Evaluation of 192Ir-Based Hypofractionated Stereotactic Ablative Brachytherapy as A Neoadjuvant Treatment for Operable Peripheral Non-small Cell Lung Cancer
-
摘要:目的
评价模板辅助192铱源大分割立体后装放射消融术(SABT)在可手术周围型非小细胞肺癌新辅助治疗中的临床效果。
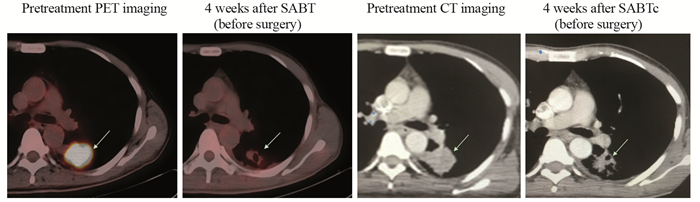
方法收集经病理证实的可手术周围型非小细胞肺癌初治患者,给予模板辅助SABT(30 Gy/1 F),并计划在SABT术后4~6周进行外科手术切除肿瘤。记录所有患者SABT围手术期的不良反应,并分别于治疗前、外科手术前行正电子发射断层扫描(PET/CT)和动态灌注CT(DCECT)扫描,通过比较肿瘤区体积(VGTV)、最大标准摄取值(SUVmax)、肿瘤血容量(TBV)和肿瘤血流量(TBF)来评价新辅助治疗效果。
结果所有患者在模板辅助SABT围手术期未出现严重并发症;4~6周后新辅助治疗效果评价指标明显下降(VGTV(P < 0.001)、SUVmax(P < 0.001)、TBV(P < 0.001)和TBF(P=0.009))。
结论对于可手术周围型非小细胞肺癌,模板辅助SABT新辅助治疗疗效明显,各评价指标明显下降,同时未观察到严重的不良反应。
Abstract:ObjectiveTo evaluate the safety, feasibility and efficacy of template-assisted 192Ir-based hypofractionated stereotactic ablative brachytherapy (SABT) combined with surgery for peripheral non-small cell lung cancer (NSCLC) patients.
MethodsWe enrolled the patients who were pathologically confirmed as operable peripheral NSCLC. The patients underwent template-assisted SABT (30 Gy delivered in one fraction) and were scheduled for tumor resection 4-6 weeks after SABT. The perioperative adverse reactions of SABT were recorded to evaluate the safety and feasibility of SABT as a neoadjuvant therapy. Imaging with 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (18F-FDG-PET/CT) and dynamic contrast-enhanced computed tomography (DCECT) were scheduled before SABT and surgery to evaluate the efficacy of SABT by comparing the gross tumor volume, maximum standardized uptake value, tumor blood volume and tumor blood flow.
ResultsAll patients did not experience any serious adverse event. After 4-6 weeks, the indicators for the efficacy of neoadjuvant therapy significantly decreased in all patients: gross tumor volume (P < 0.001), maximum standardized uptake value (P < 0.001), tumor blood volume (P < 0.001) and tumor blood flow (P=0.009).
ConclusionThe efficacy of template-assisted SABT as a neoadjuvant therapy is significant in operable peripheral NSCLC patients, without any serious adverse reaction.
-
Key words:
- Brachytherapy /
- Neoadjuvant /
- Non-small cell lung cancer /
- Safety /
- Efficacy
-
作者贡献石翔翔: 采集数据结果并起草论文; 庞皓文、孙小杨、任培蓉: 协助完善数据及后装放疗计划的设计、制作和实施; 吴敬波: 审核后装放疗计划; 林盛: 试验项目管理工作并指导论文写作
-
表 1 接受SABT新辅助治疗入组患者的一般情况
Table 1 Baseline characteristics of patients who underwent neoadjuvant SABT

表 2 SABT围手术期并发症(n=13)
Table 2 Complications of SABT in perioperative period (n=13)

表 3 GTV相关参数在SABT新辅助治疗前后的变化(x±s)
Table 3 VGTV, SUVmax, TBF and TBV before and after SABT (x±s)

-
[1] Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. Ca A Cancer J Clin, 2016, 66(2): 115-32. doi: 10.3322/caac.21338
[2] Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012[J]. Ca A Cancer J Clin, 2015, 65(2): 87-108. doi: 10.3322/caac.21262
[3] Berghmans T, Paesmans M, Sculier JP. Prognostic factors in stage Ⅲ non-small cell lung cancer: a review of conventional, metabolic and new biological variables[J]. Ther Adv Med Oncol, 2011, 3(3): 127-38. doi: 10.1177/1758834011401951
[4] 王亚旗, 王兴, 阎石, 等.非小细胞肺癌新辅助治疗联合外科治疗的进展[J].中国肺癌杂志, 2017, 20(5): 352-60. http://d.old.wanfangdata.com.cn/Periodical/zgfazz201705012 Wang YQ, Wang X, Yan S, et al. Progress of neoadjuvant therapy combined with surgery in non-small cell lung cancer[J]. Zhongguo Fei Ai Za Zhi, 2017, 20(5): 352-60. http://d.old.wanfangdata.com.cn/Periodical/zgfazz201705012
[5] Tanvetyanon T, Clark JI, Campbell SC, et al. Neoadjuvant therapy: an emerging concept in oncology[J]. South Med J, 2005, 98(3): 338-44. doi: 10.1097/01.SMJ.0000145313.92610.12
[6] Manning MA, Zwicker RD, Arthur DW, et al. Biologic treatment planning for high-dose-rate brachytherapy[J]. Int J Radiat Oncol, Biol Phys, 2001, 49(3): 839-45. doi: 10.1016/S0360-3016(00)01453-X
[7] Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial[J]. Lancet Oncol, 2015, 16(7): 795-803. doi: 10.1016/S1470-2045(15)00054-6
[8] Roses RE, Datta J, Czerniecki BJ. Radiation as immunomodulator: implications for dendritic cell-based immunotherapy[J]. Radiat Res, 2014, 182(2): 211-8. doi: 10.1667/RR13495.1
[9] Almo SC, Guha C. Considerations for combined immune checkpoint modulation and radiation treatment[J]. Radiat Res, 2015, 182(3): 230-8. doi: 10.1667/RR13667.1
[10] Wattenberg MM, Fahim A, Ahmed MM, et al. Unlocking the combination: potentiation of radiation-induced antitumor responses with immunotherapy[J]. Radiat Res, 2014, 182(2): 126-38. doi: 10.1667/RR13374.1
[11] Burnette B, Weichselbaum RR. The immunology of ablative radiation[J]. Semin Radiat Oncol, 2015, 25(1): 40-5. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3227385
[12] Xiang L, Zhang JW, Lin S, et al. Computed Tomography-Guided Interstitial High-Dose-Rate Brachytherapy in Combination With Regional Positive Lymph Node Intensity-Modulated Radiation Therapy in Locally Advanced Peripheral Non-Small Cell Lung Cancer: A Phase 1 Clinical Trial[J]. Int J Radiat Oncol Biol Phys, 2015, 92(5): 1027-34. doi: 10.1016/j.ijrobp.2015.04.019
[13] Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage Ⅰ nonsmall cell lung carcinoma[J]. Cancer, 2004, 101(7): 1623-31. doi: 10.1002/(ISSN)1097-0142
[14] Kim MS, Kim W, Park I, H et al. Radiobiological mechanisms of stereotactic body radiation therapy and stereotactic radiation surgery[J]. Radiat Oncol J, 2015, 33(4): 265-75. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4707209/
[15] Martínez-Monge R, Pagola M, Vivas I, et al. CT-guided permanent brachytherapy for patients with medically inoperable early-stage non-small cell lung cancer (NSCLC)[J]. Lung Cancer, 2008, 61(2): 209-13. doi: 10.1016/j.lungcan.2007.12.016
[16] Sharma DN, Rath GK, Thulkar S, et al. Computerized tomography-guided percutaneous high-dose-rate interstitial brachytherapy for malignant lung lesions[J]. J Cancer Res Ther, 2011, 7(2): 174-9. doi: 10.4103/0973-1482.82914
[17] Palma DA, Nguyen TK, Kwan K, et al. Short report: interim safety results for a phase Ⅱ trial measuring the integration of stereotactic ablative radiotherapy (SABR) plus surgery for early stage non-small cell lung cancer (MISSILE-NSCLC)[J]. Radiat Oncol, 2017, 12(1): 30. doi: 10.1186/s13014-017-0770-7
[18] Glover J, Velez-Cubian FO, Toosi K, et al. Perioperative outcomes and lymph node assessment after induction therapy in patients with clinical N1 or N2 non-small cell lung cancer[J]. J Thorac Dis, 2016, 8(8): 2165-74. doi: 10.21037/jtd
[19] Wang ZM, Lu J, Liu T, et al. CT-guided interstitial brachytherapy of inoperable non-small cell lung cancer[J]. Lung Cancer, 2011, 74(2): 253-7. doi: 10.1016/j.lungcan.2011.03.006
[20] Shirai K, Nakagawa A, Abe T, et al. Use of FDG-PET in Radiation Treatment Planning for Thoracic Cancers[J]. Int J Mol Imaging, 2012, 2012: 609545. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3361167
[21] Sasaki R, Komaki R, Macapinlac H, et al.[18F]Fluorodeoxyglucose Uptake by Positron Emission Tomography Predicts Outcome of Non-Small-Cell Lung Cancer[J]. J Clin Oncol, 23(6): 1136-43. doi: 10.1200/JCO.2005.06.129
[22] Sanli Y, Turkmen C, Bakir B, et al. Diagnostic value of PET/CT is similar to that of conventional MRI and even better for detecting small peritoneal implants in patients with recurrent ovarian cancer[J]. Nucl Med Commun, 2012, 33(5): 509-15. doi: 10.1097/MNM.0b013e32834fc5bf
[23] Hwang SO, Lee SW, Kim HJ, et al. The Comparative Study of Ultrasonography, Contrast-Enhanced MRI, and18F-FDG PET/CT for Detecting Axillary Lymph Node Metastasis in T1 Breast Cancer[J]. J Breast Cancer, 2013, 16(3): 315-21. doi: 10.4048/jbc.2013.16.3.315
[24] Krystal GW, Alesi E, Tatum JL. Early FDG/PET scanning as a pharmacodynamic marker of anti-EGFR antibody activity in colorectal cancer[J]. Mol Cancer Ther, 2012, 11(7): 1385-8. doi: 10.1158/1535-7163.MCT-12-0011
[25] Miles KA, Griffiths MR. Perfusion CT: a worthwhile enhancement?[J]. Br J Radiol, 2003, 76(904): 220-31. doi: 10.1259/bjr/13564625
[26] 邓东, 杨新官, 张小波, 等. 16层螺旋CT灌注成像强化指标和肿瘤微血管密度与肺癌淋巴结转移的关系[J].中华放射学杂志, 2010, 44(1): 24-8. doi: 10.3760/cma.j.issn.1005-1201.2010.01.007 Deng D, Yang XG, Zhang XB, et al. Correlation of 16-slice spiral CT perfusion enhancement parameters and histological microvessel density with lymphatic involvement in peripheral lung cancer[J]. Zhonghua Fang She Xue Za Zhi, 2010, 44(1): 24-8. doi: 10.3760/cma.j.issn.1005-1201.2010.01.007



 下载:
下载:

