Adenovirus-mediated Hes1 Expression Inhibits Proliferation and Migration of Hepatocellular Carcinoma Cells
-
摘要:目的
探讨Hes1基因对肝细胞癌细胞系Hep1-6细胞增殖、迁移与侵袭的影响。
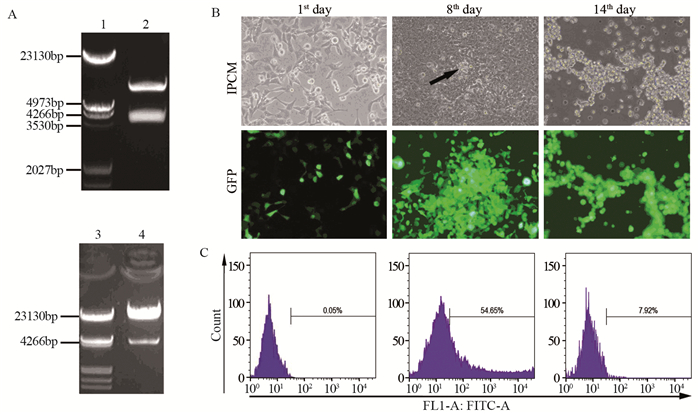
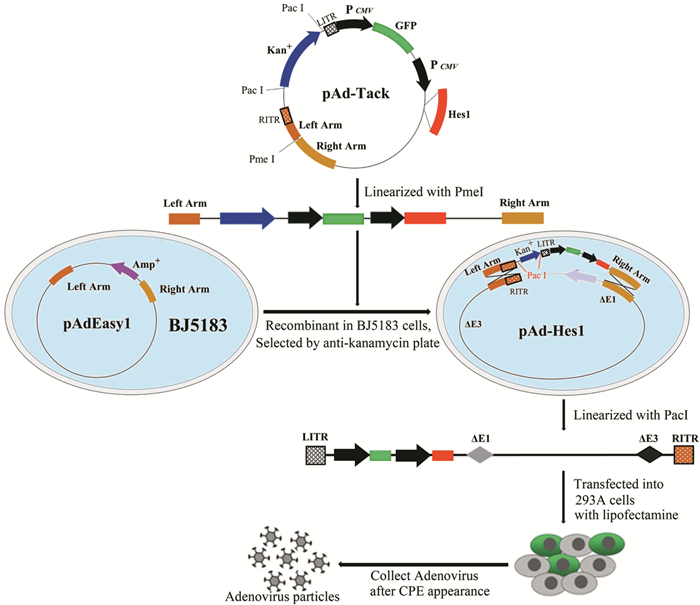
方法通过重组腺病毒载体介导Hes1基因在Hep1-6细胞系中过表达,随后测定细胞克隆形成率、增殖速率及体外迁移与侵袭能力的改变。
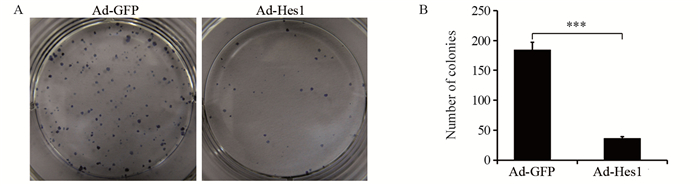
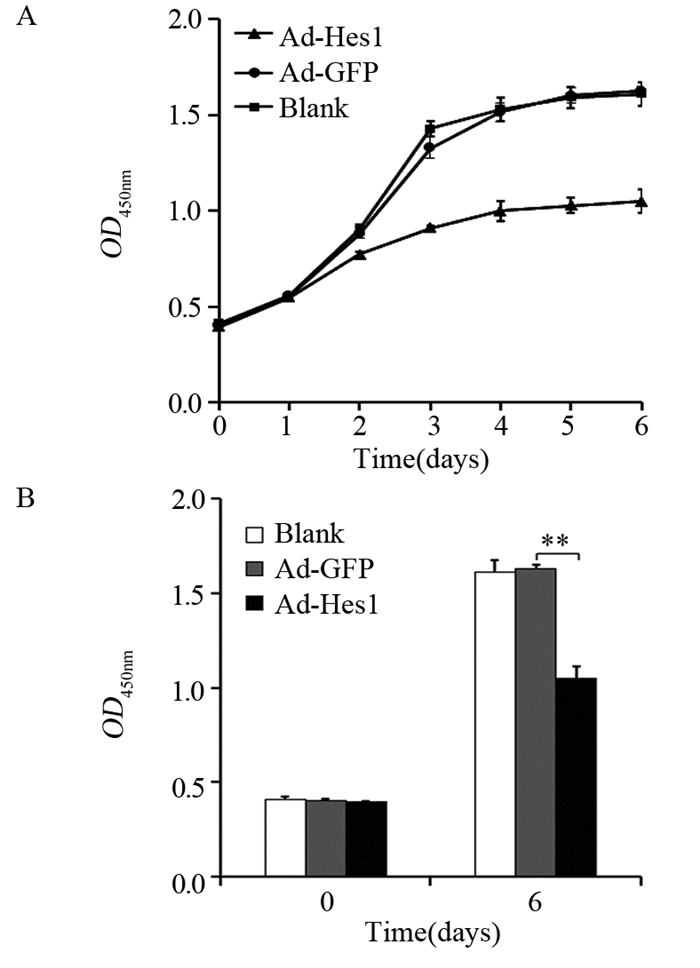
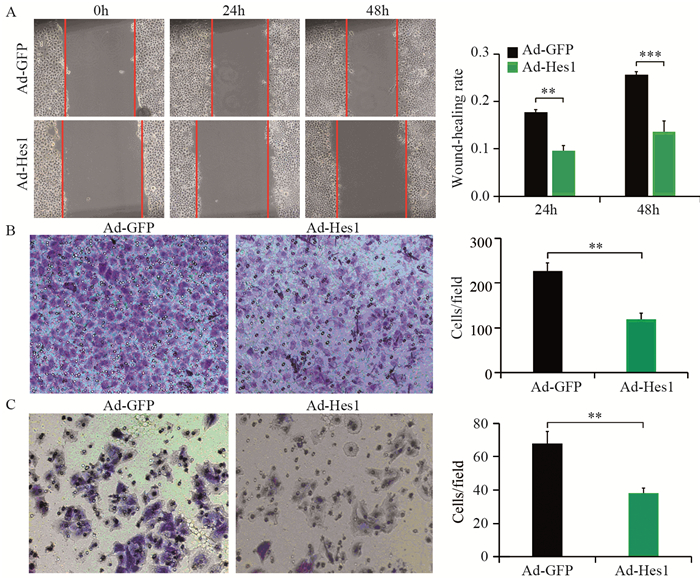
结果Hep1-6细胞分别感染Ad-Hes1和阴性对照Ad-GFP后,感染Ad-Hes1的细胞系克隆形成数显著少于对照组(P < 0.001);感染病毒6天后,CCK-8检测感染Ad-Hes1的细胞系在OD450 nm的吸光度值显著低于对照组(P < 0.01);感染Ad-Hes1的细胞系24 h和48 h的划痕愈合率显著低于对照组(P < 0.001);感染Ad-Hes1的细胞系在Transwell小室培养48 h后迁移进入Transwell下室的细胞数量显著低于对照组(P < 0.001);感染Ad-Hes1的细胞系在铺有Matrigel基质胶的Transwell小室培养48 h后侵袭进入Transwell下室的细胞数量显著低于对照组(P < 0.01)。
结论Hes1有抑制Hep1-6细胞增殖、迁移与侵袭的作用。
Abstract:ObjectiveTo investigate the effects of Hes1 gene on the proliferation, migration and invasion of hepatocellular carcinoma cell line Hep1-6.
MethodsCell proliferation, cell colony formation, migration and invasion were measured after Hes1 gene were overexpressed in Hep1-6 cells mediated by recombinant adenovirus.
ResultsThe clone numbers of Hep1-6 cells infected with Ad-Hes1 were significantly less than the control group infected with Ad-GFP (P < 0.001). Six days after Ad-Hes1 infection, OD450nm of Hep1-6 cells were significantly lower than that of control group (P < 0.01). The wound healing rates of Hep1-6 cells infected with Ad-Hes1 at 24h and 48h were significantly lower than those of the control group (P < 0.001). The number of Hep1-6 cells infected with Ad-Hes1 migrating into the lower chamber of Transwell after 48 h of culture was significantly lower than that of the control group (P < 0.001). The number of Hep1-6 cells infected with Ad-Hes1 invading into the lower chamber of Transwell after 48h of culture was significantly lower than that in the control group (P < 0.01).
ConclusionHes1 could suppress the proliferation, migration and invasion of Hep1-6 cells.
-
Key words:
- Hes1 /
- Adenovirus vector /
- HCC /
- Proliferation /
- Migration /
- Invasion
-
作者贡献于兵:设计研究方案、实施研究过程、撰写论文尹刚:分析实验数据、修改论文胡以平:提出研究思路、审核论文
-
-
[1] Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019[J]. CA Cancer J Clin, 2019, 69(1): 7-34. doi: 10.3322/caac.21551
[2] Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. doi: 10.3322/caac.21338
[3] Giakoustidis A, Giakoustidis D, Mudan S, et al. Molecular signalling in hepatocellular carcinoma: Role of and crosstalk among WNT/ß-catenin, Sonic Hedgehog, Notch and Dickkopf-1[J]. Can J Gastroenterol Hepatol, 2015, 29(4): 209-217. doi: 10.1155/2015/172356
[4] Nowell CS, Radtke F. Notch as a tumour suppressor[J]. Nat Rev Cancer, 2017, 17(3): 145-159. doi: 10.1038/nrc.2016.145
[5] Kim W, Khan SK, Gvozdenovic-Jeremic J, et al. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis[J]. J Clin Invest, 2017, 127(1): 137-152. https://www.jci.org/articles/view/88486
[6] Qi R, An H, Yu Y, et al. Notch1 signaling inhibits growth of human hepatocellular carcinoma through induction of cell cycle arrest and apoptosis[J]. Cancer Res, 2003, 63(23): 8323-8329. http://cn.bing.com/academic/profile?id=c1bd9841d6ffdc0292e3908c16b501fc&encoded=0&v=paper_preview&mkt=zh-cn
[7] Viatour P, Ehmer U, Saddic LA, et al. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway[J]. J Exp Med, 2011, 208(10): 1963-1976. doi: 10.1084/jem.20110198
[8] Li L, Tang P, Li S, et al. Notch signaling pathway networks in cancer metastasis: a new target for cancer therapy[J]. Med Oncol, 2017, 34(10): 180. doi: 10.1007/s12032-017-1039-6
[9] Rani A, Greenlaw R, Smith RA, et al. HES1 in immunity and cancer[J]. Cytokine Growth Factor Rev, 2016, 30: 113-117. doi: 10.1016/j.cytogfr.2016.03.010
[10] Murata K, Hattori M, Hirai N, et al. Hes1 directly controls cell proliferation through the transcriptional repression of p27Kip1[J]. Mol Cell Biol, 2005, 25(10): 4262-4271. doi: 10.1128/MCB.25.10.4262-4271.2005
[11] Liu ZH, Dai XM, Du B. Hes1: a key role in stemness, metastasis and multidrug resistance[J]. Cancer Biol Ther, 2015, 16(3): 353-359. doi: 10.1080/15384047.2015.1016662
[12] Zou JH, Xue TC, Sun C, et al. Prognostic significance of Hes-1, a downstream target of notch signaling in hepatocellular carcinoma[J]. Asian Pac J Cancer Prev, 2015, 16(9): 3811-3816. doi: 10.7314/APJCP.2015.16.9.3811
[13] Singh A, Paul MS, Dutta D, et al. Regulation of notch signaling by a chromatin modeling protein Hat-trick[J]. Development, 2019, 146(14). pii: dev170837. https://www.researchgate.net/publication/333465365_Regulation_of_notch_signaling_by_a_chromatin_modeling_protein_Hat-trick
[14] Siebel C, Lendahl U. Notch Signaling in Development, Tissue Homeostasis, and Disease[J]. Physiol Rev, 2017, 97(4): 1235-1294. doi: 10.1152/physrev.00005.2017
[15] Katoh M, Katoh M. Precision medicine for human cancers with Notch signaling dysregulation[J]. Int J Mol Med, 2020, 45(2): 279-297. http://cn.bing.com/academic/profile?id=38653227b4ffef9dfb8afa687b7575de&encoded=0&v=paper_preview&mkt=zh-cn
[16] Meurette O, Mehlen P. Notch Signaling in the Tumor Microenvironment[J]. Cancer Cell, 2018, 34(4): 536-548. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_2518892
[17] Geisler F, Strazzabosco M. Emerging roles of Notch signaling in liver disease[J]. Hepatology, 2015, 61(1): 382-392. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1002/hep.27268
[18] Li X, Cao Y, Li M, et al. Upregulation of HES1 Promotes Cell Proliferation and Invasion in Breast Cancer as a Prognosis Marker and Therapy Target via the AKT Pathway and EMT Process[J]. J Cancer, 2018, 9(4): 757-766. doi: 10.7150/jca.22319
[19] Kamińska A, Pardyak L, Marek S, et al. Bisphenol A and dibutyl phthalate affect the expression of juxtacrine signaling factors in rat testis[J]. Chemosphere, 2018, 199: 182-190. doi: 10.1016/j.chemosphere.2018.02.011
[20] Tian C, Yu Y, Jia Y, et al. HES1 activation suppresses proliferation of leukemia cells in acute myeloid leukemia[J]. Ann Hematol, 2015, 94(9): 1477-1483. doi: 10.1007/s00277-015-2413-0
[21] Wakabayashi N, Kageyama R, Habu T, et al. A novel cis-acting element regulates Hes-1 gene expression in P19 embryonal carcinoma cells treated with retinoic acid[J]. J Biochem, 2000, 128(6): 1087-1095. doi: 10.1093/oxfordjournals.jbchem.a022837
[22] Giovannini C, Gramantieri L, Minguzzi M, et al. CDKN1C/P57 is regulated by the Notch target gene Hes1 and induces senescence in human hepatocellular carcinoma[J]. Am J Pathol, 2012, 181(2): 413-422. doi: 10.1016/j.ajpath.2012.04.019



 下载:
下载: