度学术
2. 张伟康,胡志耀,钟剑锋,姜德培,文学,王新友,陈泓磊. BLM基因与胃癌免疫浸润水平及临床预后的相关性分析. 消化肿瘤杂志(电子版). 2024(02): 194-200 .  百度学术
百度学术
3. 闫微婕,杜贞华,黄忠献,林文俐. 动脉灌注化疗在胃癌新辅助治疗中的应用. 中国肿瘤临床. 2024(15): 801-805 .  百度学术
百度学术
4. 高婷婷,丁井永,李珊,于德全. 扶正固本祛邪加减方联合调强放疗治疗Ⅲ~Ⅳ期胃癌的临床效果观察. 新疆医科大学学报. 2023(06): 832-836 .  百度学术
百度学术
5. 谢刚,张静,卢今. 艾迪注射液降低胃癌患者化疗过程中骨髓抑制发生率及其影响因素的研究. 中国医院用药评价与分析. 2023(06): 673-677+681 .  百度学术
百度学术
6. 王龙彪,刘洪,董天雄. 中心体扩增细胞占比和C反应蛋白-白蛋白比值对胃癌根治术治疗预后的预测价值. 中华普通外科学文献(电子版). 2023(05): 352-356 .  百度学术
百度学术
7. 冯立宗,陈永强,张志鹏,张坚. 基于倾向性匹配对进展期胃癌病人根治性手术联合腹腔热灌注化疗安全性的评估. 青岛大学学报(医学版). 2023(05): 671-674 .  百度学术
百度学术
其他类型引用(25)




 下载:
下载: