Clinical Observation of mXELIRI and mFOLFIRI Combined with Cetuximab in First-line Treatment of Metastatic Left-side Colon Cancer
-
摘要:目的
初步比较mXELIRI与mFOLFIRI方案联合西妥昔单抗一线治疗转移性左半结肠癌的临床疗效及安全性。
方法回顾性分析68例接受西妥昔单抗联合mXELIRI或mFOLFIRI方案治疗的转移性左半结肠癌患者临床资料,比较西妥昔单抗联合两种化疗方案的客观缓解率(ORR)、疾病控制率(DCR)及无进展生存时间(PFS),并评估安全性。
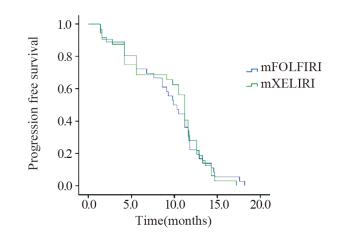
结果mXELIRI组和mFOLFIRI组的客观缓解率分别为62.5%和58.4%,疾病控制率为87.5%和88.9%,中位PFS分别为10.5和9.9月,差异均无统计学意义(均P > 0.05);mXELIRI组手足综合征发生率明显高于mFOLFIRI组(37.5% vs. 13.9%, P=0.025)。
结论西妥昔单抗联合mXELIRI或mFOLFIRI方案一线治疗转移性左半结肠癌的疗效及安全性相当。
Abstract:ObjectiveTo compare the clinical efficacy and safety between mXELIRI and mFOLFIRI combined with cetuximab in the first-line treatment of metastatic left-side colorectal cancer.
MethodsWe retrospectively analyzed the clinical data of 68 patients with metastatic left-side colorectal cancer treated with cetuximab combined with mXELIRI or mFOLFIRI. We compared the objective response rate (ORR), disease control rate (DCR) and progression-free survival time (PFS) between two regimens, and evaluated the safety.
ResultsThe ORR of mXELIRI and mFOLFIRI groups were 62.5% and 58.4%, the DCR were 87.5% and 88.9%, and the median PFS were 10.5 and 9.9 months, respectively (all P > 0.05). The incidence of hand-foot syndrome (HFS) of mXELIRI group was significantly higher than that of mFOLFIRI group (37.5% vs. 13.9%, P=0.025).
ConclusionCetuximab combined with mXELIRI and mFOLFIRI in the first-line treatment of metastatic left-side colorectal cancer have equivalent efficacy and safety.
-
Key words:
- Cetuximab /
- Bevacizumab /
- Irinotecan /
- Xeloda /
- Colorectal cancer
-
作者贡献程建平:方案制定、病例筛选和随访、论文撰写赵晓琳、李珍、杨梦媛、曹世长:病例数据整理、病例管理于久飞:选题、把握研究方向
-
表 1 68例转移性左半结直肠癌患者的临床病理特征(n(%))
Table 1 Clinicopathological features of 68 patients with metastatic left-side colorectal cancer (n(%))

表 2 68例转移性左半结直肠癌患者两组方案的不良反应(n(%))
Table 2 Chemotherapy-related side effects of 68 patients with metastatic left-side colorectal cancer treated with two regimens (n(%))

-
[1] Bajetta E, Bartolomeo MD, Mariani L, et al. Randomized multicenter Phase Ⅱ trial of two different schedules of irinotecan combined with capecitabine as first-line treatment in metastatic colorectal carcinoma[J]. Cancer, 2004, 100(2): 279-287.
[2] Fuchs CS, Moore MR, Harker G, et al. Phase Ⅲ Comparison of Two Irinotecan Dosing Regimens in Second-Line Therapy of Metastatic Colorectal Cancer[J]. J Clin Oncol, 2003, 21(5): 807-814.
[3] Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009, 45(2): 228-247.
[4] Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: The Common Terminology Criteria for Adverse Events Version 4.0[J]. J Am Acad Dermatol, 2012, 67(5): 1025-1039.
[5] Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer a randomized clinical trial[J]. JAMA, 2017, 317(23): 2392-2401. doi: 10.1001/jama.2017.7105
[6] Qin S, Li J, Wang L, et al. Efficacy and tolerability of first-line cetuximab plus leucovorin, fluorouracil, and oxaliplatin (FOLFOX-4) versus FOLFOX-4 in patients with RAS wild-type metastatic colorectal cancer: the open-label, randomized, Phase ⅢTAILOR trial[J]. J Clin Oncol, 2018, 36(30): 3031-3039. doi: 10.1200/JCO.2018.78.3183
[7] Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial[J].JAMA, 2017, 317(23): 2392-2401. doi: 10.1001/jama.2017.7105
[8] Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial[J]. Lancet Oncol, 2014, 15(10): 1065-1075. doi: 10.1016/S1470-2045(14)70330-4
[9] Kamran SC, Clark JW, Zheng H, et al. Primary tumor sidedness is an independent prognostic marker for survival in metastatic colorectal cancer: Results from a large retrospective cohort with mutational analysis[J]. Cancer Med, 2018, 7(7): 2934-2942. doi: 10.1002/cam4.1558
[10] Signorelli C, Chilelli MG, Sperduti I, et al. Correlation of Tumor Location to Clinical Outcomes in Colorectal Cancer: A Single-institution Retrospective Analysis[J]. Anticancer Res, 2019, 39(9): 4917-4924. doi: 10.21873/anticanres.13679
[11] Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials[J]. JAMA Oncol, 2017, 3(2): 194-201. doi: 10.1001/jamaoncol.2016.3797
[12] Fuchs CS, Marshall J, Barrueco J, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study[J]. J Clin Oncol, 2008, 26(4): 689-690. doi: 10.1200/JCO.2007.15.5390
[13] Cui C, Shu C, Yang Y, et al. XELIRI compared with FOLFIRI as a second-line treatment in patients with metastatic colorectal cancer[J]. Oncol Lett, 2014, 8(4): 1864-1872. doi: 10.3892/ol.2014.2335
[14] Ducreux M, Adenis A, Pignon JP, et al. Efficacy and safety of bevacizumab-based combination regimens in patients with previously untreated metastatic colorectal cancer: Final results from a randomised phase ii study of bevacizumab plus 5-fluorouracil, leucovorin plus irinotecan versus bevacizumab plus capecitabine plus irinotecan (FNCLCC ACCORD 13/0503 study)[J]. Eur J Cancer, 2013, 49(6): 1236-1245. doi: 10.1016/j.ejca.2012.12.011
[15] Xu RH, Muro K, Morita S, et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): a multicentre, open-label, randomised, non-inferiority, phase 3 trial[J]. Lancet Oncol, 2018, 19(5): 660-671. doi: 10.1016/S1470-2045(18)30140-2
[16] Rudek MA, Dasari A, Laheru D, et al. Phase I Study of ABT-751 in Combination with CAPIRI (Capecitabine and Irinotecan) and Bevacizumab in Patients with Advanced Colorectal Cancer[J]. J Clin Pharmacol, 2016, 56(8): 966-973. doi: 10.1002/jcph.681
[17] Garcia-Alfonso P, Muñoz-Martin A, Mendez-Ureña M, et al. Capecitabine in combination with irinotecan (XELIRI), administered as a 2-weekly schedule, as first-line chemotherapy for patients with metastatic colorectal cancer: a phase Ⅱ study of the Spanish GOTI group[J]. Br J Cancer, 2009, 101(7): 1039-1043. doi: 10.1038/sj.bjc.6605261
[18] Nakayama G, Mitsuma A, Sunagawa Y, et al. Randomized Phase II Trial of CapOX plus Bevacizumab and CapIRI plus Bevacizumab as First-Line Treatment for Japanese Patients with Metastatic Colorectal Cancer (CCOG-1201 Study)[J]. Oncologist, 2018, 23(8): 919-927. doi: 10.1634/theoncologist.2017-0640
[19] Moosmann N, Heinemann V. Cetuximab plus XELIRI or XELOX for first-line therapy of metastatic colorectal cancer[J]. Clin Colorectal Cancer, 2008, 7(2): 110-117. doi: 10.3816/CCC.2008.n.015
[20] Moosmann N, von Weikersthal LF, Vehling-Kaiser U, et al. Cetuximab Plus Capecitabine and Irinotecan Compared With Cetuximab Plus Capecitabine and Oxaliplatin As First-Line Treatment for Patients With Metastatic Colorectal Cancer: AIO KRK-0104—A Randomized Trial of the German AIO CRC Study Group[J]. J Clin Oncol, 2011, 29(8): 1050-1058. doi: 10.1200/JCO.2010.31.1936
[21] von Einem JC, Heinemann V, von Weikersthal LF, et al. Left-sided primary tumors are associated with favorable prognosis in patients with KRAS codon 12/13 wild-type metastatic colorectal cancer treated with cetuximab plus chemotherapy: an analysis of the AIO KRK-0104 trial[J]. J Cancer Res Clin Oncol, 2014, 140(9): 1607-1614. doi: 10.1007/s00432-014-1678-3



 下载:
下载:

