-
摘要:目的
筛选与挖掘卵巢癌多药耐药相关基因及其生物信息。
方法基于GSE41499、GSE33482、GSE15372和GSE28739等4套来源不同的卵巢癌化疗耐药与敏感基因芯片表达谱数据集,综合运用差异基因表达分析、基因通路富集分析和文本挖掘等生物信息学方法预测卵巢癌多药耐药相关基因及通路。
结果MAPK信号通路、泛素介导的蛋白质水解、轴突导向、焦点粘连、神经营养素信号通路、癌症通路、肾细胞癌、柠檬酸循环、类萜骨干生物合成、错配修复和亨廷顿氏舞蹈病等11条基因通路是出现频率相对较高的显著性上调通路(P<0.05),甘油脂、戊醣酸途径、果糖和甘露糖代谢、谷胱甘肽代谢、蛋白酶体、p53信号通路和溶酶体等7条基因通路是出现频率相对较高的显著性下调通路(P<0.05);进一步的文本挖掘发现,ACO1、BDNF、CXCR4、HMGCR和NRP1等5个上调表达基因(P<0.05)和CDKN2C、FAS和SKP2等3个下调表达基因(P<0.05)可能与卵巢癌多药耐药形成相关。
结论卵巢癌多药耐药机制的形成可能涉及到多种不同的通路和基因,其中ACO1、BDNF、CXCR4、HMGCR、NRP1、CDKN2C、FAS和SKP2等基因可能在其中发挥着关键作用,后续研究将对其进行实验和临床双重验证。
Abstract:ObjectiveTo screen and mine the genes related to multidrug resistance (MDR) in ovarian cancer(OC) and their biological information.
MethodsBased on four different microarray expression profiles(GSE41499, GSE33482, GSE15372 and GSE28739) between resistant samples and sensitive samples relatedto OC, we performed a comprehensive bioinformatics analysis through gene expression analysis, geneticpathway enrichment analysis and text mining to predict the pathways and their genes related to MDR inOC.
ResultsEleven significantly upregulated pathways were found frequently among four OC microarraydatasets, including MAPK signaling pathway, ubiquitin-mediated proteolysis, axon guidance, focal adhesion,neurotrophin signaling pathway, pathways in cancer, renal cell carcinoma, citrate cycle, terpenoid backbonebiosynthesis, mismatch repair and Huntington’s disease(P<0.05); and seven significantly downregulatedpathways were found frequently, including glycerolipid metabolism, pentose phosphate pathway, fructoseand mannose metabolism, glutathione metabolism, proteasome, p53 signaling pathway and lysosome(P<0.05). By further text mining methods, we found five significantly upregulated genes, including ACO1,BDNF, CXCR4, HMGCR and NRP1(P<0.05), as well as three significantly downregulated genes, includingCDKN2C, FAS and SKP2(P<0.05), might be associated with MDR in OC.
ConclusionOC MDR mightbe involved in various pathways and genes. ACO1, BDNF, CXCR4, HMGCR, NRP1, CDKN2C, FAS andSKP2 might play crucial roles in those pathways. Follow-up study would validate the roles of those genes inthe experiments and clinical practice.
-
Key words:
- Ovarian cancer /
- MDR /
- Gene /
- Bioinformatics analysis /
- Screen and mine
-
0 引言
化疗是卵巢癌重要的治疗手段。尽管卵巢癌患者对化疗的初始反应率较高,但多数患者仍会复发和死亡。有研究指出,经过紫杉醇类和铂类药物等一线化疗方案化疗后,晚期患者初始反应率高达80%,完全缓解率达40%~60%,但70%~80%患者终会复发,其中位无进展生存时间为18月[1-2]。近年来,尽管卵巢癌化疗方案不断得到改进,但是其疗效并没有得到根本性改善,5年生存率仍徘徊在30%~45%[3]。普遍存在的肿瘤化疗耐药现象是导致化疗失败、制约生存率提高的中心环节。如何解决卵巢癌化疗耐药性问题,是目前医学界面临的难点之一。
卵巢癌化疗耐药机制至今仍未明确。基因芯片技术的出现,为卵巢癌化疗耐药机制研究提供新的途径。基因芯片技术是一种高通量、超微量的检测技术,在疾病诊疗、预防和机制研究中占有独特的优势。利用基因芯片技术检测卵巢癌发生、进展、组织学亚型、药物反应、预后等方面基因表达水平差异[4-6],是目前分子生物学领域中常用的研究手段,并由此积累了大量的实验数据。从生物信息学角度出发,对这些实验数据进行再利用,挖掘出潜在的有用的生物信息,不仅解决了实验成本高、周期长等缺点,还能为卵巢癌化疗耐药机制研究提供新的借鉴。本研究在多套已公开发表的基因芯片表达数据基础上,充分利用差异基因表达分析、基因通路富集分析和文本挖掘等生物信息学研究方法,旨在筛选出卵巢癌多药耐药相关基因及其生物信息,从而为阐明卵巢癌多药耐药机制提供理论依据。
1 资料与方法
1.1 资料来源
1.1.1 数据来源及获取途径
我们系统检索GEO(Gene Expression Omnibus)数据库[7],使用ovariancancer作为检索关键词,限制研究类型为expressionprofiling by array,限制种属为homo sapiens,检索出2014年2月以前世界范围内已公开报道的与卵巢癌耐药相关基因表达谱,结果共检索出30套与卵巢癌耐药相关的基因芯片表达数据集。
1.1.2 纳入标准
(1) 数据集必须是卵巢癌全基因组的表达mRNA芯片数据;(2) 这些数据必须是关于卵巢癌化疗耐药与敏感的对照研究;(3) 所下载的数据为原始数据集或者经过标准化处理;(4) 数据集病例(耐药)—对照(敏感)各组必须包括或超过3个样本;(5) 必须给出各样品明确的化疗耐药与敏感的信息。符合以上标准的数据集将纳入到本研究中。最终,只有4套数据集符合我们的纳入标准,分别为GSE41499、GSE33482、GSE15372和GSE28739。其中,GSE41499是关于PEO1和PEO4细胞株的基因表达数据集,GSE33482是关于A2780和A2780cis细胞株的基因表达数据集,GSE15372是基于A2780和Round5 A2780细胞株的基因表达数据集,GSE28739是有关卵巢上皮癌冰冻组织以铂类为基础初始化疗耐药和敏感的基因表达数据集。
1.2 方法
1.2.1 差异基因筛选
采用Benjamini-Hochberg(BH)法[8]进行差异基因表达分析,该多重假设检验方法在GEO2R[9] (http://www.ncbi.nlm.nih.gov/geo/geo2r/)工具中运行,以校正后P<0.05作为筛选差异表达基因的标准。另外结合倍数分析(foldchange,FC)方法进行分析,当ogFC<0时,基因呈下调表达;当logFC>0时,基因呈上调表达。
1.2.2 基因通路富集
在DAVID[10]工具中的KEGG(Kyoto Encyclopedia of Genes and Genomes)[11]库中进行基因通路富集分析。采用Fisher精确检验对通路的显著性进行检验,以P<0.05作为筛选差异表达基因的标准。
1.2.3 文本挖掘
从文献水平上挖掘出差异基因与一般性耐药(drug resistance)、多药耐药(drugresistance,multiple)和肿瘤耐药(drug resistance,neoplasm)的关系,该过程在COREMINE(http://www.coremine.com/medical/#search)工具中完成,利用CytoScape2.6.1软件[12]分别辅助构建差异基因与耐药共有文献联系图。以P<0.05作为差异有统计学意义的判断标准。从疾病水平揭示差异基因、肿瘤多药耐药与卵巢癌三者之间的联系,该过程在IPAD(Integrated Pathway Analysis Database,http://bioinfo.hsc.unt.edu/ipad/)数据库[13]中完成。
2 结果
2.1 筛选差异基因
GSE41499筛选出5 003个差异表达基因,包括2 505个上调表达基因和2 498个下调表达基因;GSE33482筛选出3 372个差异表达基因,包括1 487个上调表达基因和1 885个下调表达基因;GSE15372筛选出2 029个差异表达基因,包括798个上调表达基因和1 231个下调表达基因;GSE28739筛选出267个差异表达基因,包括180个上调表达基因和87个下调表达基因。
2.2 基因通路富集分析
GSE41499富集出76条基因通路,包括54条上调通路和22条下调通路;GSE33482富集出40条基因通路,包括12条上调通路和28条下调通路;GSE15372富集出50条基因通路,包括25条上调通路和25条下调通路;GSE28739富集出11条基因通路,包括5条上调通路和6条下调通路。
4套数据集中,MAPK信号通路、泛素介导的蛋白质水解、轴突导向、焦点粘连、神经营养素信号通路、癌症通路、肾细胞癌、柠檬酸循环、类萜骨干生物合成、错配修复和亨廷顿氏舞蹈病等11条基因通路是出现频率相对较高的显著性上调通路(P<0.05),甘油脂、戊醣酸途径、果糖和甘露糖代谢、谷胱甘肽代谢、蛋白酶体、p53信号通路和溶酶体等7条基因通路是出现频率相对较高的显著性下调通路(P<0.05)。各基因通路所涉及的差异表达基因,见表 1~2。
表 1 共同上调通路及其基因Table 1 Up-regulated pathways and their corresponding genes 表 2 共同下调通路及其基因Table 2 Down-regulated pathways and their corresponding genes
表 2 共同下调通路及其基因Table 2 Down-regulated pathways and their corresponding genes
2.3 文本挖掘
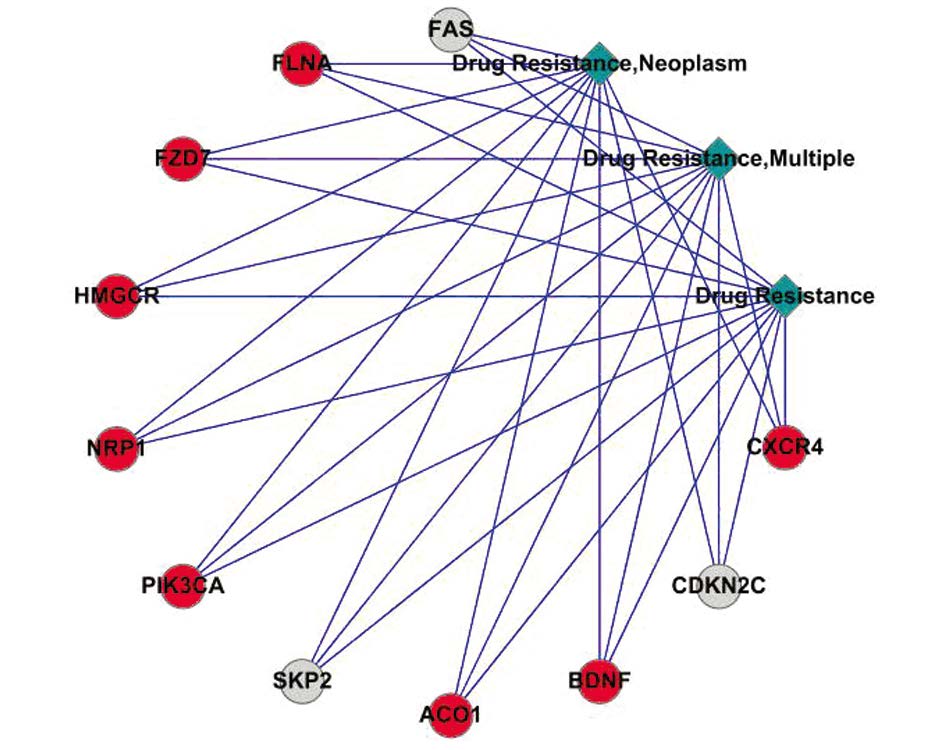
通过COREMINE工具检索表 1和表 2中的差异表达基因与一般性耐药(drug resistance)、多药耐药(drug resistance,multiple)和肿瘤耐药(drugresistance,neoplasm)的共有文献关系,并利用CytoScape2.6.1软件辅助构建网络联系图,见图 1。结果显示,FLNA、CXCR4、NRP1、PIK3CA、BDNF、ACO1、FZD7和HMGCR等上调表达基因与肿瘤多药耐药存在较强的共有文献联系,FAS、SKP2和CDKN2C等下调表达基因与肿瘤多药耐药存在较强的共有文献联系。
![]() 图 1 从文献水平上反映差异基因与一般性耐药、多药耐药和肿瘤耐药的关系图Figure 1 Co-occurrence analysis of differentially expressedgenes to exact keyword expression ‘drug resistance’, ‘drugresistance, multiple’ and ‘drug resistance, neoplasm’The red circles represented upregulated genes, and the grey circlesrepresented downregulated genes
图 1 从文献水平上反映差异基因与一般性耐药、多药耐药和肿瘤耐药的关系图Figure 1 Co-occurrence analysis of differentially expressedgenes to exact keyword expression ‘drug resistance’, ‘drugresistance, multiple’ and ‘drug resistance, neoplasm’The red circles represented upregulated genes, and the grey circlesrepresented downregulated genes分别利用I PAD数据库对表 1 和表 2 中的上调表达基因和下调表达基因进行检索。结果发现,ACLY、ACO1、ACO2、BDNF、CAV1、CSF2RA、CXCR4、FGF5、FH、FYN、FZD2、HMGCR、NDUFV1、NRP1、RFC5和TGFB2等上调表达基因以及PGD、TTK、MCM6、PFKL、PPAP2C、CDKN2C、ALDH9A1、SKP2、FAS和RRM2等下调表达基因均与卵巢癌相关,见表 3。
表 3 差异基因与卵巢癌的关系Table 3 Relationship between differentially expressed genes and ovarian cancer
从文献水平和疾病水平均反映了ACO1 、BDNF、CXCR4、HMGCR和NRP1等5个上调表达基因和CDKN2C、FAS和SKP2等3个下调表达基因既与肿瘤多药耐药相关,又与卵巢癌相关,可能均参与卵巢癌多药耐药机制的形成。
3 讨论
卵巢癌多药耐药问题一直是近年来研究的热点。多药耐药是卵巢癌化疗耐药的主要形式,且其分子机制复杂,可能涉及多基因、多信号通路的共同参与。通过广泛搜索,本研究查找到4套公开发表的样本来源不同的卵巢癌化疗耐药与敏感对照的基因芯片表达数据集,并筛选出卵巢癌耐药相关的共同通路,包括11条共同上调和7条共同下调通路,涉及到ACO1、BDNF、CXCR4、HMGCR和NRP1等5个上调表达基因和CDKN2C、FAS和SKP2等3个下调表达基因,这些基因可能参与卵巢癌多药耐药形成。
3.1 基因通路与卵巢癌多药耐药
卵巢癌多药耐药往往涉及到信号转导、代谢途径、蛋白酶体途径和DNA损伤与修复等通路。某些通路如MAPK信号通路等的激活[14-15]或者某些通路如P53信号通路等的失活[16-18],通过影响细胞的生长、增殖、分化和凋亡,参与卵巢癌多药耐药的形成。其中,MAPK信号通路是目前研究报道较多的与卵巢癌化疗耐药相关的通路。MAPK家族包括ERK、ERK3、ERK5/BMK1、ERK7、ERK8、JNK、NLK、P38 MAPK等8个亚族,由这些亚族组成多条信号通路,其中包括ERK1/2信号途径、JNK/SAPK信号途径、P38 MAPK信号途径等三条主要途径[19]。卵巢上皮性肿瘤细胞中的ERK1/2和JNK持续性激活依赖于铂类药物剂量,进而诱导DNA损伤和细胞凋亡,反过来,ERK1/2和JNK等MAPK信号通路的持续性激活,亦可诱导卵巢癌细胞对铂类药物的耐药性,而抑制ERK1/2信号通路激活,可增强卵巢癌对铂类药物的敏感度[14-15]。也有研究指出,ERK1/2通路的激活和P38MAPK通路的失活,可诱导卵巢癌细胞对铂类药物产生耐药现象[20]。
P53是一种抑癌基因,参与诱导细胞周期阻滞,促进细胞凋亡和DNA修复等。有研究指出,P53的功能状态与卵巢癌铂类药物化疗敏感度密切相关[16-18]。Vogelstein等[21]于2000年首次提出P53基因调控网络(即P53信号通路)的概念,认为P53基因调控网络中的基因相互作用共同调节细胞的生命活动。在P53信号通路中,MDM2-P53-P21WAF1/CIPI是较为重要的通路,该通路中任何基因的异常,均可能导致肿瘤的发生。已有研究表明,P53信号通路与卵巢癌发病和预后相关[22-23]。其他通路如错配修复的激活,是诱导卵巢癌铂类化疗耐药的一个重要因素[24-25],以上这些发现与本研究结果基本一致。
3.2 差异基因与卵巢癌多药耐药
基因异质性是肿瘤异质性的主要形式,主要体现在基因变异、基因扩增与丢失、基因表达谱的改变和基因的编码产物蛋白质水平的变化等方面。卵巢癌化疗耐药的发生,往往伴随着一系列基因的改变,比如基因变异、扩增、缺失或者甲基化等。它们通过某些通路的激活或失活,影响细胞增殖、迁移和浸润,导致肿瘤细胞对化疗药物反应产生差异,从而出现化疗耐药现象。通过本研究的文本挖掘,我们发现了8个新型的潜在的卵巢癌耐药基因,包括5个上调和3个下调表达基因。这些基因与卵巢癌化疗耐药关系尚未得到实验和临床验证。究其原因,我们分析如下:(1)以往研究多集中在MDR1、MRP、LRP、GST-π、ERCC1和VEGF等基因,且为单一研究的结果;(2)MDR1、MRP、LRP、GST-π、ERCC1和VEGF等基因可能与肿瘤多药耐药有关,然而在预测卵巢癌化疗耐药方面特异性不强;(3)通过多套芯片数据筛选和文本挖掘方法,可能为挖掘新型的潜在的卵巢癌耐药基因提供了新的途径。
近年来,有关CXCR4和NRP1的基因功能颇受到研究者的关注。CXCR4编码趋化因子受体4(chemokine receptor 4),该受体与其配体基质细胞衍生因子-1(stromal cell derived factor-1,SDF-1)特异性结合,参与肿瘤细胞增殖、浸润和定向迁移等,与肿瘤的进展和预后相关。Kwong等[26]发现,联合应用CTCE-9908(CXCR4受体拮抗剂)和紫杉醇可增加细胞毒性作用,诱导卵巢癌细胞有丝分裂障碍,故有学者认为,CXCR4可当作卵巢癌分子治疗的药物靶点[27]。NRP1是血管内皮生长因子(vascular endothelial growth factor,VEGF)的一个受体,具有促进癌症干细胞的自我更新能力,在肿瘤生长中具有重要的意义[28]。NRP1在癌组织中持续过度表达,严重影响患者的预后,是包括卵巢癌在内的人类多种恶性肿瘤潜在的抗肿瘤治疗靶点[29-30]。
综上所述,本研究运用逐一对比分析的研究方法,对4套来源不同的卵巢癌化疗耐药与敏感基因芯片表达谱数据集进行差异基因表达分析和基因通路富集分析,筛出影响卵巢上皮癌多药耐药的上调或下调基因及其相应的上调或下调通路,进一步从文献水平和疾病水平进行文本挖掘,筛选出8个与卵巢癌多药耐药存在较强联系的差异基因,包括5个上调(ACO1、BDNF、CXCR4、HMGCR和NRP1)和3个下调表达基因(CDKN2C、FAS和SKP2)。后续研究将对这些差异基因和通路进行实验和临床双重验证。
-
表 1 共同上调通路及其基因
Table 1 Up-regulated pathways and their corresponding genes

表 2 共同下调通路及其基因
Table 2 Down-regulated pathways and their corresponding genes

表 3 差异基因与卵巢癌的关系
Table 3 Relationship between differentially expressed genes and ovarian cancer

-
[1] Rubin SC, Randall TC, Armstrong KA, et al. Ten-year followup of ovarian cancer patients after second-look laparotomy with negative findings[J]. Obstet Gynecol, 1999, 93(1): 21-4. [1] Rubin SC, Randall TC, Armstrong KA, et al. Ten-year followupof ovarian cancer patients after second-look laparotomy withnegative findings[J]. Obstet Gynecol, 1999, 93(1): 21-4.
[2] Lin H, Changchien CC. Management of relapsed/refractory epithelial ovarian cancer: current standards and novel approaches[J]. Taiwan J Obstet Gynecol, 2007, 46: 379-88. [2] Lin H, Changchien CC. Management of relapsed/refractoryepithelial ovarian cancer: current standards and novelapproaches[J]. Taiwan J Obstet Gynecol, 2007, 46: 379-88.
[3] Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010[J]. CACancer J Clin, 2010, 60: 277-300.
[3] Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010[J]. CA Cancer J Clin, 2010, 60: 277-300. [4] Elgaaen BV, Olstad OK, Sandvik L, et al. ZNF385B and VEGFAare strongly differentially expressed in serous ovarian carcinomasand correlate with survival[J]. PLoS One, 2012, 7(9): e46317.
[4] Elgaaen BV, Olstad OK, Sandvik L, et al. ZNF385B and VEGFA are strongly differentially expressed in serous ovarian carcinomas and correlate with survival[J]. PLoS One, 2012, 7(9): e46317. [5] Huang RY, Chen GB, Matsumura N, et al. Histotype-specific copynumberalterations in ovarian cancer[J]. BMC Med Genomics,2012, 5: 47.
[5] Huang RY, Chen GB, Matsumura N, et al. Histotype-specific copynumber alterations in ovarian cancer[J]. BMC Med Genomics, 20 12, 5: 47. [6] Marchion DC, Cottrill HM, Xiong Y, et al. BAD phosphorylationdetermines ovarian cancer chemosensitivity and patientsurvival[J]. Clin Cancer Res, 2011, 17: 6356-66.
[6] Marchion DC, Cottrill HM, Xiong Y, et al. BAD phosphorylation determines ovarian cancer chemosensitivity and patient survival[J]. Clin Cancer Res, 2011, 17: 6356-66. [7] Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository[J]. Nucleic Acids Res, 2002, 30(1): 207-10. [7] Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus:NCBI gene expression and hybridization array data repository[J].Nucleic Acids Res, 2002, 30(1): 207-10.
[8] Benjamini Y, Hochberg Y. Controlling the false discovery rate-a practical and powerful approach to multiple testing[J]. J R STAT SOC B, 1995, 57(1): 289-300. [8] Benjamini Y, Hochberg Y. Controlling the false discovery rate-apractical and powerful approach to multiple testing[J]. J R STATSOC B, 1995, 57(1): 289-300.
[9] Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive forfunctional genomics data sets-update[J]. Nucleic Acids Res, 2013,41(Dataase issue): D991-5.
[9] Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets-update[J]. Nucleic Acids Res, 2013, 41 (Dataase issue): D991-5. [10] Huang DW, Sherman BT, Tan Q, et al. DAVID BioinformaticsResources: expanded annotation database and novel algorithms tobetter extract biology from large gene lists[J]. Nucleic Acids Res,2007, 35(Web Server issue): W169-75.
[10] Huang DW, Sherman BT, Tan Q, et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists[J]. Nucleic Acids Res, 20 07, 35(Web Server issue): W169-75. [11] Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes[J]. Nucleic Acids Res, 2000, 28(1): 27-30. [11] Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes andgenomes[J]. Nucleic Acids Res, 2000, 28(1): 27-30.
[12] Shannon P, Markiel A, Ozier O, et al. Cytoscape: a softwareenvironment for integrated models of biomolecular interactionnetworks[J]. Genome Res, 2003, 13(11): 2498-504.
[12] Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks[J]. Genome Res, 2003, 13(11): 2498-504. [13] Zhang F, Drabier R. IPAD: the Integrated Pathway Analysis Database for Systematic Enrichment Analysis[J]. BMC Bioinformatics, 2012, 13 Suppl 15: S7. [13] Zhang F, Drabier R. IPAD: the Integrated Pathway AnalysisDatabase for Systematic Enrichment Analysis[J]. BMCBioinformatics, 2012, 13 Suppl 15: S7.
[14] Rosanò L, Cianfrocca R, Spinella F, et al. Acquisition ofchemoresistance and EMT phenotype is linked with activation ofthe endothelin A receptor pathway in ovarian carcinoma cells[J].Clin Cancer Res, 2011, 17(8): 2350-60.
[14] Rosanò L, Cianfrocca R, Spinella F, et al. Acquisition of chemoresistance and EMT phenotype is linked with activation of the endothelin A receptor pathway in ovarian carcinoma cells[J]. Clin Cancer Res, 2011, 17(8): 2350-60. [15] Masoumi-Moghaddam S, Amini A, Wei AQ, et al. Sprouty2 protein in prediction of post-treatment ascites in epithelial ovarian cancer treated with adjuvant carbotaxol chemotherapy[J]. Am J Cancer Res, 2015, 5(8): 2498-507. [15] Masoumi-Moghaddam S, Amini A, Wei AQ, et al. Sprouty2protein in prediction of post-treatment ascites in epithelial ovariancancer treated with adjuvant carbotaxol chemotherapy[J]. Am JCancer Res, 2015, 5(8): 2498-507.
[16] Yang-Hartwich Y, Soteras MG, Lin ZP, et al. p53 proteinaggregation promotes platinum resistance in ovarian cancer[J].Oncogene, 2015, 34(27): 3605-16.
[16] Yang-Hartwich Y, Soteras MG, Lin ZP, et al. p53 protein aggregation promotes platinum resistance in ovarian cancer[J]. Oncogene, 2015, 34(27): 3605-16. [17] Woo MG, Xue K, Liu J, et al. Calpain-mediated processing ofp53-associated parkin-like cytoplasmic protein (PARC) affectschemosensitivity of human ovarian cancer cells by promoting p53subcellular trafficking[J]. J Biol Chem, 2012, 287(6): 3963-75.
[17] Woo MG, Xue K, Liu J, et al. Calpain-mediated processing of p53-associated parkin-like cytoplasmic protein (PARC) affects chemosensitivity of human ovarian cancer cells by promoting p53 subcellular trafficking[J]. J Biol Chem, 2012, 287(6): 3963-75. [18] Leung EL, Fraser M, Fiscus RR, et al. Cisplatin alters nitric oxide synthase levels in human ovarian cancer cells: involvement in p53 regulation and cisplatin resistance[J]. Br J Cancer, 2008, 98(11): 18 03-9. [18] Leung EL, Fraser M, Fiscus RR, et al. Cisplatin alters nitric oxidesynthase levels in human ovarian cancer cells: involvement in p53regulation and cisplatin resistance[J]. Br J Cancer, 2008, 98(11):1803-9.
[19] Fecher LA, Amaravadi RK, Flaherty KT. The MAPK pathway inmelanoma[J]. Curr Opin Oncol, 2008, 20(2): 183-9.
[19] Fecher LA, Amaravadi RK, Flaherty KT. The MAPK pathway in melanoma[J]. Curr Opin Oncol, 2008, 20(2): 183-9. [20] Villedieu M, Deslandes E, Duval M, et al. Acquisition ofchemoresistance following discontinuous exposures to cisplatin isassociated in ovarian carcinoma cells with progressive alterationof FAK, ERK and p38 activation in response to treatment[J].Gynecol Oncol, 2006, 101(3): 507-19.
[20] Villedieu M, Deslandes E, Duval M, et al. Acquisition of chemoresistance following discontinuous exposures to cisplatin is associated in ovarian carcinoma cells with progressive alteration of FAK, ERK and p38 activation in response to treatment[J]. Gynecol Oncol, 2006, 101(3): 507-19. [21] Vogelstein B, Lane D, Levine AJ. Surfing the p53 network[J].Nature, 2000, 408(6810): 307-10.
[21] Vogelstein B, Lane D, Levine AJ. Surfing the p53 network[J]. Nature, 2000, 408(6810): 307-10. [22] Green JA, Berns EM, Coens C, et al. Alterations in the p53pathway and prognosis in advanced ovarian cancer: a multifactorialanalysis of the EORTC Gynaecological Cancer group(study 55865)[J]. Eur J Cancer, 2006, 42(15): 2539-48.
[22] Green JA, Berns EM, Coens C, et al. Alterations in the p53 pathway and prognosis in advanced ovarian cancer: a multifactorial analysis of the EORTC Gynaecological Cancer group (study 55865)[J]. Eur J Cancer, 2006, 42(15): 2539-48. [23] Bartel F, Jung J, Böhnke A, et al. Both germ line and somaticgenetics of the p53 pathway affect ovarian cancer incidence andsurvival[J]. Clin Cancer Res, 2008, 14(1): 89-96.
[23] Bartel F, Jung J, Böhnke A, et al. Both germ line and somatic genetics of the p53 pathway affect ovarian cancer incidence and survival[J]. Clin Cancer Res, 2008, 14(1): 89-96. [24] Pani E, Stojic L, El-Shemerly M, et al. Mismatch repair status and the response of human cells to cisplatin[J]. Cell Cycle, 2007, 6( 14): 1796-802. [24] Pani E, Stojic L, El-Shemerly M, et al. Mismatch repair statusand the response of human cells to cisplatin[J]. Cell Cycle, 2007,6(14): 1796-802.
[25] Helleman J, van Staveren IL, Dinjens WN, et al. Mismatch repair andtreatment resistance in ovarian cancer[J]. BMC Cancer, 2006, 6: 201.
[25] Helleman J, van Staveren IL, Dinjens WN, et al. Mismatch repair and treatment resistance in ovarian cancer[J]. BMC Cancer, 2006, 6: 201. [26] Kwong J, Kulbe H, Wong D, et al. An antagonist of the chemokinereceptor CXCR4 induces mitotic catastrophe in ovarian cancercells[J]. Mol Cancer Ther, 2009, 8(7): 1893-905
[26] Kwong J, Kulbe H, Wong D, et al. An antagonist of the chemokine receptor CXCR4 induces mitotic catastrophe in ovarian cancer cells[J]. Mol Cancer Ther, 2009, 8(7): 1893-905. [27] Ray P, Lewin SA, Mihalko LA, et al. Noninvasive imaging reveals inhibition of ovarian cancer by targeting CXCL12-CXCR4[J]. Neoplasia, 2011, 13(12): 1152-61. [27] Ray P, Lewin SA, Mihalko LA, et al. Noninvasive imaging revealsinhibition of ovarian cancer by targeting CXCL12-CXCR4[J].Neoplasia, 2011, 13(12): 1152-61.
[28] Beck B, Driessens G, Goossens S, et al. A vascular niche and aVEGF-Nrp1 loop regulate the initiation and stemness of skintumours[J]. Nature, 2011, 478(7369): 399-403.
[28] Beck B, Driessens G, Goossens S, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours[J]. Nature, 2011, 478(7369): 399-403. [29] Grandclement C, Borg C. Neuropilins: a new target for cancer therapy[J]. Cancers (Basel), 2011, 3(2): 1899-928. [29] Grandclement C, Borg C. Neuropilins: a new target for cancertherapy[J]. Cancers (Basel), 2011, 3(2): 1899-928.
[30] Siamakpour-Reihani S, Owzar K, Jiang C, et al. Prognostic significance of differential expression of angiogenic genes in women with high-grade serous ovarian carcinoma[J]. Gynecol Oncol, 2015, 139(1): 23-9. [30] Siamakpour-Reihani S, Owzar K, Jiang C, et al. Prognosticsignificance of differential expression of angiogenic genes inwomen with high-grade serous ovarian carcinoma[J]. GynecolOncol, 2015, 139(1): 23-9.
-
期刊类型引用(0)
其他类型引用(1)



 下载:
下载: