-
摘要:
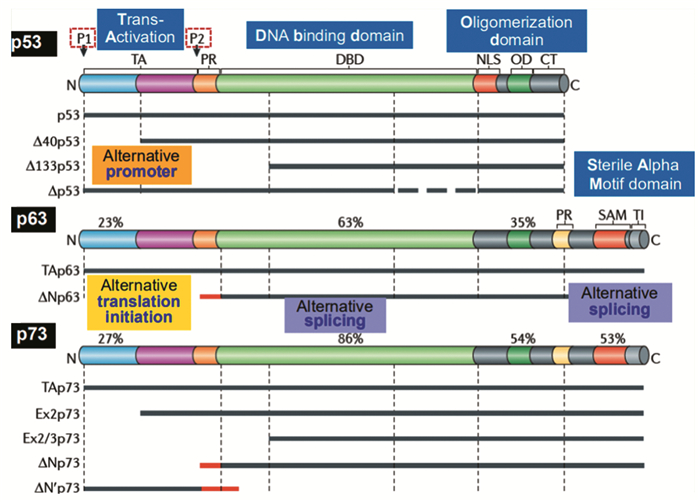
p73蛋白是p53蛋白家族的主要成员之一,其编码基因TP73与TP53基因高度同源。与p53蛋白类似,p73蛋白功能涉及细胞生命活动的各个方面;但又与p53不同,p73蛋白在机体正常的细胞活动和肿瘤的发生发展中不仅扮演着抑癌因子的角色,其功能的复杂性和重要性丝毫不亚于p53蛋白。肿瘤发生涉及多种细胞生物学过程,例如细胞凋亡、自噬、迁移和代谢等。这些正常的细胞生物学过程受多条细胞信号通路的严密调节而维持机体稳态,一旦调节通路发生致癌性异常将最终导致肿瘤发生。因此,本文主要聚焦在近几年p73与肿瘤发生的重要研究成果及其异于p53蛋白的独特功能。
Abstract:P73 protein is one of the main members of p53 protein family, and its coding gene TP73 is highly homologous to TP53 gene. On one hand, similar to p53, p73 protein is involved in all aspects of cell life. On the other hand, unlike the p53, not only p73 protein plays the role of tumor suppressor in normal cell activities and tumor development, but also the function of p73 protein is no less complex and important than that of p53 protein. Tumorigenesis involves a variety of cellular biological processes, such as apoptosis, autophagy, cell migration and cell metabolism. These normal cellular biological processes are tightly regulated by multiple cellular signaling pathways to maintain homeostasis. Once a carcinogenic abnormality occurs, it will eventually lead to tumorigenesis. Therefore, this paper mainly focuses on the important research results of p73 and tumorigenesis and its unique function different from p53 protein in recent years.
-
Key words:
- TP73 gene /
- Tumorigenesis /
- Biological process
-
Competing interests: The authors declare that they have no competing interests.作者贡献:许杰:论文构思及撰写郝牧:课题资金支持和论文修改
-
[1] Levine AJ, Tomasini R, McKeon FD, et al. The p53 family: guardians of maternal reproduction[J]. Nat Rev Mol Cell Biol, 2011, 12(4): 259-265. doi: 10.1038/nrm3086
[2] Napoli M, Flores ER. The p53 family reaches the final frontier: the variegated regulation of the dark matter of the genome by the p53 family in cancer[J]. RNA Biol, 2020, 17(11): 1636-1647. doi: 10.1080/15476286.2019.1710054
[3] Hall C, Muller PAJ. The Diverse Functions of Mutant 53, Its Family Members and Isoforms in Cancer[J]. Int J Mol Sci, 2019, 20(24): 6188. doi: 10.3390/ijms20246188
[4] Biscotti MA, Barucca M, Carducci F, et al. The p53 gene family in vertebrates: Evolutionary considerations[J]. J Exp Zool B Mol Dev Evol, 2019, 332(6): 171-178. doi: 10.1002/jez.b.22856
[5] Vikhreva P, Melino G, Amelio I. p73 Alternative Splicing: Exploring a Biological Role for the C-Terminal Isoforms[J]. J Mol Biol, 2018, 430(13): 1829-1838. doi: 10.1016/j.jmb.2018.04.034
[6] Zhang YX, Pan WY, Chen J. p53 and its isoforms in DNA double-stranded break repair[J]. J Zhejiang Univ Sci B, 2019, 20(6): 457-466. doi: 10.1631/jzus.B1900167
[7] Wang H, Liu Y, Wang D, et al. The Upstream Pathway of mTOR-Mediated Autophagy in Liver Diseases[J]. Cells, 2019, 8(12): 1597. doi: 10.3390/cells8121597
[8] Huang F, Wang BR, Wang YG. Role of autophagy in tumorigenesis, metastasis, targeted therapy and drug resistance of hepatocellular carcinoma[J]. World J Gastroenterol, 2018, 24(41): 4643-4651. doi: 10.3748/wjg.v24.i41.4643
[9] Datta S, Chakraborty S, Panja C, et al. Reactive nitrogen species control apoptosis and autophagy in K562 cells: implication of TAp73α induction in controlling autophagy[J]. Free Radic Res, 2018, 52(4): 491-506. doi: 10.1080/10715762.2018.1449210
[10] Dulloo I, Hooi PB, Sabapathy K. Hypoxia-induced DNp73 stabilization regulates Vegf-A expression and tumor angiogenesis similar to TAp73[J]. Cell Cycle, 2015, 14(22): 3533-3539. doi: 10.1080/15384101.2015.1078038
[11] Stantic M, Wolfsberger J, Sakil H, et al. ΔNp73 enhances HIF-1α protein stability through repression of the ECV complex[J]. Oncogene, 2018, 37(27): 3729-3739. doi: 10.1038/s41388-018-0195-2
[12] Marini A, Rotblat B, Sbarrato T, et al. TAp73 contributes to the oxidative stress response by regulating protein synthesis[J]. Proc Natl Acad Sci U S A, 2018, 115(24): 6219-6224. doi: 10.1073/pnas.1718531115
[13] Nemajerova A, Moll UM. Tissue-specific roles of p73 in development and homeostasis[J]. J Cell Sci, 2019, 132(19): jcs2333378.
[14] Gunaratne PH, Pan Y, Rao AK, et al. Activating p53 family member TAp63: A novel therapeutic strategy for targeting p53-altered tumors[J]. Cancer, 2019, 125(14): 2409-2422. doi: 10.1002/cncr.32053
[15] Bunch B, Krishnan N, Greenspan RD, et al. TAp73 expression and P1 promoter methylation, a potential marker for chemoresponsiveness to cisplatin therapy and survival in muscle-invasive bladder cancer (MIBC)[J]. Cell Cycle, 2019, 18(17): 2055-2066. doi: 10.1080/15384101.2019.1638693
[16] Steder M, Alla V, Meier C, et al. DNp73 exerts function in metastasis initiation by disconnecting the inhibitory role of EPLIN on IGF1R-AKT/STAT3 signaling[J]. Cancer Cell, 2013, 24(4): 512-527. doi: 10.1016/j.ccr.2013.08.023
[17] Uboveja A, Satija Y K, Siraj F, et al. p73-NAV3 axis plays a critical role in suppression of colon cancer metastasis[J]. Oncogenesis, 2020, 9(2): 12. doi: 10.1038/s41389-020-0193-4
[18] Lee Y, Kwon YH. Regulation of apoptosis and autophagy by luteolin in human hepatocellular cancer Hep3B cells[J]. Biochem Biophys Res Commun, 2019, 517(4): 617-622. doi: 10.1016/j.bbrc.2019.07.073
[19] Lopriore P, Capitanio N, Panatta E, et al. TAp73 regulates ATP7A: possible implications for ageing-related diseases[J]. Aging (Albany NY), 2018, 10(12): 3745-3760.
[20] Nemajerova A, Amelio I, Gebel J, et al. Non-oncogenic roles of TAp73: from multiciliogenesis to metabolism[J]. Cell Death Differ, 2018, 25(1): 144-153. doi: 10.1038/cdd.2017.178
[21] Campbell SL, Wellen KE. Metabolic Signaling to the Nucleus in Cancer[J]. Mol Cell, 2018, 71(3): 398-408. doi: 10.1016/j.molcel.2018.07.015
[22] Itahana Y, Itahana K. Emerging Roles of p53 Family Members in Glucose Metabolism[J]. Int J Mol Sci, 2018, 19(3): 776. doi: 10.3390/ijms19030776
[23] Humpton TJ, Hock AK, Maddocks O, et al. p53-mediated adaptation to serine starvation is retained by a common tumour-derived mutant[J]. Cancer Metab, 2018, 6: 18. doi: 10.1186/s40170-018-0191-6
[24] Amelio I, Markert EK, Rufini A, et al. p73 regulates serine biosynthesis in cancer[J]. Oncogene, 2014, 33(42): 5039-5046. doi: 10.1038/onc.2013.456
[25] Sampath D, Calin GA, Puduvalli VK, et al. Specific activation of microRNA106b enables the p73 apoptotic response in chronic lymphocytic leukemia by targeting the ubiquitin ligase Itch for degradation[J]. Blood, 2009, 113(16): 3744-3753. doi: 10.1182/blood-2008-09-178707
[26] Zhang X, Ren D, Wu X, et al. miR-1266 Contributes to Pancreatic Cancer Progression and Chemoresistance by the STAT3 and NF-κB Signaling Pathways[J]. Mol Ther Nucleic Acids, 2018, 11: 142-158. doi: 10.1016/j.omtn.2018.01.004
[27] Zhang X, Zhang M, Wang G, et al. Tumor promoter role of miR647 in gastric cancer via repression of TP73[J]. Mol Med Rep, 2018, 18(4): 3744-3750.
[28] Knouf EC, Garg K, Arroyo JD, et al. An integrative genomic approach identifies p73 and p63 as activators of miR-200 microRNA family transcription[J]. Nucleic Acids Res, 2012, 40(2): 499-510. doi: 10.1093/nar/gkr731



 下载:
下载:

